Methyl Antcinate A Suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line
Abstract
:1. Introduction
2. Results
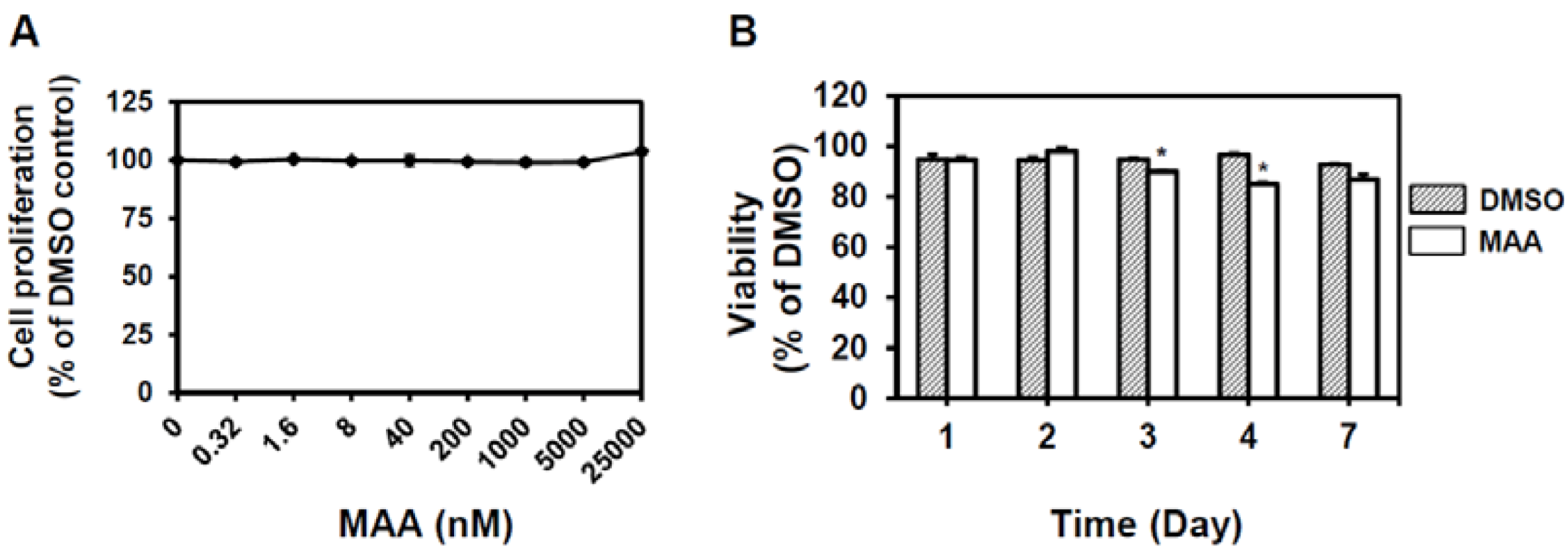
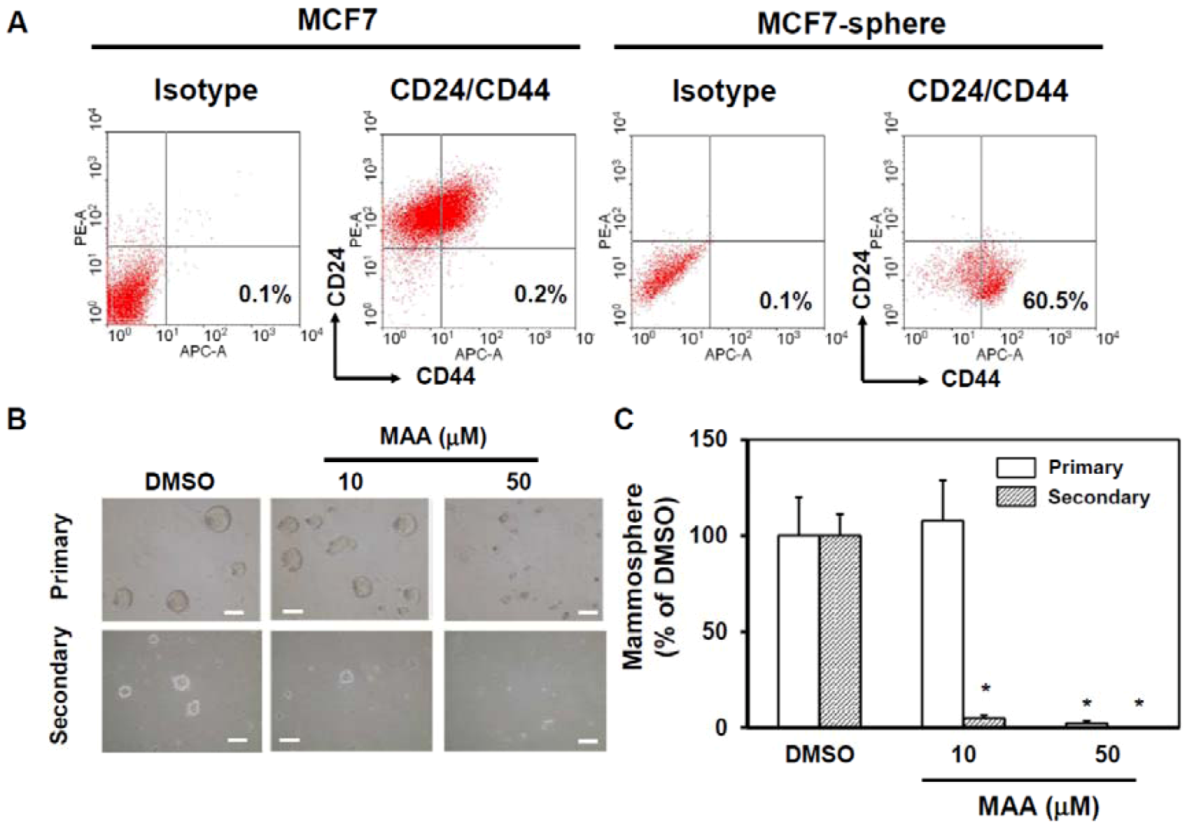
2.1. MAA Suppressed Self-Renewal Capability of MCF7 Mammospheres
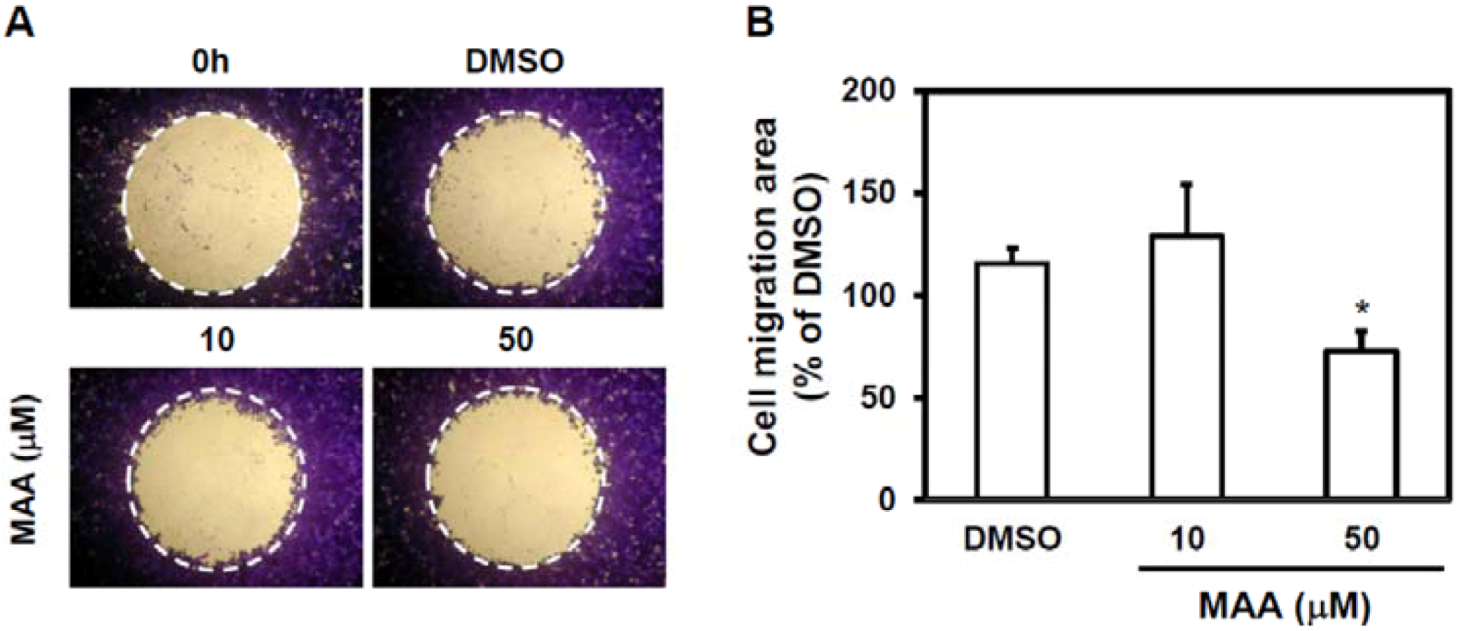
2.2. MAA Inhibited Cell Migration Ability of MCF7 Sphere Cells
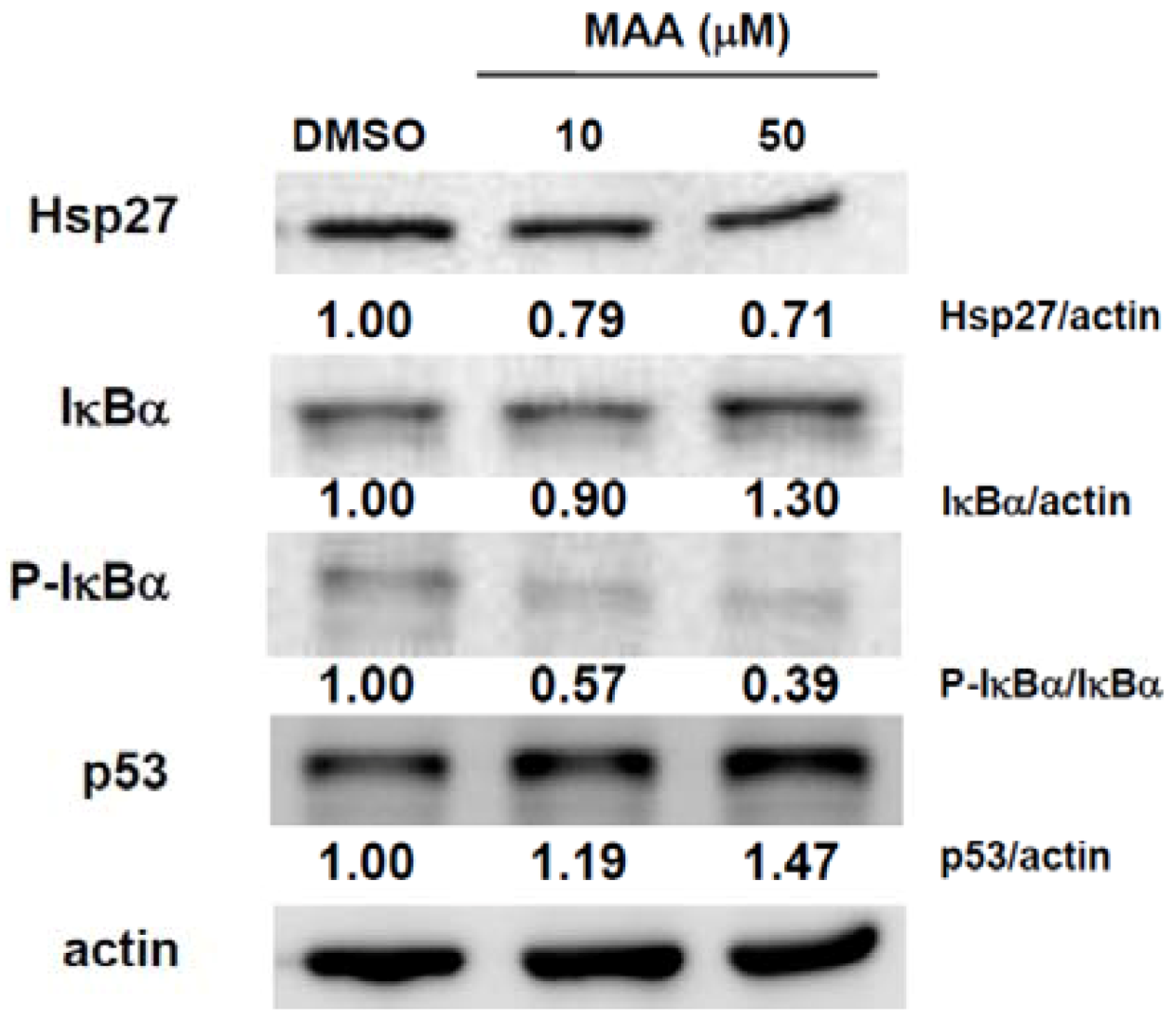
2.3. MAA Inhibited the Expression of Heat Shock Protein 27 (Hsp27) and Increased the Expression of IκBα and p53 in MCF7 Sphere Cells
3. Discussion
4. Experimental
4.1. Preparation of MAA
4.2. Cell Culture, Proliferation and Viability Assay
4.3. Mammosphere Assay
4.4. Flow Cytometric Analysis of CD24 and CD44 Expression
4.5. Cell Migration Assay
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Chang, T.T.; Chou, W.N. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol. Res. 1995, 99, 3. [Google Scholar]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J. Ethnopharmacol. 2007, 114, 78–85. [Google Scholar]
- Geethangili, M.; Tzeng, Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. Based Complement. Alternat. Med. 2011, 2011, 212641. [Google Scholar]
- Tsai, W.C.; Rao, Y.K.; Lin, S.S.; Chou, M.Y.; Shen, Y.T.; Wu, C.H.; Geethangili, M.; Yang, C.C.; Tzeng, Y.M. Methylantcinate A induces tumor specific growth inhibition in oral cancer cells via Bax-mediated mitochondrial apoptotic pathway. Bioorg. Med. Chem. Lett. 2010, 20, 6145–6148. [Google Scholar]
