Synthesis and Antiproliferative Activity of C-3 Functionalized Isobenzofuran-1(3H)-ones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Isobenzofuran-1(3H)-ones
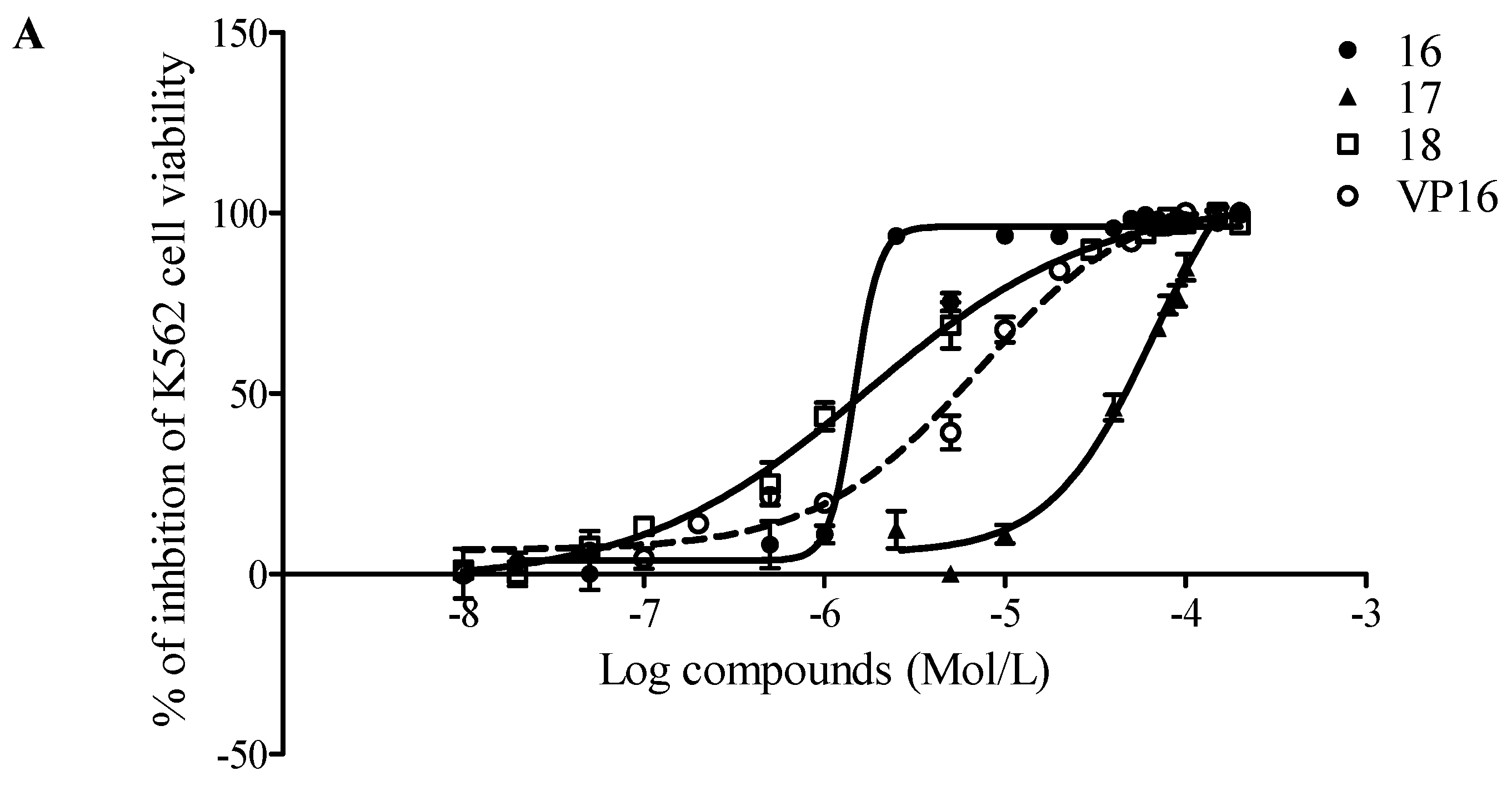
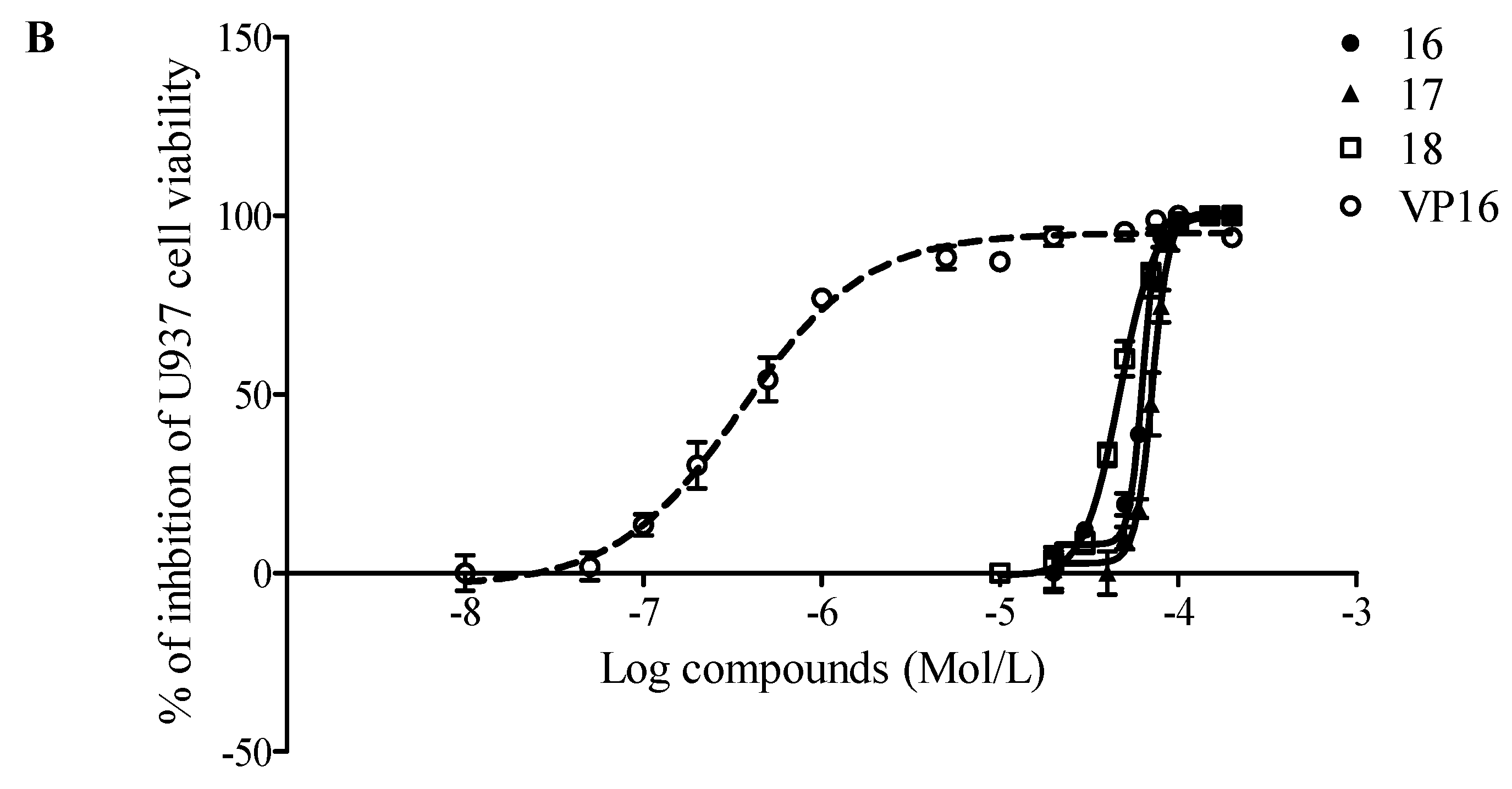
2.2. Evaluation of Antiproliferative Activity
2.3. Structure-Activity Relationship and in Silico Calculations of Drug-Like Properties
3. Experimental
3.1. General
3.2. Synthesis of Compounds 11–13, Exemplified by the Synthesis of 3-(2-Hydroxy-6-oxocyclohex-1-enyl)isobenzofuran-1(3H)-one (11)
3.3. Synthesis of Compounds 14–16, Exemplified by the Synthesis of 3-(2,6-Dihydroxyphenyl)-isobenzofuran-1(3H) one (14)
3.4. Synthesis of Compounds 17–19, Exemplified by the Synthesis of 2-(3-Oxo-1,3-dihydroisobenzofuran-1-yl)-1,3-phenylene diacetate (17)
3.5. Synthesis of 3-(2-Hydroxy-4,4-dimethyl-6-oxo-cyclohexen-1-yl)isobenzofuran-1(3H)-one (20)
3.6. Synthesis of Compounds 21 and 22, Exemplified by the Synthesis 3-(2-Hydroxy-5-oxocyclopent-1-enyl)isobenzofuran-1(3H)-one (21)
3.7. Antiproliferative Activity Assays
4. Conclusions
Acknowledgments
References
- Huang, X.-Z.; Zhu, Y.; Guan, X.-L.; Tian, K.; Guo, J.-M.; Wang, H.-B.; Fu, G.-M. A novel antioxidant isobenzofuranone derivative from fungus Cephalosporium sp. AL031. Molecules 2012, 17, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Ford, E.; Worapong, J.; Harper, J.K.; Arif, A.M.; Grant, D.M.; Fung, P.C.W.; Chau, R.M.W. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora possessing antifungal and antioxidant activities. Phytochemistry 2002, 60, 170–183. [Google Scholar] [CrossRef]
