Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield
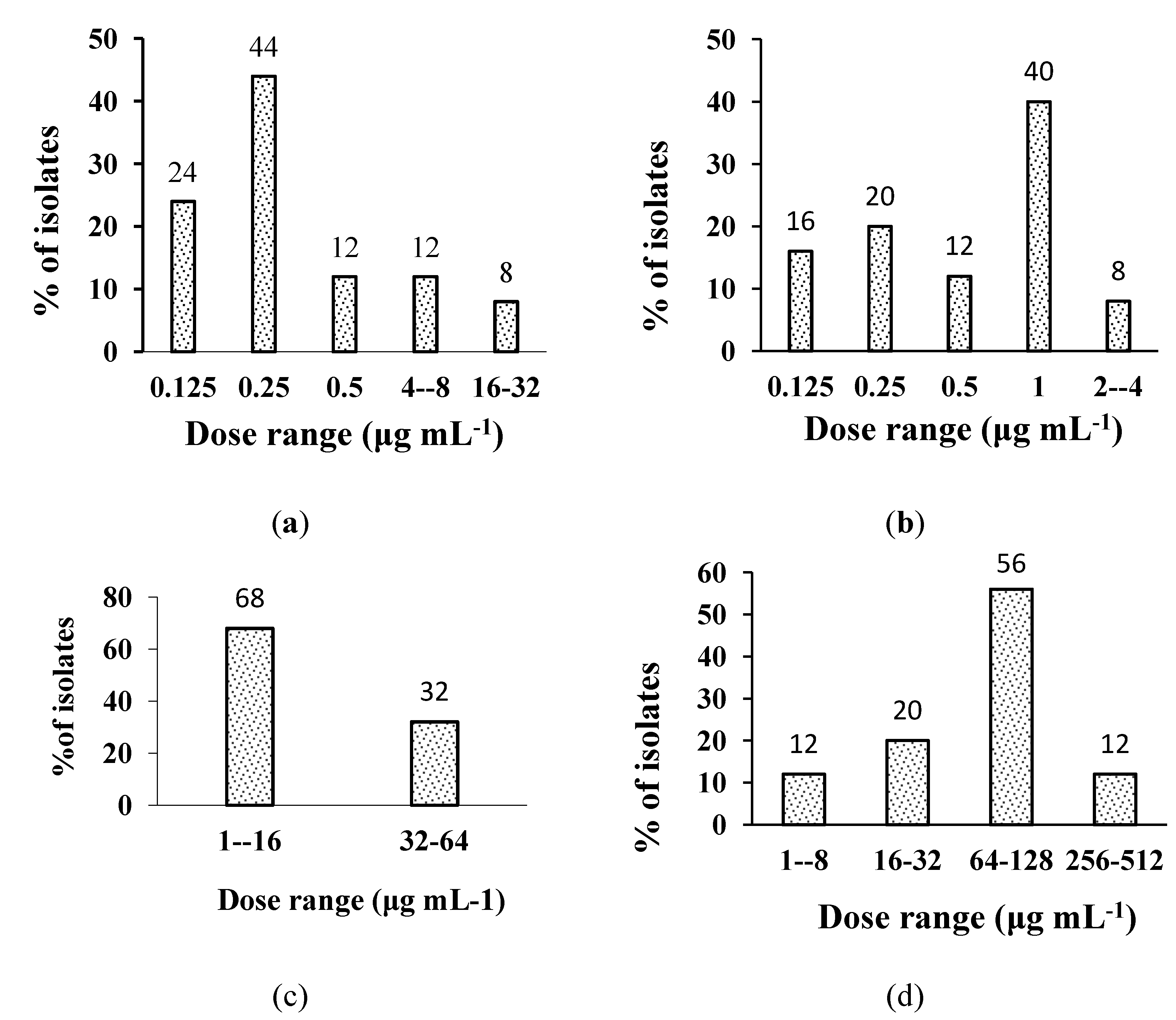
2.2. In Vitro Anti-H. pylori Activity of the Extracts
| Sr. No | Plant name | Family | Part used | Therapeutic application | Extract | % yield |
|---|---|---|---|---|---|---|
| 1 | Acacia nilotica (L.) Delile | Fabaceae | Leaves, flowers | Chewing of young leaves is quite effective against nausea. Flowers and Pods decoction is used as expectorant [8,9] | K1 | 21.0 ± 0.6 |
| K2 | 24.0 ± 1.2 | |||||
| K3 | 33.0 ± 1.5 | |||||
| K4 | 30.0 ± 1.2 | |||||
| 2 | Calotropis procera (Aiton) W.T. Aiton | Apocynaceae | Leaves, flowers | Powdered flowers are used in cold, cough and asthma. Leave juice is taken to relieve intermittent fevers [10,11] | K5 | 37.0 ± 1.2 |
| K6 | 19.0 ± 1.0 | |||||
| K7 | 27.0 ± 1.0 | |||||
| L1 | 17.0 ± 2.2 | |||||
| 3 | Fagonia arabica L. | Zygophyllaceae | Whole plant | decoction is effectively used against fever and also used as best blood purifier and as well as cooling agent [12] | L2 | 22.0 ± 1.0 |
| L3 | 36.0 ± 1.7 | |||||
| L4 | 30.0 ± 1.5 | |||||
| L5 | 31.0 ± 1.6 | |||||
| 4 | Adhatoda vasica Nees | Acanthaceae | Whole plant | Decoction of whole plant is used as remedy of all kinds of bronchial diseases [13,14] | L6 | 18.0 ± 1.8 |
| L7 | 14.0 ± 1.0 | |||||
| M1 | 15.0 ± 0.9 | |||||
| 5 | Casuarina equisetifolia L. | Casuarinaceae | Fruit | A decoction from the astringent bark and fruit is used as a remedy for diarrhea, sore throat, cough and swellings [15] | M2 | 21.0 ± 2.0 |
| M3 | 35.0 ± 1.5 | |||||
| M4 | 29.0 ± 1.6 | |||||
| M5 | 32.0 ± 1.7 | |||||
| M6 | 13.5 ± 1.8 | |||||
| M7 | 14.3 ± 1.5 |
| Extracts | Range in clinical isolates | Range in reference strains | MIC50 in clinical isolates | MIC50 in reference strains | MIC90 in clinical isolates | MIC90 in reference strains |
|---|---|---|---|---|---|---|
| AMX | 0.25–8 | 0.125–0.5 | 0.125–0.5 | 0.125 | 0.25 | 0.5 |
| CLT | 0.125–32 | 0.5–1 | 0.5 | 1 | 8 | 1 |
| TET | 1–64 | 4–8 | 8 | 4 | 32 | 16 |
| MNT | 1–512 | 16–32 | 32 | 32 | 32 | 32 |
| L1 | 16–128 | 16–32 | 8 | 16 | 64 | 64 |
| L2 | 8.00–128 | 32–64 | 64 | 128 | 128 | 128 |
| L3 | 4.0–64 | 16–32 | 32 | 8 | 16 | 16 |
| L4 | 32–256 | 32–64 | 128 | 256 | 512 | 256 |
| L5 | 8.00–128 | 16–32 | 64 | 4 | 16 | 4 |
| L6 | 128.0–1024 | 128–256 | 56 | 128 | 128 | 128 |
| L7 | 64–512 | 16–32 | 32 | 16 | 32 | 16 |
