Production of Polygalacturonases by Aspergillus section Nigri Strains in a Fixed Bed Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Aspergillus Strains
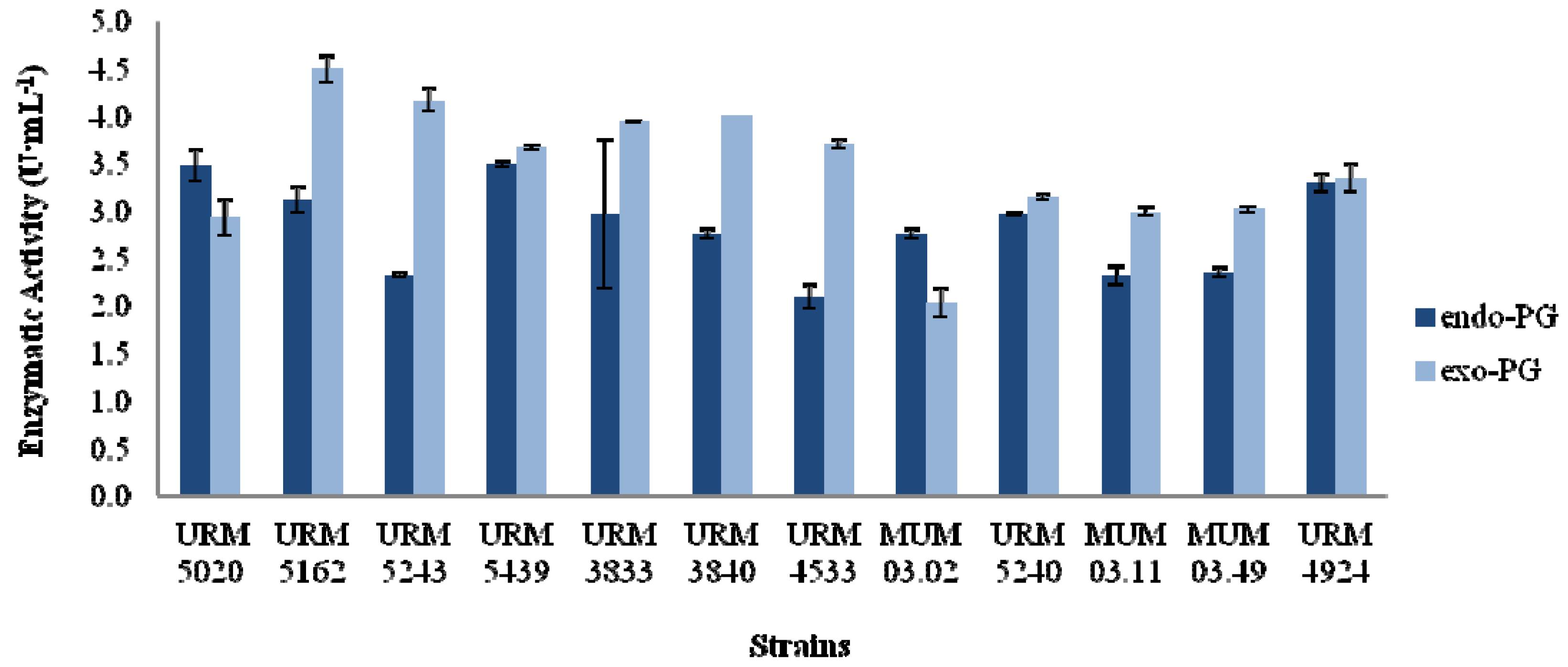
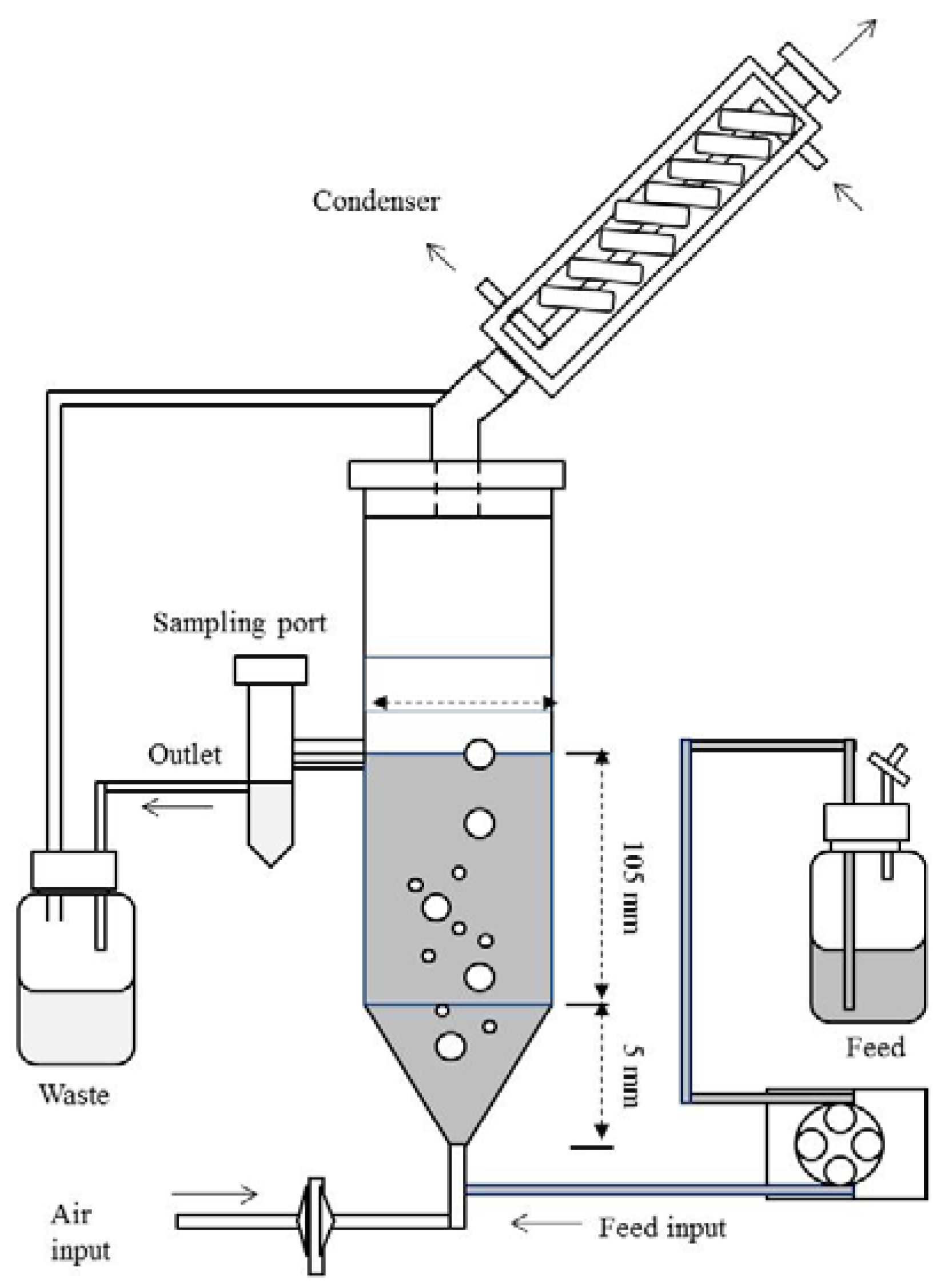
2.2. Fixed Bed Reactor
 ) and endo-polygalacturonase (
) and endo-polygalacturonase (  ) by Aspergillus niger strain URM 5162 in fixed bed reactor. (a) immobilised cells without aeration; (b) immobilised cells with aeration; (c) immobilised cells with aeration and adding pectin; and (d) free cells with aeration. pH variation during the polygalacturonases production (
) by Aspergillus niger strain URM 5162 in fixed bed reactor. (a) immobilised cells without aeration; (b) immobilised cells with aeration; (c) immobilised cells with aeration and adding pectin; and (d) free cells with aeration. pH variation during the polygalacturonases production (  ).
).
 ) and endo-polygalacturonase (
) and endo-polygalacturonase (  ) by Aspergillus niger strain URM 5162 in fixed bed reactor. (a) immobilised cells without aeration; (b) immobilised cells with aeration; (c) immobilised cells with aeration and adding pectin; and (d) free cells with aeration. pH variation during the polygalacturonases production (
) by Aspergillus niger strain URM 5162 in fixed bed reactor. (a) immobilised cells without aeration; (b) immobilised cells with aeration; (c) immobilised cells with aeration and adding pectin; and (d) free cells with aeration. pH variation during the polygalacturonases production (  ).
