Secondary Metabolites from Sida rhombifolia L. (Malvaceae) and the Vasorelaxant Activity of Cryptolepinone
Abstract
:1. Introduction
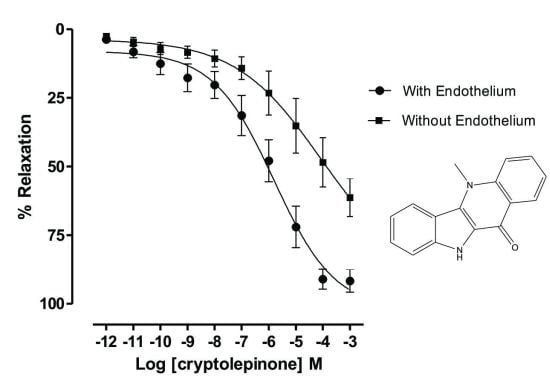
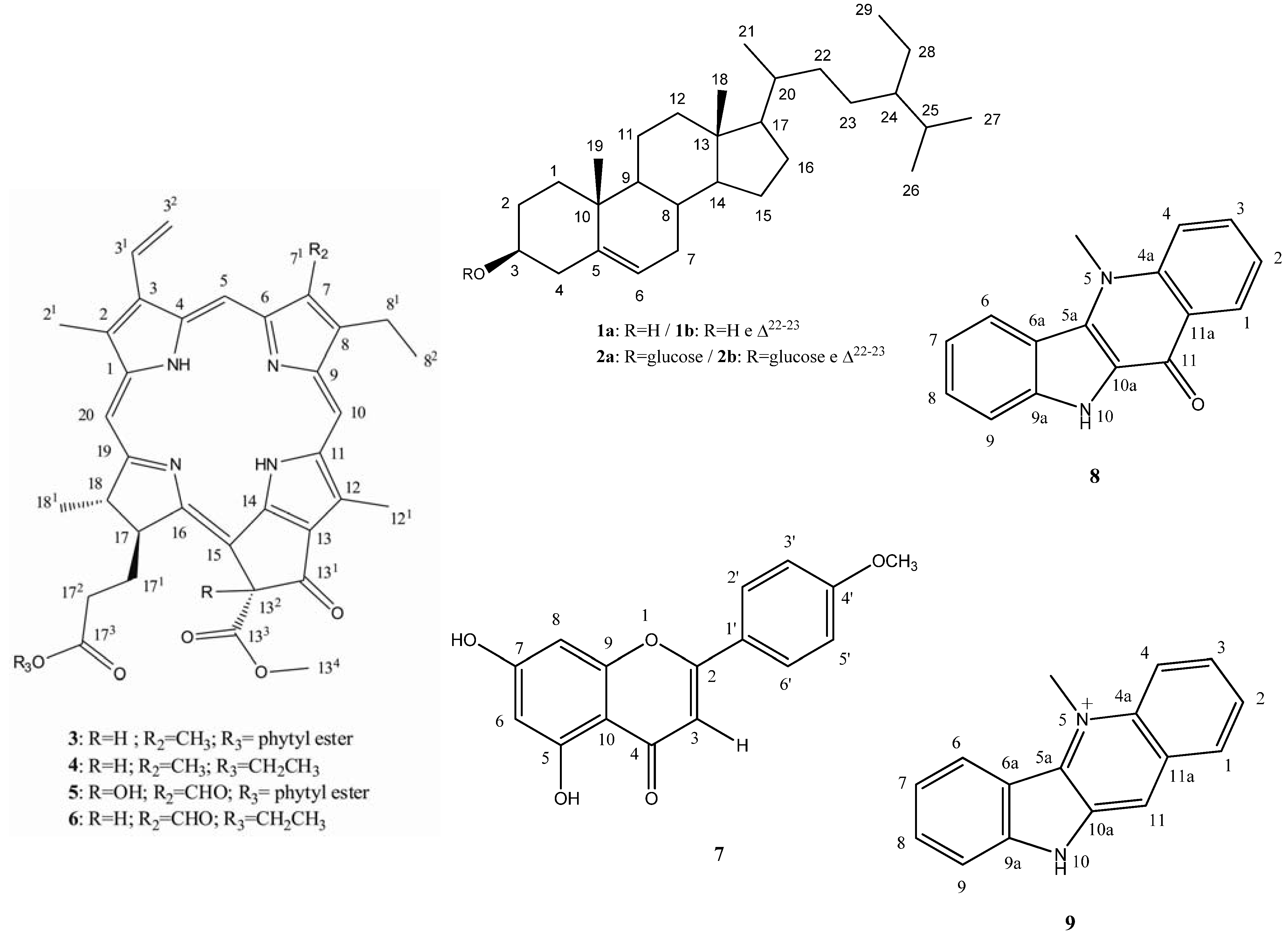
2. Results and Discussion
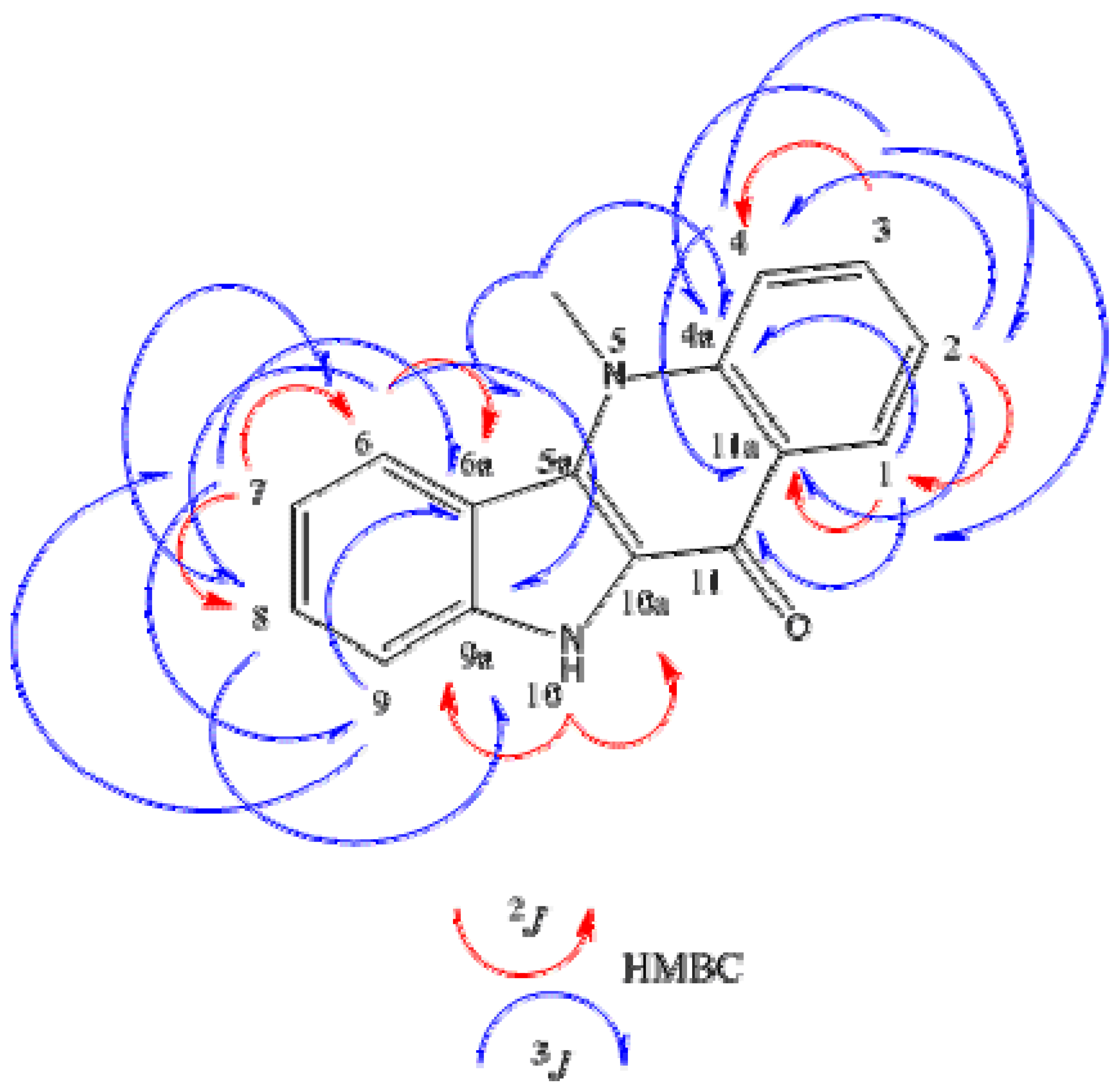
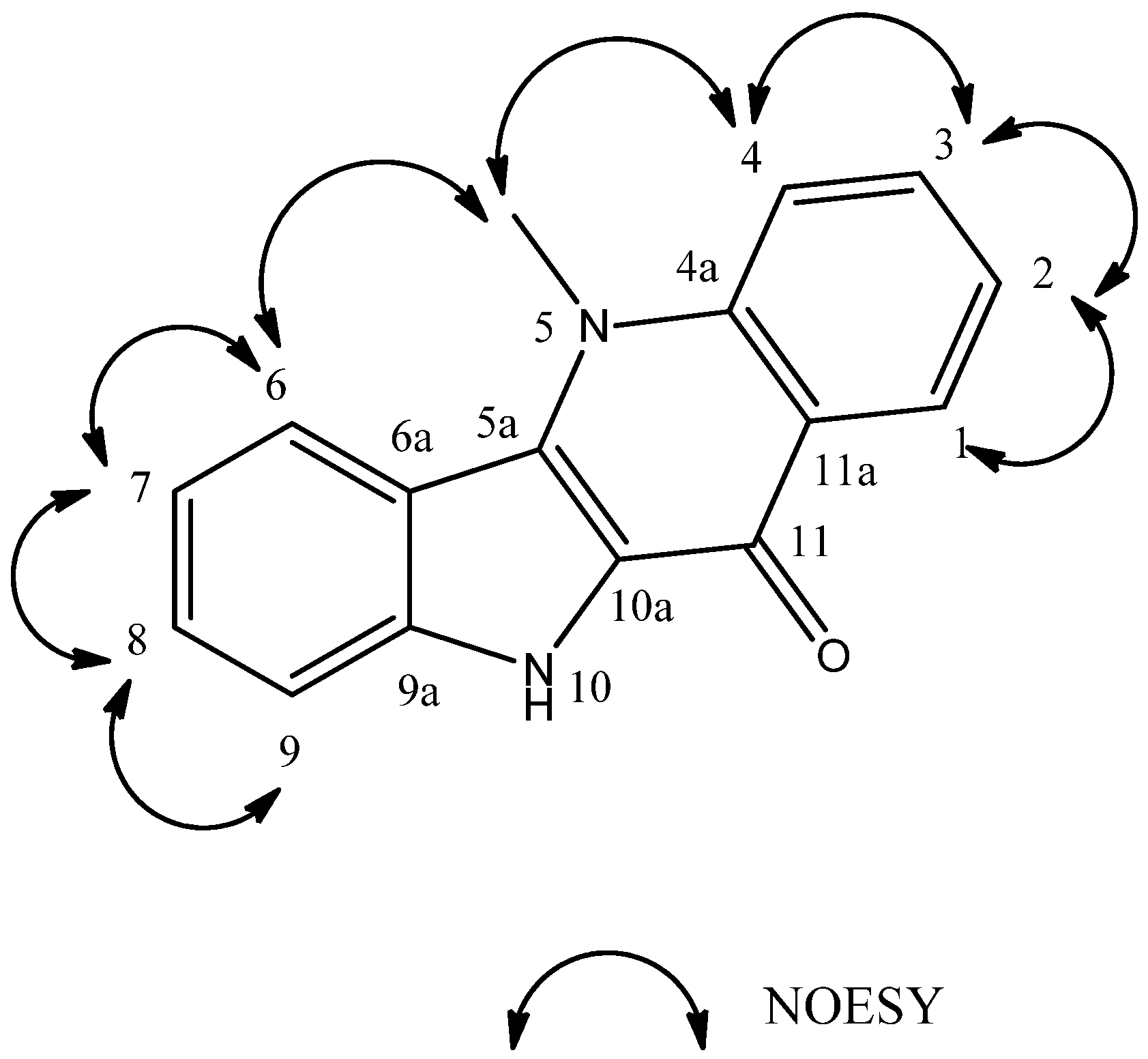
| HMQC | HMBC | NOESY | |||||
2J (H  C) C) | 3J (H  C) C) | (H↔H) | |||||
| n° | δC | δH | |||||
| 1 | 124.99 | 8.44 (dd, 1H, J = 8.1 and 1.4 Hz) | C-11a | C-11 | C-4a | H-2 | |
| 2 | 120.04 | 7.35 (ddt, 1H, J = 8.1; 7.3 and 0.8 Hz) | C-1 | C-4 | C-11a | H-1/H-3 | |
| 3 | 130.71 | 7.77 (ddt, 1H, J = 8.7; 7.3 and 1.4 Hz) | C-4 | C-1 | C-4a | H-2/H-4 | |
| 4 | 115.13 | 7.95 (brd, 1H, J = 8.7 Hz) | C-2 | C-11a | H-3 | ||
| 4a | 139.73 | - | |||||
| 5 | - | - | |||||
| 5a | 129.89 | - | |||||
| 6 | 122.45 | 8.38 (brd, 1H, J = 8,4 Hz) | C-6a | C-8 | C-9a | H-7 | |
| 6a | 115.70 | - | |||||
| 7 | 118.62 | 7.20 (ddt, 1H, J = 8.4; 7.6 and 1.0 Hz) | C-6 | C-8 | C-9 | C-6a | H-6/H-8 |
| 8 | 126.54 | 7.47 (ddt, 1H J = 8.3; 7.6 and 1.0 Hz) | C-6 | C-9a | H-7/H-9 | ||
| 9 | 112.36 | 7.57 (brd, 1H J = 8.3 Hz) | C-6a | C-7 | H-8 | ||