| M1 | 32–256 | 64–128 | 32 | 64 | 64 | 128 |
| M2 | 8–128 | 32–64 | 32 | 16 | 32 | 32 |
| M3 | 8–64 | 8–32 | 64 | 16 | 16 | 16 |
| M4 | 32–256 | 16–32 | 128 | 16 | 32 | 32 |
| M5 | 64–256 | 64–128 | 64 | 256 | 512 | 256 |
| M6 | 128–512 | 128 | 256 | 256 | 512 | 512 |
| M7 | 64–512 | 128 | 16 | 128 | 128 | 128 |
| K3 | 8–64 | 16 | 64 | 8 | 32 | 32 |
| Plant Isolate | Dose range µg mL−1 (% of H. pylori Isolates Inhibited) |
|---|---|
| L1 | 16–32 (36); 64–128 (64) |
| M1 | 32–64(40); 128–256(60) |
| L2 | 8.0–64 (64); 128 (36) |
| M2 | 8.0–64 (66); 128 (34) |
| L3 | 4–16 (72); 32–64 (28) |
| M3 | 8–16 (56); 32–64 (44) |
| L4 | 32–64 (52); 128–256 (48) |
| M4 | 32–64 (60); 128–256 (40) |
| L5 | 8–16 (52); 64–128 (28) |
| M5 | 64–128 (56); 256 (44) |
| L6 | 128–256 (20); 512–1024 (80) |
| M6 | 128 (16); 256–512 (84) |
| L7 | 64–-128 (28); 256–512 (72) |
| M7 | 64–128 (24); 256–512 (76) |
2.3. Urease Inhibitory Activity
| Sample Extracts | Concentration (µg mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| L3 | 9.20 ± 0.29 | 19.45 ± 0.55 | 38.31 ± 0.45 | 72.22 ± 0.13 | 86.56 ± 0.12 | ----- | ----- |
| L4 | ---- | ----- | ----- | 7.23 ± 0.22 | 19.21 ± 0.34 | 32.21 ± 0.46 | 58.21 ± 0.42 |
| L5 | 9.33 ± 0.88 | 18.41 ± 0.66 | 36.21 ± 0.56 | 68.21 ± 0.15 | |||
| M3 | 8.21 ± 0.76 | 16.11 ± 0.89 | 33.23 ± 0.59 | 67.32 ± 0.75 | 88.21 ± 0.12 | ----- | ------ |
| M4 | ------ | ------ | ------ | 12.23 ±0.96 | 23.21 ± 0.46 | 39.12 ± 0.53 | 48.22 ± 0.24 |
| M5 | ------ | 12.21 ± 0.23 | 24.51 ± 0.82 | 48.23 ± 0.52 | 86.21 ± 0.46 | ------ | ------ |

2.4. Discussion
3. Experimental
3.1. Collection of Plant Materials
3.2. Preparation of Plant Extracts
3.3. H. pylori Isolates
3.4. MIC Determination
3.5. Urease Inhibition Assay and Kinetics
3.5.1. Isolation of H. pylori Urease
3.5.2. Urease Inhibitory Assay
4. Conclusions
Acknowledgments
- Samples Availability: Samples of the plant parts used in this study can be available from the authors.
References
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar]
- Mégraud, F; Lehn, N.; Lind, T.; Bayerdorffer, E.; O’morain, C.; Spiller, R.; Unge, P.; van Zanten, S.V.; Wrangstadh, M.; Burman, C.F. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: The MACH 2 study. Antimicrob. Agents Chemother. 1999, 43, 2747–2752. [Google Scholar]