).
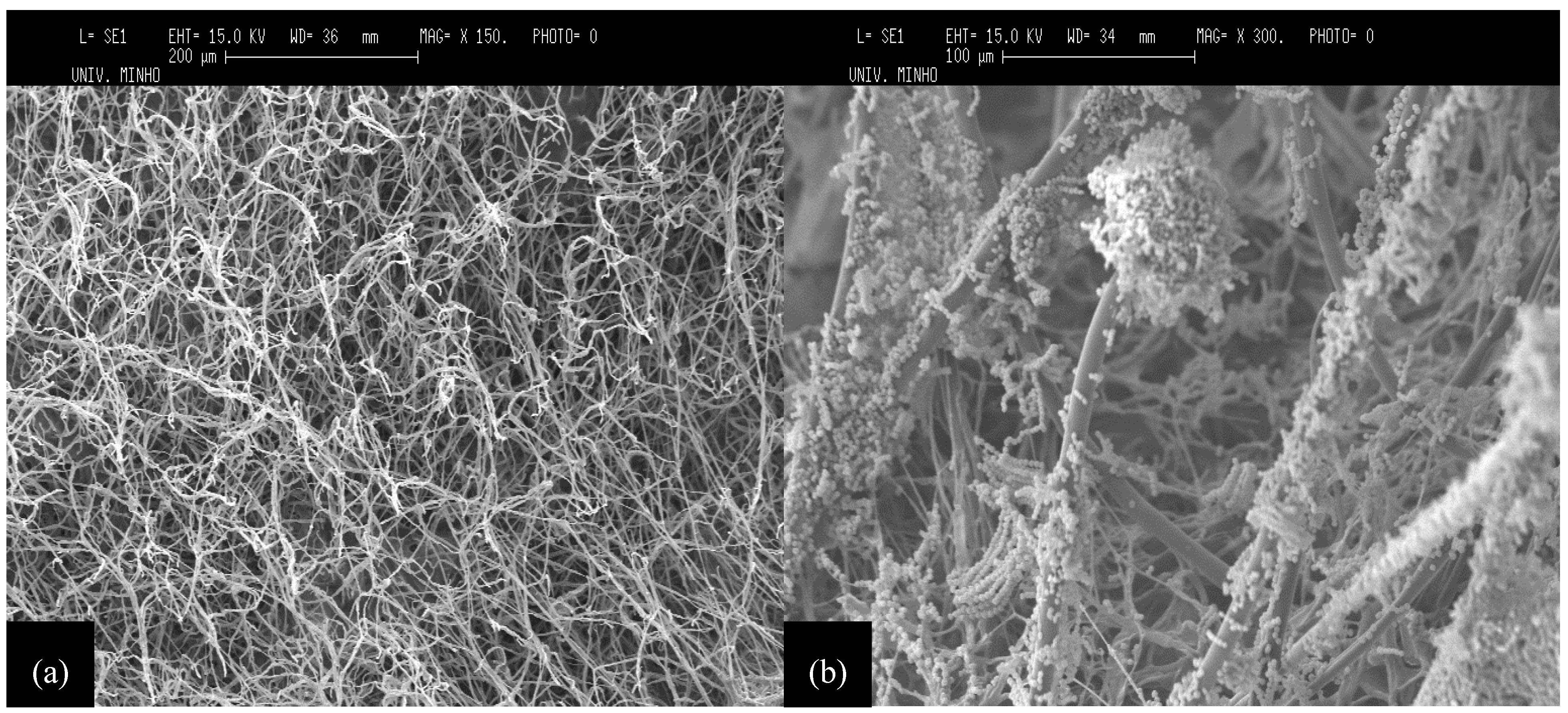
2.3. Microscopic Observation of Cell Growth in Orange Peels
3. Experimental
3.1. Microorganisms
3.2. Inoculum Standardisation
3.3. Miniaturised Screening Method
| Number | Strains | Number | Strains |
|---|---|---|---|
| URM 13 | Aspergillus niger | URM 5555 | A. niger |
| URM 18 | A. niger | URM 5741 | A. niger |
| URM 19 | A. niger | URM 5837 | A. niger |
| URM 20 | A. niger | URM 5838 | A. niger |
| URM 238 | A. niger | URM 5842 | A. niger |
| URM 949 | A. niger | URM 5910 | A. niger |
| URM 2228 | A. niger | URM 6054 | A. niger |
| URM 2813 | A. niger | URM 3452 | A. japonicus |
| URM 2908 | A. niger | URM 3833 | A. japonicus |
| URM 3701 | A. niger | URM 3840 | A. japonicus |
| URM 3753 | A. niger | URM 3916 | A. japonicus |
| URM 3755 | A. niger | URM 4533 | A. japonicus |
| URM 3806 | A. niger | URM 4599 | A. japonicus |
| URM 3811 | A. niger | URM 4663 | A. japonicus |
| URM 3820 | A. niger | URM 5242 | A. japonicus |
| URM 3853 | A. niger | URM 5620 | A. japonicus |
| URM 3856 | A. niger | URM 5633 | A. japonicus |
| URM 3885 | A. niger | URM 5723 | A. japonicus |
| URM 4312 | A. niger | URM 5751 | A. japonicus |
| URM 4924 | A. niger | URM 3776 | A. aculeatus |
| URM 5020 | A. niger | URM 4953 | A. aculeatus |
| URM 5149 | A. niger | URM 5240 | A. aculeatus |
| URM 5162 | A. niger | MUM 03.02 | A. japonicus |
| URM 5207 | A. niger | MUM 03.11 | A. aculeatus |
| URM 5238 | A. niger | MUM 05.10 | A. brasiliensis |
| URM 5239 | A. niger | MUM 03.12 | A. ellipticus |
| URM 5243 | A. niger | MUM 03.49 | A. ibericus |
| URM 5253 | A. niger | MUM 06.152 | A. tubingensis |
| URM 5437 | A. niger | MUM 06.153 | A. vadensis |
| URM 5438 | A. niger | MUM 08.01 | A. uvarum |
| URM 5439 | A. niger |
3.4. Polygalacturonases Production in Fixed Bed Reactor
3.4.1. Procedures of Immobilisation Biomass on Natural Support
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. Submerged Fermentation
3.5. Enzymatic Assays
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References
- Neves, M.F.; Trombin, V.G.; Milan, P.; Lopes, F.F.; Cressoni, F.; Kalaki, R. O Retrato da Citricultura Brasileira; CitrusBR: São Paulo, Brazil, 2011; p. 138. [Google Scholar]
- Zhou, J.M.; Ge, X.Y.; Zhang, W.G. Improvement of polygalacturonase production at high temperature by mixed culture of Aspergillus niger and Saccharomyces cerevisiae. Bioresour. Technol. 2011, 102, 10085–10088. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torres, P.; Converti, A.; Dominguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food. Chem. 2008, 56, 2380–2387. [Google Scholar]