- Hsieh, Y.C.; Rao, Y.K.; Wu, C.C.; Huang, C.Y.; Geethangili, M.; Hsu, S.L.; Tzeng, Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem. Res. Toxicol. 2010, 23, 1256–1267. [Google Scholar]
- Lee, Y.P.; Tsai, W.C.; Ko, C.J.; Rao, Y.K.; Yang, C.R.; Chen, D.R.; Yang, M.H.; Yang, C.C.; Tzeng, Y.M. Anticancer effects of eleven triterpenoids derived from Antrodia camphorata. Anticancer Res. 2012, 32, 2727–2734. [Google Scholar]
- Hermann, P.C.; Bhaskar, S.; Cioffi, M.; Heeschen, C. Cancer stem cells in solid tumors. Semin. Cancer Biol. 2010, 20, 77–84. [Google Scholar] [CrossRef]
- Bomken, S.; Fiser, K.; Heidenreich, O.; Vormoor, J. Understanding the cancer stem cell. Br. J. Cancer 2010, 103, 439–445. [Google Scholar]
- Dalerba, P.; Cho, R.W.; Clarke, M.F. Cancer stem cells: Models and concepts. Annu. Rev. Med. 2007, 58, 267–284. [Google Scholar] [CrossRef]
- Chuthapisith, S.; Eremin, J.; El-Sheemey, M.; Eremin, O. Breast cancer chemoresistance: Emerging importance of cancer stem cells. Surg. Oncol. 2010, 19, 27–32. [Google Scholar] [CrossRef]
- Nakai, E.; Park, K.; Yawata, T.; Chihara, T.; Kumazawa, A.; Nakabayashi, H.; Shimizu, K. Enhanced MDR1 expression and chemoresistance of cancer stem cells derived from glioblastoma. Cancer Invest. 2009, 27, 901–908. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.J.; Chan, K.W.; Guan, X.Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PκB survival pathway. Oncogene 2008, 27, 1749–1758. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef]
