Polyphenolic Extracts of Edible Flowers Incorporated onto Atelocollagen Matrices and Their Effect on Cell Viability
Abstract
:1. Introduction
2. Results and Discussion
| Name (compound number) | Wild Chive | Introduced Sage | European Elderberry | Common Dandelion |
|---|---|---|---|---|
| A. schoenoprasum | S. pratensis | S. nigra | T. officinale | |
| Gallic acid (1) | 201.76 | 22.67 | 176.61 | 441.40 |
| Coumaric acid (2) | 207.29 | / | / | / |
| Ferulic acid (3) | 887.44 | / | / | / |
| Rutin (4) | 20.26 | / | / | 18.66 |
| Resveratrol (50 | / | / | / | 274.92 |
| Vanillic acid (6) | / | / | 299.38 | 82.88 |
| Sinapic acid (7) | / | / | / | 593.04 |
| Catechin (8) | / | 37.56 | / | / |
| Caffeic acid (9) | / | / | 913.19 | / |
| Total phenolic content (mg/g) | 17.50 | 17.10 | 18.40 | 17.20 |
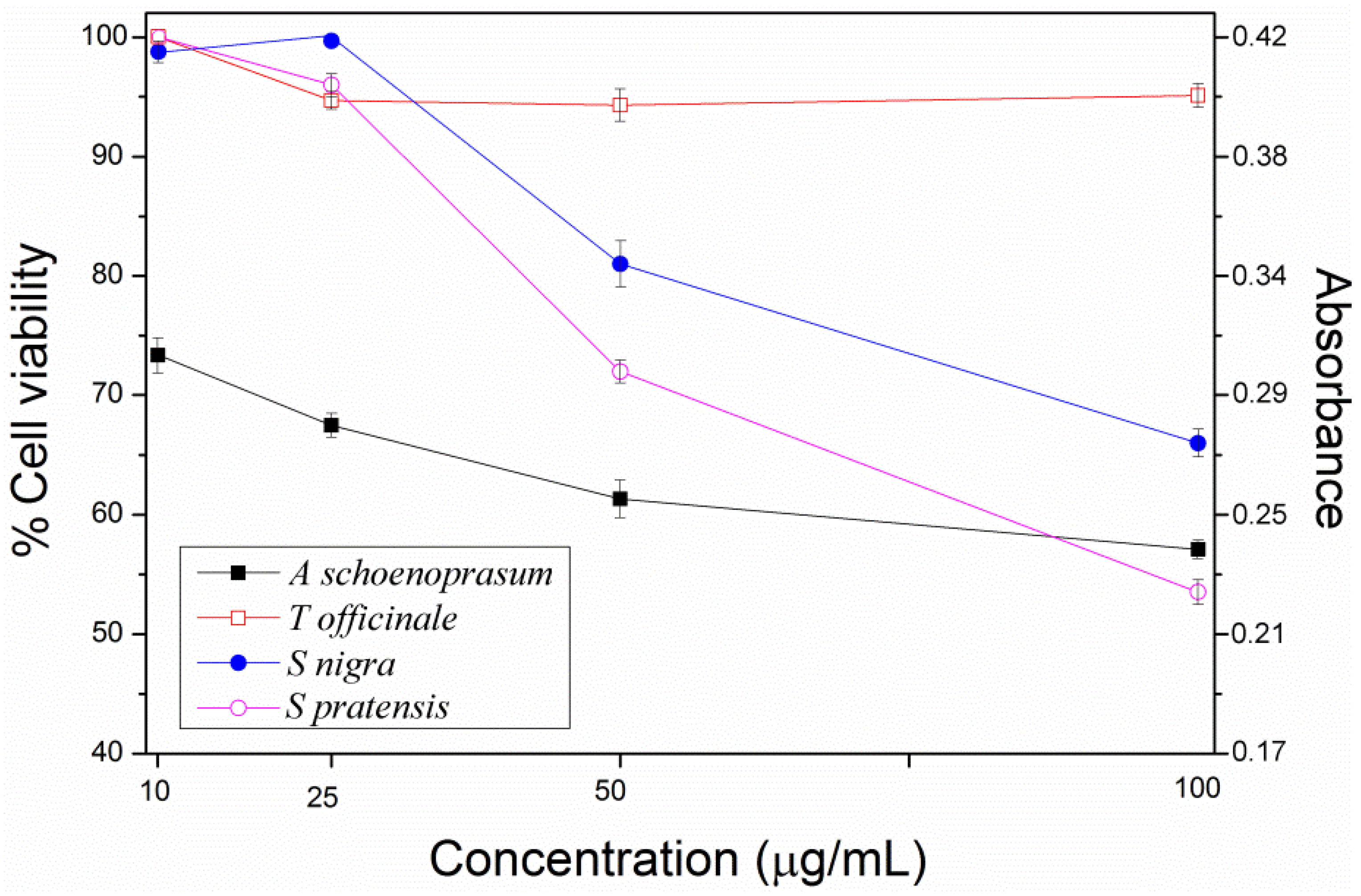
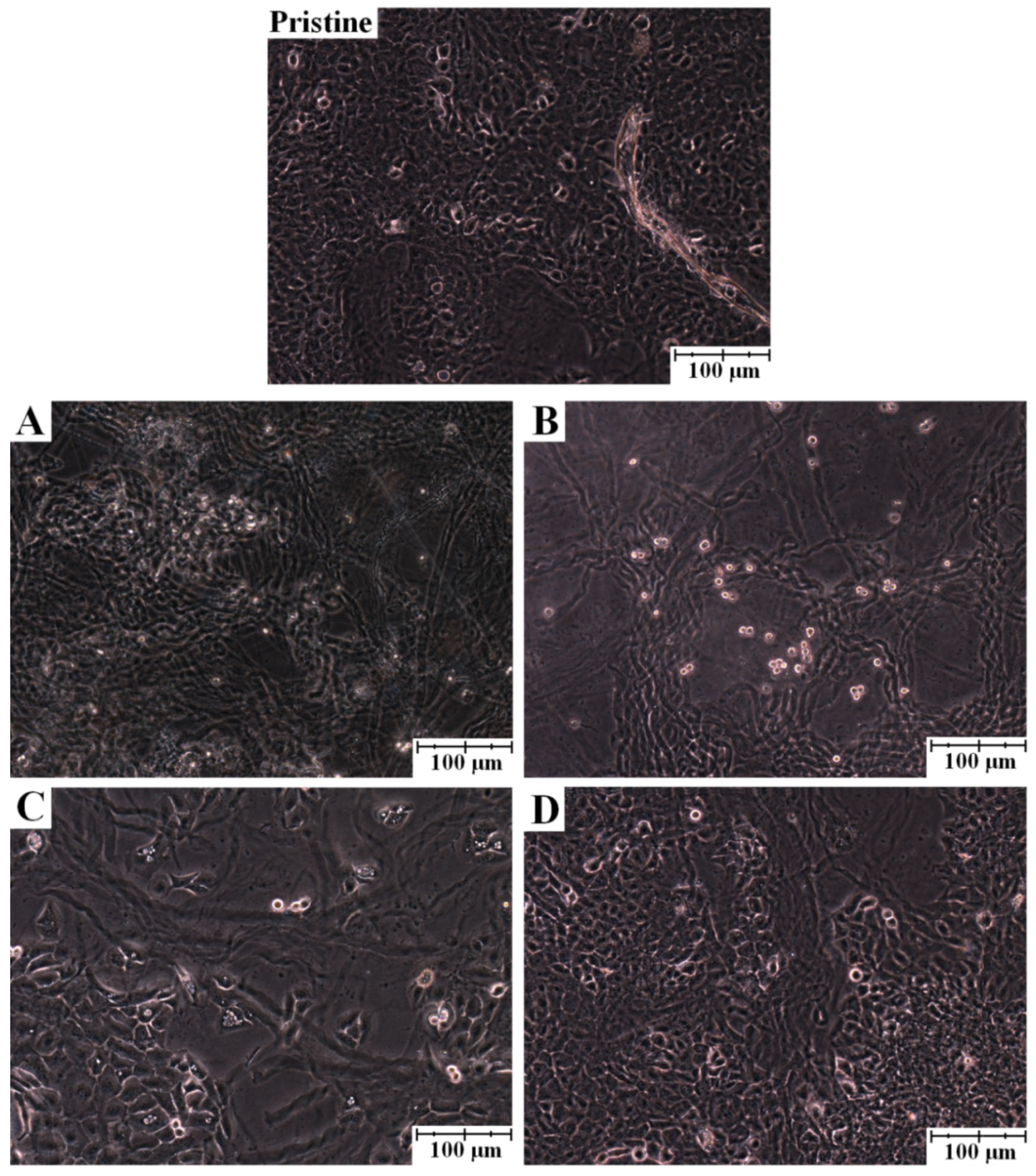
3. Experimental
3.1. Materials
3.2. Extraction Conditions
3.3. Determination of Polyphenols
3.4. High Performance Liquid Chromatography (HPLC)
3.5. Atelocollagen Thin Films Preparation
3.6. HaCaT Cell Incubation
3.7. Cell Viability
3.8. Antimicrobial Assay in Vitro
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, S.; Maiti, T.K. Caprine (Goat) collagen: A potential biomaterial for skin tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 355–373. [Google Scholar] [CrossRef]
- Bernal, A.; Balková, R.; Kuřítka, I.; Sáha, P. Preparation and characterisation of a new double-sided bio-artificial material prepared by casting of poly(vinyl alcohol) on collagen. Polym. Bull. 2012, 70, 431–453. [Google Scholar]
- López-García, J.; Humpolíček, P.; Lehocký, M.; Junkar, I.; Mozetič, M. Different source atelocollagen thin films: Preparation, process optimisation and its influence on the interaction with eukaryotic cells. Mater. Tehnol. 2013, 47, 473–479. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue enginnering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Langer, R.; Tirell, D.A. Designing materials for biology and medicine. Nature 2004, 18, 487–492. [Google Scholar] [CrossRef]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, 311–324. [Google Scholar] [CrossRef]