- Holck, J.; Hjernø, K.; Lorentzen, A.; Vigsnæs, L.K.; Hemmingsen, L.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length difference on human faecal microbiota composition after in vitro fermentation. Process Biochem. 2011, 46, 1039–1049. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Solid state production of polygalacturonase by Lentinus edodes using fruit processing wastes. Process Biochem. 2000, 35, 825–830. [Google Scholar] [CrossRef]
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A review. Process Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Rodríguez, R.; Contreras-Esquivel, J.C. Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem. Eng. J. 2012, 60, 90–95. [Google Scholar]
- Gomes, J.; Zeni, J.; Cence, K.; Toniazzo, G.; Treichel, H.; Valduga, E. Evaluation of production and characterization of polygalacturonase by Aspergillus niger ATCC 9642. Food Bioprod. Process. 2011, 89, 281–287. [Google Scholar] [CrossRef]
- Laaksonen, O.; Sandell, M.; Nordlund, E.; Heinio, R.L.; Malinen, H.L.; Jaakkola, M.; Kallio, H. The effect of enzymatic treatment on blackcurrant (Ribes nigrum) juice flavour and its stability. Food Chem. 2012, 130, 31–41. [Google Scholar] [CrossRef]
- Fontana, R.C.; da Silveira, M.M. Production of polygalacturonases by Aspergillus oryzae in stirred tank and internal- and external-loop airlift reactors. Bioresour. Technol. 2012, 123, 157–163. [Google Scholar] [CrossRef]
- Alimardani-Theuil, P.; Gainvors-Claisse, A.; Duchiron, F. Yeasts: An attractive source of pectinases-From gene expression to potential applications: A review. Process Biochem. 2011, 46, 1525–1537. [Google Scholar] [CrossRef]
- Maciel, M.H.C.; Herculano, P.N.; Porto, T.S.; Teixeira, M.F.S.; Moreira, K.A.; Souza-Motta, C.M. Production and partial characterization of pectinases from forage palm by Aspergillus niger URM4645. Afr. J. Biotechnol. 2011, 10, 2469–2475. [Google Scholar]
- Rodríguez Couto, S.; Toca-Herrera, J.L. Laccase production at reactor scale by filamentous fungi. Biotechnol. Adv. 2007, 25, 558–569. [Google Scholar] [CrossRef]
- Cunha, F.M.; Esperança, M.N.; Zangirolami, T.C.; Badino, A.C.; Farinas, C.S. Sequential solid-stateand submerged cultivation of Aspergillus niger on sugarcane bagasse for the production of cellulase. Bioresour. Technol. 2012, 112, 270–274. [Google Scholar] [CrossRef]
- Nighojkar, S.; Phanse, Y.; Sinha, D.; Nighojkar, A.; Kumar, A. Production of polygalacturonase by immobilized cells of Aspergillus niger using orange peel as inducer. Process Biochem. 2006, 41, 1136–1140. [Google Scholar] [CrossRef]
- Shrinivas, D.; Kumar, R.; Naik, G.R. Enhanced production of alkaline thermostable keratinolytic protease from calcium alginate immobilized cells of thermoalkalophilic Bacillus halodurans JB 99 exhibiting dehairing activity. J. Ind. Microbiol. Biotechnol. 2012, 39, 93–98. [Google Scholar]