- Amin, M.; Iqbal, M.S.; Hughes, R.W.; Khan, S.A.; Reynolds, P.A.; Enne, V.I.; Rahman, S.; Mirza, A.S. Mechanochemical synthesis and in vitro anti-Helicobacter pylori and urease inhibitory activities of novel zinc(II)-famotidine complex. J. Enzyme Inhib. Med. Chem. 2010, 25, 383–3890. [Google Scholar]
- Mégraud, F. The challenge of Helicobacter pylori resistance to antibiotics: The comeback of bismuth-based quadruple therapy. Therap. Adv. Gastroenterol. 2012, 5, 103–109. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar]
- Tabaka, M.; Armonb, R.; Neeman, I. Cinnamon extracts’ inhibitory effect on Helicobacter pylori. J. Ethnopharmacol. 1999, 67, 269–77. [Google Scholar]
- Rojas, J.J.; Ochoa, V.J.; Saul, A.O.; Munoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement. Altern. Med. 2006, 6, 2. [Google Scholar]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar]
- Mahesh, B; Satesh, S. Antimicrobial activity of some medicinal plants against plant and human pathogen. Word J. Agric. Sci. 2008, 4, 839–843. [Google Scholar]
- Yesmin, M.N.; Din, S.N.; Mubassara, S.; Akond, M.A. Antioxidant and Antibacterial Activities of Calotropis procera Linn. Am. Eurasian J. Agric. Environ. Sci. 2008, 4, 550–553. [Google Scholar]
- Ahmad, N.; Anwar, F.; Hameed, S.; Boyce, M.C. Antioxidant and antimicrobial attributes of different solvent extracts from leaves and flowers of Akk [Calotropis procera (Ait.)Ait. F.)]. J. Med. Plants Res. 2011, 5, 4879–4887. [Google Scholar]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Effect of Fagonia arabica (Dhamasa) on in vitro thrombolysis. BMC Complement. Altern. Med. 2007, 7, 36–41. [Google Scholar]
- Dhuley, J.N. Antitusive effect of Adhatoda vasica extract on mechanical or chemical stimulation induced coughing in animals. J. Ethnopharmacol. 1999, 67, 361–365. [Google Scholar]
- Victoria, E.E. Pest infestation on the biochemical modulation of Adhatoda vasica. J. Biopestic. 2010, 3, 413–419. [Google Scholar]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannins of Casuarina and Stachyurus species. Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. Perkin Trans. I 1983, 8, 1765–1772. [Google Scholar]
- Shafiq, A.T.; Ahmad, M.N.; Obaidullah, K.A.; Choudhary, M.I.; Ahmad, W.; Ahmad, M. Urease inhibitors from Indigofera gerardiana Wall. J. Enzyme Inhib. Med. Chem. 2011, 26, 480–484. [Google Scholar]
- Ayaz, M.; Lodhi, M.A.; Riaz, M.; Ulhaq, A.; Malik, A.; Chaudhary, M.I. Novel urease inhibitors from Daphne oleoids. J. Enzyme Inhib. Med. Chem. 2006, 21, 527–529. [Google Scholar]