- Garcia, J.L.; Asadinezhad, A.; Pacherník, J.; Lehocký, M.; Junkar, I.; Humpolíček, P.; Sáha, P.; Valášek, P. Cell proliferation of HaCaT keratinocytes on collagen films modified by argon plasma treatment. Molecules 2010, 15, 2845–2856. [Google Scholar] [CrossRef]
- Štajner, D.; Čanadanović-Brunet, J.; Pavlović, A. Allium schoenoprasum L., as a natural antioxidant. Phytother. Res. 2004, 18, 522–524. [Google Scholar] [CrossRef]
- Anačkov, G.; Božin, B.; Zorić, L.; Vukov, D.; Mimica-Dukić, N.; Merkulov, L.; Igić, R.; Jovanović, M.; Boža, P. Chemical composition of essential oil and leaf anatomy of Salvia bertolonii vis. and Salvia pratensis L. (Sect. Plethiosphace, Lamiaceae). Molecules 2009, 14, 1–9. [Google Scholar]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plants. Res. 2010, 4, 1805–1809. [Google Scholar]
- Kirschner, J.; Stepanek, J. Typification of Leontodon taraxacum L. (Taraxacum officinale FH Wigg.) and the generic name Taraxacum: A review and a new typification proposal. Taxon 2011, 60, 216–220. [Google Scholar]
- Shirshova, T.I.; Beshlei, I.V.; Deryagina, V.P.; Ryzhova, N.I.; Matistov, N.V. Chemical composition of Allium schoenoprasum leaves and inhibitory effect of their extract on tumor growth in mice. Pharm. Chem. J. 2013, 46, 672–675. [Google Scholar] [CrossRef]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Chemical constituents of three Allium species from Romania. Molecules 2013, 18, 114–127. [Google Scholar]
- Kuceková, Z.; Mlček, J.; Humpolíček, P.; Rop, O. Edible flowers–Antioxidant activity and impact on cell viability. Cent. Eur. J. Biol. 2013, 8, 1023–1031. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int. 2009, 42, 1331–1336. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of colour and phenolic compounds during Garnacha Tintorera grape raisining. Food Chem. 2013, 141, 3230–3240. [Google Scholar] [CrossRef]
- Rop, O.; Mlček, J.; Juřiková, T.; Valsikova, M.; Sochor, J.; Reznicek, J.; Kramarova, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J. Med. Plants Res. 2010, 4, 2431–2437. [Google Scholar]
- Osorio, C.; Carriazo, J.G.; Almanza, O. Antioxidant activity of corozo (Bactris guineensis) fruit byelectron paramagnetic resonance (EPR) spectroscopy. Eur. Food Res. Technol. 2011, 233, 103–108. [Google Scholar] [CrossRef]