| 9a | 138.25 | - | |||||
| 10 (N-H) | - | 11.89 (s, 1H) | C-10a | C-9a | |||
| 10a | 123.03 | - | |||||
| 11 | 166.45 | - | |||||
| 11a | 122.90 | - | |||||
| N-CH3 | 35.41 | 4.36 (s, 3H) | C-4a | C-5a | H-4/H-6 | ||
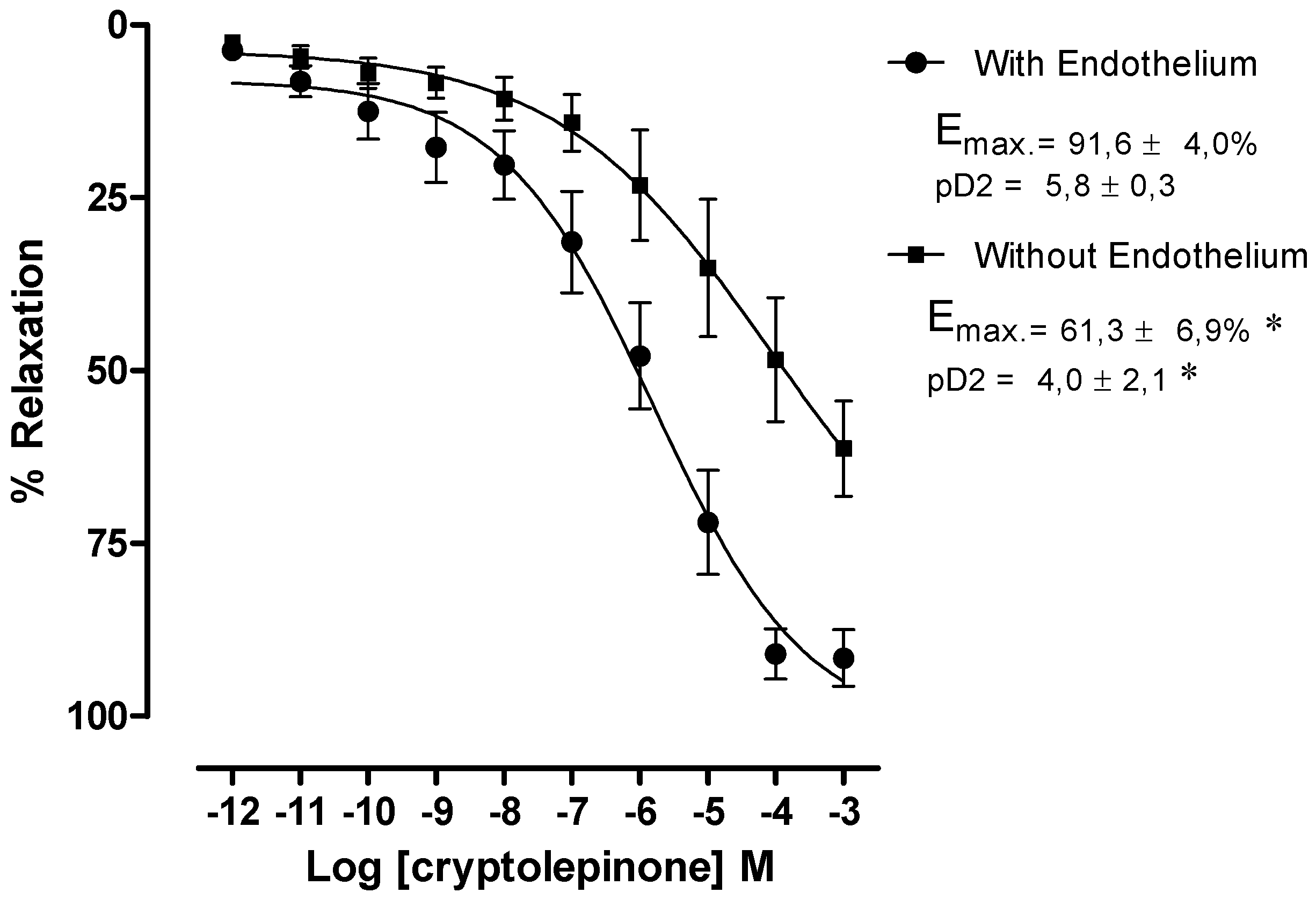
3. Experimental
3.1. General
3.2. Collection, Extraction and Isolation
3.3. Bioactivity Assay
4. Conclusions
Acknowledgments
References
- Silva, D.A.; Silva, T.M.S.; Lins, A.C.S.; Costa, D.A.; Cavalcante, J.M.S.; Matias, W.N.; Souza, M.F.V.; Braz Filho, R. Constituintes Químicos e Atividade Antioxidante de Sida galheirensis Ulbr. (Malvaceae). Quim. Nova 2006, 29, 1250–1256. [Google Scholar]
- Thounaojam, M.C.; Jadeja, R.N.; Ansarullah; Patel, V.B.; Devkar, R.V.; Ramachandran, A.V. Potential of Sida rhomboidea.Roxb Leaf Extract in Controlling Hypertriglyceridemia in Experimental Models. Pharmacognosy Res. 2009, 1, 208–212. [Google Scholar]
- Iswantini, D.; Darusman, L.K.; Hidayat, R. Indonesian Sidaguri (Sida rhombifolia L.) as Antigout and Inhibition Kinetics of Flavonoids Crude Extract on the Activity of Xanthine Oxidase. J. Biol. Sci. 2009, 9, 504–508. [Google Scholar]