- Velasco-Velazquez, M.A.; Popov, V.M.; Lisanti, M.P.; Pestell, R.G. The role of breast cancer stem cells in metastasis and therapeutic implications. Am. J. Pathol. 2011, 179, 2–11. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar]
- Millarte, V.; Farhan, H. The Golgi in cell migration: Regulation by signal transduction and its implications for cancer cell metastasis. ScientificWorldJournal 2012, 2012, 498278. [Google Scholar]
- Wei, L.; Liu, T.T.; Wang, H.H.; Hong, H.M.; Yu, A.L.; Feng, H.P.; Chang, W.W. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast Cancer Res. 2011, 13, R101. [Google Scholar]
- Cicalese, A.; Bonizzi, G.; Pasi, C.E.; Faretta, M.; Ronzoni, S.; Giulini, B.; Brisken, C.; Minucci, S.; di Fiore, P.P.; Pelicci, P.G. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 2009, 138, 1083–1095. [Google Scholar] [CrossRef]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef]
- Lee, T.K.; Castilho, A.; Cheung, V.C.; Tang, K.H.; Ma, S.; Ng, I.O. Lupeol targets liver tumor-initiating cells through phosphatase and tensin homolog modulation. Hepatology 2011, 53, 160–170. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Kim, B.Y.; Lee, H.S.; Ahn, J.S.; Chang, Y.S. Inhibition of phospholipase cgamma1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla. J. Nat. Prod. 2000, 63, 753–756. [Google Scholar]