- Mlček, J.; Rop, O. Fresh edible flowers of ornamental plants–A new source of nutraceutical foods. Trends Food Sci. Tech. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of post-harvest practices on flavonoid content of red and white onion cultivars. Food Control 2010, 21, 878–884. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 2011, 22, 1108–1113. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Austin, M.B.; Stewart, C.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Bioch. 2008, 46, 356–370. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Simal-Gándara, J.; Boso, S.; Martínez, M.C.; Santiago, J.L.; Cancho-Grande, B. Flavonoids in Gran Negro berries collected from shoulders and tips within the cluster, and comparison with Brancellao and Mouratón varieties. Food Chem. 2012, 133, 806–815. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Kuceková, Z.; Mlček, J.; Humpolíček, P.; Rop, O.; Valášek, P.; Sáha, P. Phenolic compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and their antiproliferative effects. Molecules 2011, 16, 9207–9217. [Google Scholar] [CrossRef]
- Araim, O.; Ballantyne, J.; Waterhouse, A.L.; Sumpio, B.E. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J. Vasc. Surg. 2002, 35, 1226–1232. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Simal-Gándara, J.; Boso, S.; Martínez, M.C.; Santiago, J.L.; Cancho-Grande, B. Anthocyanins and flavonols berries from Vitis vinifera L. cv. Brancellao separately collected from two different positions within the cluster. Food Chem. 2012, 135, 47–56. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Regueiro, J.; Simal-Gándara, J.; Tomás, E.; Rivas-Gonzalo, J.C.; Escribano-Bailón, T. Relationship between the sensory-determined astringency and the flavanolic composition of red wines. J. Agric. Food Chem. 2012, 60, 12355–12361. [Google Scholar] [CrossRef]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity and antimicrobial activity of leaf extracts from six Vitis Viniferea L. varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Hashimoto, S.; Miyazawa, M.; Kameoka, H. Volatile flavour component of chive Allium Schoenprasum. J. Food Sci. 1983, 48, 1858–1863. [Google Scholar] [CrossRef]