- Juszkiewicz, A.; Zaborska, A.; Laptus, A.; Olech, Z. A study of the inhibition of jack bean urease by garlic extract. Food Chem. 2004, 85, 553–558. [Google Scholar] [CrossRef]
- Irfan, M.; Ali, M.; Ahmed, H.; Anis, I.; Khan, A.; Chaudhary, M.I.; Shah, M.R. Urease inhibitors from Hypericum oblongifolium Wall. J. Enzyme Inhib. Med. Chem. 2010, 25, 296–299. [Google Scholar]
- Shabbir, G.; Anwar, F.; Sultana, B.; Khalid, Z.M.; Afzal, M.; Khan, M.Q.; Ashrafuzzaman, M. Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]. Molecules 2011, 16, 7302–7319. [Google Scholar]
- Amtul, Z.; Rasheed, M.; Choudhary, M.I.; Rosanna, S.; Khan, K.M.; Rehman, A. Kinetics of novel competitive inhibitors of urease enzymes by a focused library of oxadiazoles/thiadiazoles and triazoles. Biochem. Biophys. Res. Commun. 2004, 319, 1053–1063. [Google Scholar]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green Synthesis of Silver Nanoparticles through Reduction with Solanum xanthocarpum L. Berry Extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar]
- Zaborska, W.; Krajewska, B.; Leszko, M.; Olech, Z. Inhibition of urease by Ni2+ ions: Analysis of reaction progress curves. J. Mol. Catal. B Enzym. 2001, 13, 103–108. [Google Scholar]
- Malviya, S.; Kharia, A.; Verma, M. Medicinal attributes of Acacia nilotica Linn. A comprehensive review on ethnopharmacological claims. J. Pharm. Life Sci. 2011, 2, 830–837. [Google Scholar]
- Javier, P.G. Rescue therapy for Helicobacter pylori infection. Gastroenterol. Res. Pract. 2012, 2012, 974594. [Google Scholar]
- Fidock, D.A.; Rosenthal, P.J.; Croft, S.L.; Brun, R.; Nwaka, S. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. Drug Discov. 2004, 3, 509–510. [Google Scholar]
- Dabur, R.; Gupta, A.; Mandal, T.K.; Singh, D.D.; Bajpai, V.; Gurav, A.M.; Lavekar, G.S. Antimicrobial activities of some Indian medical plants. Afr. J. Trad. Complement. Altern. Med. 2007, 4, 313–318. [Google Scholar]
- Zaborska, W.; Kot, M.; Superata, K. Evaluation of the inhibition mechanism. J. Enzym. Inhib. Med. Chem. 2002, 17, 247–253. [Google Scholar] [CrossRef]
- Kareem, S.; OAkpan, I.; Ojo, O.P. Antimicrobial activities of Calotropis procera on selected pathogenic microorganisms. Afr. J. Biomed. Res. 2008, 11, 105–110. [Google Scholar]