- Jadhava, A.N.; Pawara, R.S.; Avulaa, B.; Khan, I.A. Ecdysteroid Glycosides from Sida rhombifolia L. Chem. Biodivers. 2007, 4, 2225–2230. [Google Scholar] [CrossRef]
- Prakash, A.; Varma, R.K.; Ghosal, S. Chemical Constituents of the Malvaceae. Part III. Alkaloidal Constituents of Sida acuta, Sida humilis, Sida rhombifolia and Sida spinosa. Planta Med. 1981, 43, 384–388. [Google Scholar]
- Martin, G.E.; Guido, J.E.; Robins, R.H.; Sharaf, M.H.M.; Schiff, P.L., Jr.; Tackie, A.N. Submicro Inverse-Detection Gradient NMR: A Powerful New Way of Conducting Structure Elucidation Studies with <0.05 micromol Samples. J. Nat. Prod. 1998, 61, 555–559. [Google Scholar] [CrossRef]
- Tousek, J.; Vanmiert, S.; Pieters, L.; Baelen, G.V.; Hostyn, S.; Maes, B.U.W.; Lemiere, G.; Dommisse, R.; Marek, R. Structural and Solvent Effects on the 13C and 15N NMR Chemical Shifts of Indoloquinoline Alkaloids: Experimental and DFT Study. Magn. Reson. Chem. 2008, 46, 42–51. [Google Scholar] [CrossRef]