- Ma, F.; Gao, Y.; Qiao, H.; Hu, X.; Chang, J. Antiplatelet activity of 3-butyl-6-bromo-1(3H)-isobenzofuranone on rat platelet aggregation. J. Thromb. Thrombolysis 2012, 33, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, J.A.; Braz-Filho, R.; Rincón-Velandia, J.; Guerrero-Pabón, M.F. 3-butil-isobenzofuranona: Un compuesto aislado de Apium graveolens com actividad anticonvulsivante. Rev. Colomb. Cienc. Quim. Farm. 2005, 34, 69–76. [Google Scholar]
- Arnone, A.; Assante, G.; Nasini, G.; Strada, S.; Vercesi, A. Cryphonectric acid and other minor metabolites from a hypovirulent strain of Cryphonectria parasítica. J. Nat. Prod. 2002, 65, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Demuner, A.J.; Barbosa, L.C.A.; Veiga, T.A.M.; Barreto, R.W.; King-Diaz, B.; Lotina-Hennsen, B. Phytotoxic constituents from Nimbya alternantherae. Biochem. Syst. Ecol. 2006, 34, 790–795. [Google Scholar] [CrossRef]
- Logrado, L.P.L.; Santos, C.O.; Romeiro, L.A.S.; Costa, A.M.; Ferreira, J.R.O.; Cavalcanti, B.C.; de Moraes, O.M.; Costa-Lotufo, L.V.; Pessoa, C. Synthesis and cytotoxicity screening of substituted isobenzofuranones designed from anarcadic acids. Eur. J. Med. Chem. 2010, 45, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Barbosa, L.C.A.; Maltha, C.R.A.; Rocha, M.E.; Bezerra, D.P.; Costa-Lotufo, L.V.; Pessoa, C.; Moraes, M.O. Synthesis and cytotoxic activity of some 3-benzyl-5-arylidenefuran-2(5H)-ones. Molecules 2007, 12, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.C.A.; Pereira, U.A.; Maltha, C.R.A.; Teixeira, R.R.; Valente, V.M.M.; Ferreira, J.R.O.; Costa-Lotufo, V.; Moraes, M.O.; Pessoa, C. Synthesis and Biological Evaluation of 2,5-bis(alkylamino)-1,4-benzoquinones. Molecules 2010, 15, 5629–5643. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.C.A.; Nogueira, L.B.; Maltha, C.R.A.; Teixeira, R.R.; Silva, A.A. Synthesis and phytogrowth properties of oxabicyclic analogues related to helminsthosporin. Molecules 2009, 14, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Barbosa, L.C.A.; Forlani, G.; Piló-Veloso, D.; de Mesquita Carneiro, J.W. Synthesis of photosynthesis-inhibiting nostoclide analogues. J. Agric. Food Chem. 2008, 56, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Pahari, P.; Senapati, B.; Mal, D. Regiospecific synthesis of isopestacin, a naturally occurring isobenzofuranone antioxidante. Tetrahedron Lett. 2004, 45, 5109–5112. [Google Scholar] [CrossRef]
- Mal, D.; Pahari, P.; De, S.R. Regiospecific synthesis of 3-(2,6-dihydroxyphenyl)phtalides: Application to the synthesis of isopestacin and cryphonectric acid. Tetrahedron 2007, 63, 11781–11792. [Google Scholar] [CrossRef]
- Oliver, J.E.; Wilzer, K.R.; Waters, R.M. Synthesis of 1-(2,6-Dihydroxyphenyl)-1-alkanones and Benzophenone by Aromatization of 2-Acyl-3-hydroxy-2-cyclohexene-1-ones with Mercuric Acetate. Synthesis 1990, 1990, 1117–1119. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V. Computing chemistry on the web. Drug Discov. Today 2005, 10, 1497–1500. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com/ (accessed on September 2012).