- Bharti, S.; Wahane, V.D.; Kumar, V.L. Protective effect of Calotropis procera latex extracts on experimentally induced gastric ulcers in rat. J. Ethnopharmacol. 2010, 127, 440–444. [Google Scholar] [CrossRef]
- Abdel-Khalik, S.M.; Miyase, T.; Hanan, E.A.; Melek, F.R. Triterpenoidsaponins from Fagonia cretica. Phytochemistry 2000, 54, 853–859. [Google Scholar]
- Satputea, R.; Bhattacharya, R.; Kashyap, R.S.; Purohit, H.J.; Deopujaric, J.Y.; Taori, G.M.; Daginawala, H.F. Antioxidant potential of Fagonia arabica against the chemical ischemia-induced in PC12 cells. Iran. J. Pharm. Res. 2012, 11, 303–313. [Google Scholar]
- Das, R.; Kaushik, A. Synergistic activity of Fagonia arabica and Heteropneustes fossilis extracts against myocardial, cerebral infarction, and embolism disorder in mice. J. Pharm. Bioallied. Sci. 2010, 2, 100–104. [Google Scholar] [CrossRef]
- Graham, D.Y.; Anderson, S.Y.; Lang, T. Garlic or jalapeno peppers for treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 1999, 94, 1200–1202. [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI), Performance standards for antimicrobial susceptibility testing (9th International Supplement); CLSI: Wayne, PA, USA, 1999; M100-S9.
- Wu, H.; Shi, D.; Wang, H.T.; Liu, J.X. Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxicillin. J. Antimicrob. Chemother. 2000, 46, 121–123. [Google Scholar]
- Mao, W.J.; Lv, P.C.; Shi, L.; Li, H.Q.; Zhu, H.L. Synthesis, molecular docking and biological evaluation of metronidazole derivatives as potent Helicobacter pylori urease inhibitors. Bioorg. Med. Chem. 2009, 17, 7531–7536. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amin, M.; Anwar, F.; Naz, F.; Mehmood, T.; Saari, N. Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants. Molecules 2013, 18, 2135-2149. https://doi.org/10.3390/molecules18022135
Amin M, Anwar F, Naz F, Mehmood T, Saari N. Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants. Molecules. 2013; 18(2):2135-2149. https://doi.org/10.3390/molecules18022135
Chicago/Turabian StyleAmin, Muhammad, Farooq Anwar, Fauqia Naz, Tahir Mehmood, and Nazamid Saari. 2013. "Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants" Molecules 18, no. 2: 2135-2149. https://doi.org/10.3390/molecules18022135
APA StyleAmin, M., Anwar, F., Naz, F., Mehmood, T., & Saari, N. (2013). Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants. Molecules, 18(2), 2135-2149. https://doi.org/10.3390/molecules18022135