- Veličković, D.T.; Randelović, N.V.; Ristić, M.S.; Šmelcerović, A.A.; Veličković, A.S. Chemical, composition and antimicrobial action of the ethanol extracts of Salvia pratensis L., Salvia glutinosa L. and Salvia aethiopis L. J. Serb. Chem. Soc. 2002, 67, 639–646. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef]
- Alonso-García, A.; Cancho-Grande, B.; Simal-Gándara, J. Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet detection for the determination of polyphenols in alcohol-free beers. J. Chromatogr. A 2004, 1054, 175–180. [Google Scholar]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Garnacha Tintorera-based sweet wines: Chromatic properties and global phenolic composition by means of UV-Vis spectrophotometry. Food Chem. 2013, 140, 217–224. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993 5: 2009. Biological evaluation of medical devices, Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Campbell, J.K.; King, J.L.; Harmston, M.; Lila, M.A.; Erdman, J.W. Synergistic effects of flavonoids on cell proliferation in Hepa-1c1c7 and LNCaP cancer cell lines. J. Food Sci. 2006, 71, 358–363. [Google Scholar]
- Kowalczyk, M.C.; Kowalczyk, P.; Tolstykh, O.; Hanausek, M.; Walaszek, Z.; Slaga, T.J. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev. Res. 2010, 3, 170–178. [Google Scholar] [CrossRef]
- Hole, A.; Grimmer, S.; Jensen, M.R.; Sahlstrøm, S. Synergistic and suppressive effects of dietary phenolic acids and other phytochemicals from cereal extracts on nuclear factor kappa B activity. Food Chem. 2012, 133, 969–977. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- García, J.L.; Pacherník, J.; Lehocký, M.; Junkar, I.; Humpolíček, P.; Sáha, P. Enhanced keratinocyte cell attachment to atelocollagen thin films through air and nitrogen plasma treatment. Prog. Colloid Polym. Sci. 2011, 138, 89–94. [Google Scholar]
- Huang, C.-C.; Wu, W.-B.; Fang, J.-Y.; Chiang, H.-S.; Chen, S.-K.; Chen, B.-H.; Chen, Y.-T.; Hung, C.-F. (−)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT Keratinocytes. Molecules 2007, 12, 1845–1858. [Google Scholar] [CrossRef]