- Karou, S.D.; Nadembega, W.M.C.; Ilboudo, D.P.; Ouermi, D.; Gbeassor, M.; de Souza, C.; Simpore, J. Sida acuta Burm. f.: A Medicinal Plant with Numerous Potencies. Afr. J. Biotechnol. 2007, 6, 2953–2959. [Google Scholar]
- Kojima, H.; Sato, N.; Hatano, A.; Ogura, H. Sterol Glucosides from Prunella vulgaris. Phytochemistry 1990, 29, 2351–2355. [Google Scholar] [CrossRef]
- Tomaz, A.C.A.; Nogueira, R.B.S.S.; Pinto, D.S.; Agra, M.F.; Souza, M.F.V.; Da-Cunha, E.V.L. Chemical Constiuents from Richardia grandiflora (Cham. & Schltdl.) Steud. (Rubiaceae). Rev. Bras. Farmacogn. 2008, 18, 47–52. [Google Scholar]
- Sakdarat, S.; Shuyprom, A.; Ayudhya, T.D.; Waterman, P.G.; Karagianis, G. Chemical Composition Investigation of the Clinacanthus nutans Lindau Leaves. Thai J. Phytopharm. 2006, 13, 13–24. [Google Scholar]
- Chee, C-F; Lee, H.B.; Ong, H.C.; Siong-Hockho, A. Photocytotoxic Pheophorbide-Related Compounds from Aglaonema simplex. Chem. Biodivers. 2005, 2, 1648–1655. [Google Scholar] [CrossRef]
- Gomes, R.A.; Ramirez, R.R.A.; Maciel, J.K.S.; Falcão-Siva, V.F.; Siqueira-Junior, J.P.; Agra, M.F.; Souza, M.F.V. Phenolic compounds from Sidastrum micranthum (A. St.-Hil.) Fryxell and Evaluation of Acacetin and 7,4'-Di-O-Methylisoscutellarein as Modulator of Bacterial Drug Resistance. Quim. Nova 2011, 34, 1385–1388. [Google Scholar]