- Soo, E.T.; Yip, G.W.; Lwin, Z.M.; Kumar, S.D.; Bay, B.H. Heat shock proteins as novel therapeutic targets in cancer. In Vivo 2008, 22, 311–315. [Google Scholar]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Shin, K.D.; Lee, M.Y.; Shin, D.S.; Lee, S.; Son, K.H.; Koh, S.; Paik, Y.K.; Kwon, B.M.; Han, D.C. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J. Biol. Chem. 2005, 280, 41439–41448. [Google Scholar]
- Gaikwad, A.; Poblenz, A.; Haridas, V.; Zhang, C.; Duvic, M.; Gutterman, J. Triterpenoid electrophiles (avicins) suppress heat shock protein-70 and x-linked inhibitor of apoptosis proteins in malignant cells by activation of ubiquitin machinery: Implications for proapoptotic activity. Clin. Cancer Res. 2005, 11, 1953–1962. [Google Scholar]
- Zhang, T.; Hamza, A.; Cao, X.; Wang, B.; Yu, S.; Zhan, C.G.; Sun, D. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 162–170. [Google Scholar] [CrossRef]
- Hsu, H.S.; Lin, J.H.; Huang, W.C.; Hsu, T.W.; Su, K.; Chiou, S.H.; Tsai, Y.T.; Hung, S.C. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer 2011, 117, 1516–1528. [Google Scholar]
- Sethi, G.; Ahn, K.S.; Pandey, M.K.; Aggarwal, B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood 2007, 109, 2727–2735. [Google Scholar]
- Shishodia, S.; Sethi, G.; Konopleva, M.; Andreeff, M.; Aggarwal, B.B. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin. Cancer Res. 2006, 12, 1828–1838. [Google Scholar] [CrossRef]