- Alafeefy, A.M.; Alqasoumi, S.I.; Ashour, A.E.; Masand, V.; Al-Jaber, N.A.; Hadda, T.B.; Mohameda, M.A. Quinazoline-etyrphostin as a new class of antitumor agents, molecular properties prediction, synthesis and biological testing. Eur. J. Med. Chem. 2012, 53, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ali, P.; Meshram, J.; Sheikh, J.; Tiwari, V.; Dongre, R.; Hadda, T.B. Predictions and correlations of structure activity relationship of some aminoantipyrine derivatives on the basis of theoretical and experimental ground. Med. Chem. Res. 2012, 21, 157–164. [Google Scholar] [CrossRef]
- Mahajan, D.T.; Masand, V.H.; Patil, K.N.; Hadda, T.B.; Jawarkar, R.D.; Thakur, S.D.; Rastija, V. CoMSIA and POM analyses of anti-malarial activity of synthetic prodiginines. Bioorg. Med. Chem. Lett. 2012, 22, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Sumrra, S.H.; Youssoufi, M.H.; Hadda, T.B. Metal based biologically active compounds: Design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur. J. Med. Chem. 2010, 45, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, R. Drugs, leads, and drug-likeness: An analysis of some recently launched drugs. Bioorg. Med. Chem. Lett. 2012, 12, 1647–1650. [Google Scholar] [CrossRef]
- Jarrahpour, A.; Fathi, J.; Mimouni, M.; Hadda, T.B.; Sheikh, J.; Chohan, Z.; Parvez, A. Petra, Osiris and molinspiration (POM) together as a successful support in drug design: Antibacterial activity and biopharmaceutical characterization of some azo schiff bases. Med. Chem. Res. 2012, 21, 1984–1990. [Google Scholar] [CrossRef]
- Tambunan, U.S.F.; Bramantya, N.; Parikesit, A.A. In silico modification of suberoylanilide hydroxamic acid (SAHA) as potential inhibitor for class II histone deacetylase (HDAC). BMC Bioinformatics 2011, 12, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of Intestinal Absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 11–22 are available from the authors. |



| Comp. | 1 µM | 50 µM | 100 µM |
|---|---|---|---|
| 10 | 44.16 ± 3.59 | 41.71 ± 1.01 | 26.71 ± 3.91 |
| 11 | 49.69 ± 0.68 | 45.85 ± 3.07 | 36.18 ± 1.00 |
| 12 | 31.42 ± 1.18 | 38.38 ± 3.04 | 38.54 ± 4.24 |
| 13 | 32.87 ± 3.20 | 23.93 ± 3.04 | 31.64 ± 2.93 |
| 14 | 37.71 ± 2.20 | 49.33 ± 4.70 | 56.82 ± 1.36 |
| 15 | 40.59 ± 2.47 | 44.32 ± 1.08 | 43.64 ± 3.85 |
| 16 | 20.33 ± 1.13 | 65.61 ± 2.42 | 77.73 ± 2.01 |
| 17 | 26.77 ± 0.99 | 56.10 ± 3.58 | 63.70 ± 0.21 |
| 18 | 37.88 ± 3.86 | 69.63 ± 0.92 | 80.05 ± 0.28 |