- Taşkin, M. Co-production of tannase and pectinase by free and immobilized cells of the yeast Rhodotorula glutinis MP-10 isolated from tannin-rich persimmon (Diospyros kaki L.) fruits. Bioprocess Biosyst. Eng. 2012. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, H.; Kim, G.H.; Kim, J.J. Miniaturized enzyme production and development of micro-assays for cellulolytic and xylanolytic enzymes. J. Microbiol. Methods 2011, 86, 124–127. [Google Scholar] [CrossRef]
- Pohar, A.; Žnidaršič-Plazl, P.; Plazl, I. Integrated system of a microbioreactor and a miniaturized continuous separator for enzyme catalyzed reactions. Chem. Eng. J. 2012, 189-190, 376–382. [Google Scholar]
- Mrudula, S.; Anitharaj, R. Pectinase production in solid state fermentation by Aspergillus niger using orange peel as substrate. Glob. J. Biotechnol. Biochem. 2011, 6, 64–71. [Google Scholar]
- Maller, A.; Damásio, A.R.L.; Silva, T.M.; Jorge, J.A.; Terenzi, H.F.; Polizeli, M.L.T.M. Biotechnological potential of agro-industrial wastes as a carbon source to thermostable polygalacturonase production in Aspergillus niveus. Enzyme Res. 2011, 2011, 1–6. [Google Scholar]
- Martins, E.S.; Leite, R.S.R.; Silva, R.; Gomes, E. Production and characterization of polygalacturonase from thermophilic Thermoascus aurantiacus on submerged fermentation. Ann. Microbiol. 2012, 62, 1199–1205. [Google Scholar] [CrossRef]
- Cordeiro, C.A.M.; Martins, M.L.L. Production of a polygalacturonase, by thermophilic Bacillus sp. and some properties of the enzyme. Ciênc. Tecnol. Aliment. 2009, 29, 135–141. [Google Scholar] [CrossRef]
- Gattás, E.A.L.; Bueno, M.R.; Ribeiro, M.H.L. Stimulation of polygalacturonase production in an immobilized system by Aspergillus sp.: Effect of pectin an glucose. Eur. Food Res. Technol. 2009, 229, 923–928. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, H.K.; Sarkar, B.C. Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Sci. Biotechnol. 2011, 20, 1289–1298. [Google Scholar] [CrossRef]
- Abbasi, H.; Shafighzadeh, H.; Rahimi, A. Continuous production of polygalacturonases (PGases) by Aspergillus awamori using wheat flour in surface culture fermentation. Iran. J. Biotechnol. 2011, 9, 50–55. [Google Scholar]
- Galiotou-Panayotou, M.P.R.; Kapantai, M. Enhanced polygalacturonase production by Aspergillus niger NRRL-364 grown on supplemented citrus pectin. Lett. Appl. Microbiol. 1993, 17, 145–148. [Google Scholar] [CrossRef]
- Fontana, R.C.; Polidoro, T.A.; da Silveira, M.M. Comparison of stirred tank and airlift bioreactors in the production of polygalacturonases by Aspergillus oryzae. Bioresour. Technol. 2009, 100, 4493–4498. [Google Scholar] [CrossRef]
- Linde, G.A.; Magagnin, G.; Costa, J.A.V.; Bertolin, T.E.; Colauto, N.B. Column bioreactor use for optimization of pectinase production in solid substrate cultivation. Braz. J. Microbiol. 2007, 38, 557–562. [Google Scholar] [CrossRef]