- Matić, I.; Žižak, Ž.; Simonović, M.; Simonović, B.; Godevadc, D.; Šavikin, K.; Juranić, Z. Cytotoxic effect of wine polyphenolic extracts and resveratrol against human carcinoma cells and normal peripheral blood mononuclear cells. J. Med. Food 2010, 13, 851–862. [Google Scholar] [CrossRef]
- Moravčíková, D.; Kuceková, Z.; Mlček, J.; Rop, O.; Humpolíček, P. Compositions of polyphenols in wild chive, meadow salsify, garden sorrel and agyoncha and their anti-proliferative effect. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 125–132. [Google Scholar]
- Gollucke, A.P.B.; Aguiar, O.; Barbisan, L.F.; Ribeiro, D.A. Use of grape polyphenols against carcinogenesis: Putative molecular mechanisms of action using in vitro and in vivo test systems. J. Med. Food 2013, 16, 199–205. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A. Normal keratinizationin a spontaneously immortalized aneuploid keratinocyte cell line. J. Cell Biol. 1998, 106, 761–771. [Google Scholar]
- Sample Availability: Not Available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
López-García, J.; Kuceková, Z.; Humpolíček, P.; Mlček, J.; Sáha, P. Polyphenolic Extracts of Edible Flowers Incorporated onto Atelocollagen Matrices and Their Effect on Cell Viability. Molecules 2013, 18, 13435-13445. https://doi.org/10.3390/molecules181113435
López-García J, Kuceková Z, Humpolíček P, Mlček J, Sáha P. Polyphenolic Extracts of Edible Flowers Incorporated onto Atelocollagen Matrices and Their Effect on Cell Viability. Molecules. 2013; 18(11):13435-13445. https://doi.org/10.3390/molecules181113435
Chicago/Turabian StyleLópez-García, Jorge, Zdenka Kuceková, Petr Humpolíček, Jiři Mlček, and Petr Sáha. 2013. "Polyphenolic Extracts of Edible Flowers Incorporated onto Atelocollagen Matrices and Their Effect on Cell Viability" Molecules 18, no. 11: 13435-13445. https://doi.org/10.3390/molecules181113435
APA StyleLópez-García, J., Kuceková, Z., Humpolíček, P., Mlček, J., & Sáha, P. (2013). Polyphenolic Extracts of Edible Flowers Incorporated onto Atelocollagen Matrices and Their Effect on Cell Viability. Molecules, 18(11), 13435-13445. https://doi.org/10.3390/molecules181113435