| 19 | 12.48 ± 3.57 | 54.56 ± 2.97 | 71.76 ± 3.73 |
| 20 | 39.87 ± 4.43 | 43.86 ± 1.92 | 49.38 ± 2.50 |
| 21 | 20.64 ± 1.91 | 18.60 ± 2.65 | 21.84 ± 3.39 |
| 22 | 46.11 ± 4.1 | 45.9 ± 3.39 | 37.61 ± 0.38 |
| Comp. | 1 µM | 50 µM | 100 µM |
|---|---|---|---|
| 10 | 28.00 ± 2.75 | 30.73 ± 2.12 | 28.81 ± 2.05 |
| 11 | 32.34 ± 1.93 | 28.58 ± 1.62 | 29.43 ± 2.76 |
| 12 | 20.38 ± 3.04 | 27.01 ± 3.68 | 24.72 ± 1.48 |
| 13 | 26.75 ± 2.12 | 45.31 ± 1.91 | 39.10 ± 0.92 |
| 14 | 25.57 ± 2.50 | 31.10 ± 3.53 | 29.86 ± 1.70 |
| 15 | 35.03 ± 2.05 | 27.03 ± 0.61 | 22.37 ± 1.13 |
| 16 | 11.64 ± 0.28 | 58.86 ± 2.62 | 90.82 ± 0.43 |
| 17 | 33.87 ± 0.92 | 61.33 ± 1.98 | 83.91 ± 0.92 |
| 18 | 36.63 ± 1.98 | 67.68 ± 1.77 | 90.78 ± 0.21 |
| 19 | 23.14 ± 3.19 | 25.46 ± 3.13 | 23.12 ± 3.43 |
| 20 | 24.33 ± 0.47 | 30.98 ± 1.04 | 34.14 ± 0.49 |
| 21 | 5.72 ± 1.27 | 21.17 ± 1.48 | 29.29 ± 2.02 |
| 22 | 27.63 ± 0.92 | 31.85 ± 0.99 | 33.58 ± 2.71 |
| Cell line | Comp. 16 | Compound 17 | Compound 18 | Etoposide (VP16) |
|---|---|---|---|---|
| K562 | 2.79 | 66.81 | 1.71 | 7.06 |
| U937 | 62.97 | 71.39 | 46.63 | 0.35 |
| Comp. | Bioavailability and Drug Score | Lipinski’s violations | Toxicity risks a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cLogP | LogS | MW | HBD | HBA | Drug-likeness | Drug-Score | M | T | I | R | ||
| 10 | 0.64 | −1.68 | 150.0 | 1 | 3 | −4.65 | 0.49 | 0 | - | - | - | - |
| 11 | 1.78 | −2.71 | 244.0 | 1 | 4 | −6.63 | 0.46 | 0 | - | - | - | - |
| 12 | 1.98 | −2.87 | 258.0 | 1 | 4 | −3.61 | 0.46 | 0 | - | - | - | - |
| 13 | 2.78 | −3.30 | 286.0 | 1 | 4 | −4.20 | 0.43 | 0 | - | - | - | - |
| 14 | 2.55 | −2.66 | 242.0 | 2 | 4 | −4.26 | 0.46 | 0 | - | - | - | - |
| 15 | 2.87 | −3.01 | 256.0 | 2 | 4 | −5.25 | 0.44 | 0 | - | - | - | - |
| 16 | 3.65 | −3.53 | 284.0 | 2 | 4 | −6.33 | 0.39 | 0 | - | - | - | - |
| 17 | 3.10 | −3.54 | 326.0 | 2 | 6 | −6.34 | 0.40 | 0 | - | - | - | - |
| 18 | 3.41 | −3.88 | 340.0 | 2 | 6 | −7.52 | 0.38 | 0 | - | - | - | - |
| 19 | 4.19 | −4.41 | 368.0 | 2 | 6 | −8.59 | 0.32 | 0 | - | - | - | - |
| 20 | 2.25 | −3.05 | 272.0 | 1 | 4 | −3.58 | 0.45 | 0 | - | - | - | - |
| 21 | 1.29 | −2.42 | 230.0 | 0 | 4 | −3.95 | 0.48 | 0 | - | - | - | - |
| 22 | 2.32 | −4.04 | 278.0 | 0 | 4 | −4.12 | 0.41 | 0 | - | - | - | - |
| Etoposide | 0.53 | −3.95 | 588.0 | 3 | 13 | −0.28 | 0.39 | 2 | - | - | - | - |
| Comp. | Molinspiration calculations | Calculations of Bioactivity Scores a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cLogP | TPSA b | NONI c | Volume | nRotB | GPCRL | ICM | KI | NRL | PI | EI | |