- Male, K.B.; Rao, Y.K.; Tzeng, Y.M.; Montes, J.; Kamen, A.; Luong, J.H. Probing inhibitory effects of Antrodia camphorata isolates using insect cell-based impedance spectroscopy: Inhibition vs. chemical structure. Chem. Res. Toxicol. 2008, 21, 2127–2133. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound MAA is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, C.-Y.; Fong, P.-C.; Yu, C.-C.; Tsai, W.-C.; Tzeng, Y.-M.; Chang, W.-W. Methyl Antcinate A Suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line. Molecules 2013, 18, 2539-2548. https://doi.org/10.3390/molecules18032539
Peng C-Y, Fong P-C, Yu C-C, Tsai W-C, Tzeng Y-M, Chang W-W. Methyl Antcinate A Suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line. Molecules. 2013; 18(3):2539-2548. https://doi.org/10.3390/molecules18032539
Chicago/Turabian StylePeng, Chih-Yu, Pin-Chung Fong, Cheng-Chia Yu, Wan-Chi Tsai, Yew-Min Tzeng, and Wen-Wei Chang. 2013. "Methyl Antcinate A Suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line" Molecules 18, no. 3: 2539-2548. https://doi.org/10.3390/molecules18032539
APA StylePeng, C.-Y., Fong, P.-C., Yu, C.-C., Tsai, W.-C., Tzeng, Y.-M., & Chang, W.-W. (2013). Methyl Antcinate A Suppresses the Population of Cancer Stem-Like Cells in MCF7 Human Breast Cancer Cell Line. Molecules, 18(3), 2539-2548. https://doi.org/10.3390/molecules18032539