- França-Silva, M.S.; Luciano, M.N.; Ribeiro, T.P.; Silva, J.S.; Santos, A.F.; França, K.C.; Nakao, L.S.; Athayde-Filho, P.F.; Braga, V.A.; Medeiros, I.A. The 2-Nitrate-1,3-Dibuthoxypropan, a New Nitric Oxide Donor, Induces Vasorelaxation in Mesenteric Arteries of the Rat. Eur. J. Pharmacol. 2012, 690, 170–175. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds sitosterol and stigmasterol (mixture), sitosterol-3-O-β-D-glucopyranoside and stigmasterol-3-O-β-D-glucopyranoside (mixture), 173-ethoxypheophorbide A, 173-ethoxypheophorbide B, cryptolepinone and a salt of cryptolepine are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chaves, O.S.; Gomes, R.A.; Tomaz, A.C.d.A.; Fernandes, M.G.; Das Graças Mendes Junior, L.; De Fátima Agra, M.; Braga, V.A.; De Fátima Vanderlei de Souza, M. Secondary Metabolites from Sida rhombifolia L. (Malvaceae) and the Vasorelaxant Activity of Cryptolepinone. Molecules 2013, 18, 2769-2777. https://doi.org/10.3390/molecules18032769
Chaves OS, Gomes RA, Tomaz ACdA, Fernandes MG, Das Graças Mendes Junior L, De Fátima Agra M, Braga VA, De Fátima Vanderlei de Souza M. Secondary Metabolites from Sida rhombifolia L. (Malvaceae) and the Vasorelaxant Activity of Cryptolepinone. Molecules. 2013; 18(3):2769-2777. https://doi.org/10.3390/molecules18032769
Chicago/Turabian StyleChaves, Otemberg Souza, Roosevelt Albuquerque Gomes, Anna Cláudia de Andrade Tomaz, Marianne Guedes Fernandes, Leônidas Das Graças Mendes Junior, Maria De Fátima Agra, Valdir Andrade Braga, and Maria De Fátima Vanderlei de Souza. 2013. "Secondary Metabolites from Sida rhombifolia L. (Malvaceae) and the Vasorelaxant Activity of Cryptolepinone" Molecules 18, no. 3: 2769-2777. https://doi.org/10.3390/molecules18032769
APA StyleChaves, O. S., Gomes, R. A., Tomaz, A. C. d. A., Fernandes, M. G., Das Graças Mendes Junior, L., De Fátima Agra, M., Braga, V. A., & De Fátima Vanderlei de Souza, M. (2013). Secondary Metabolites from Sida rhombifolia L. (Malvaceae) and the Vasorelaxant Activity of Cryptolepinone. Molecules, 18(3), 2769-2777. https://doi.org/10.3390/molecules18032769