| 10 | 0.895 | 46.533 | 1 | 126.015 | 0 | −0.59 | −0.04 | −1.12 | −0.62 | −0.95 | 0.14 |
| 11 | 1.741 | 63.604 | 1 | 211.979 | 1 | −0.60 | −0.30 | −1.40 | −0.42 | −0.68 | −0.11 |
| 12 | 1.982 | 63.604 | 1 | 228.566 | 1 | −0.58 | −0.41 | −1.35 | −0.32 | −0.60 | −0.14 |
| 13 | 3.483 | 63.604 | 1 | 261.955 | 2 | −0.39 | −0.29 | −1.15 | −0.14 | −0.35 | −0.08 |
| 14 | 3.016 | 66.761 | 2 | 205.442 | 1 | −0.29 | −0.42 | −0.31 | −0.20 | −0.56 | −0.17 |
| 15 | 3.416 | 66.761 | 2 | 222.003 | 1 | −0.29 | −0.49 | −0.30 | −0.17 | −0.56 | −0.21 |
| 16 | 4.48 | 66.761 | 2 | 255.392 | 2 | −0.13 | −0.37 | −0.20 | 0.03 | −0.37 | −0.11 |
| 17 | 2.183 | 100.903 | 2 | 243.409 | 3 | −0.01 | −0.34 | −0.18 | −0.01 | −0.25 | −0.08 |
| 18 | 2.584 | 100.903 | 2 | 259.970 | 3 | −0.04 | −0.41 | −0.21 | −0.01 | −0.27 | −0.14 |
| 19 | 3.647 | 100.903 | 2 | 293.359 | 4 | 0.03 | −0.32 | −0.19 | 0.08 | −0.17 | −0.07 |
| 20 | 2.394 | 63.604 | 1 | 244.803 | 1 | −0.50 | −0.39 | −1.31 | −0.27 | −0.66 | −0.20 |
| 21 | 0.652 | 60.447 | 0 | 195.554 | 1 | −0.26 | −0.20 | −0.85 | −0.27 | −0.49 | 0.04 |
| 22 | 2.394 | 60.447 | 0 | 233.259 | 1 | −0.12 | −0.35 | −0.54 | −0.05 | −0.45 | 0.09 |
| d Etop. | 0.698 | 160.861 | 3 | 493.508 | 5 | 0.18 | −0.48 | −0.38 | −0.33 | 0.12 | 0.30 |
© 2013 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teixeira, R.R.; Bressan, G.C.; Pereira, W.L.; Ferreira, J.G.; De Oliveira, F.M.; Thomaz, D.C. Synthesis and Antiproliferative Activity of C-3 Functionalized Isobenzofuran-1(3H)-ones. Molecules 2013, 18, 1881-1896. https://doi.org/10.3390/molecules18021881
Teixeira RR, Bressan GC, Pereira WL, Ferreira JG, De Oliveira FM, Thomaz DC. Synthesis and Antiproliferative Activity of C-3 Functionalized Isobenzofuran-1(3H)-ones. Molecules. 2013; 18(2):1881-1896. https://doi.org/10.3390/molecules18021881
Chicago/Turabian StyleTeixeira, Róbson Ricardo, Gustavo Costa Bressan, Wagner Luiz Pereira, Joana Gasperazzo Ferreira, Fabrício Marques De Oliveira, and Deborah Campos Thomaz. 2013. "Synthesis and Antiproliferative Activity of C-3 Functionalized Isobenzofuran-1(3H)-ones" Molecules 18, no. 2: 1881-1896. https://doi.org/10.3390/molecules18021881
APA StyleTeixeira, R. R., Bressan, G. C., Pereira, W. L., Ferreira, J. G., De Oliveira, F. M., & Thomaz, D. C. (2013). Synthesis and Antiproliferative Activity of C-3 Functionalized Isobenzofuran-1(3H)-ones. Molecules, 18(2), 1881-1896. https://doi.org/10.3390/molecules18021881