- Kahar, P.; Kobayashi, K.; Iwata, T.; Hiraki, J.; Kojima, M.; Okabe, M. Production of polylysine in an airlift bioreactor (ABR). J. Biosci. Bioeng. 2002, 93, 274–280. [Google Scholar]
- Uenojo, M.; Pastore, G.M. Pectinases: Aplicações industriais e perspectivas. Quim. Nova 2007, 30, 388–394. [Google Scholar] [CrossRef]
- Ming Chu, I.; Lee, C.; Li, T.S. Production and degradation of alkaline protease in batch cultures of Bacillus subtilis ATCC 14416. Enzyme Microb. Technol. 1992, 14, 755–761. [Google Scholar] [CrossRef]
- Pashova, S.; Slokoska, L.; Sheremetska, P.; Krumova, E.; Vasileva, L.; Angelova, M. Physiological aspects of immobilised Aspergillus niger cells producing polymethylgalacturonase. Process Biochem. 1999, 35, 15–19. [Google Scholar] [CrossRef]
- Pashova, S.; Slokoska, L.; Krumova, E.; Angelova, M. Induction of polymethylgalacturonase biosynthesis by immobilized cells of Aspergillus niger 26. Enzyme Microb. Technol. 1999, 24, 535–540. [Google Scholar] [CrossRef]
- Micoteca URM. Available online: www.ufpe.br/micoteca/ (accessed on 9 January 2013).
- Micoteca da Universidade do Minho. Available online: www.micoteca.deb.uminho.pt/ (accessed on 9 January 2013).
- Spier, M.R.; Greiner, R.; Rodriguez-León, J.A.; Woiciechowski, A.L.; Pandey, A.; Soccol, V.T.; Soccol, C.R. Phytase production using citric pulp and other residues of the agroindustry in SSF by fungal isolates. Food Technol. Biotechnol. 2008, 46, 178–182. [Google Scholar]
- Tuttobello, B.R.; Mill, P.J. The pectic enzymes of Aspergillus niger. 1. The production of active mixtures of pectic enzymes. Biochem. J. 1961, 79, 51–57. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maciel, M.; Ottoni, C.; Santos, C.; Lima, N.; Moreira, K.; Souza-Motta, C. Production of Polygalacturonases by Aspergillus section Nigri Strains in a Fixed Bed Reactor. Molecules 2013, 18, 1660-1671. https://doi.org/10.3390/molecules18021660
Maciel M, Ottoni C, Santos C, Lima N, Moreira K, Souza-Motta C. Production of Polygalacturonases by Aspergillus section Nigri Strains in a Fixed Bed Reactor. Molecules. 2013; 18(2):1660-1671. https://doi.org/10.3390/molecules18021660
Chicago/Turabian StyleMaciel, Marília, Cristiane Ottoni, Cledir Santos, Nelson Lima, Keila Moreira, and Cristina Souza-Motta. 2013. "Production of Polygalacturonases by Aspergillus section Nigri Strains in a Fixed Bed Reactor" Molecules 18, no. 2: 1660-1671. https://doi.org/10.3390/molecules18021660
APA StyleMaciel, M., Ottoni, C., Santos, C., Lima, N., Moreira, K., & Souza-Motta, C. (2013). Production of Polygalacturonases by Aspergillus section Nigri Strains in a Fixed Bed Reactor. Molecules, 18(2), 1660-1671. https://doi.org/10.3390/molecules18021660






