Sesquiterpene Lactones and Their Derivatives Inhibit High Glucose-Induced NF-κB Activation and MCP-1 and TGF-β1 Expression in Rat Mesangial Cells
Abstract
:1. Introduction
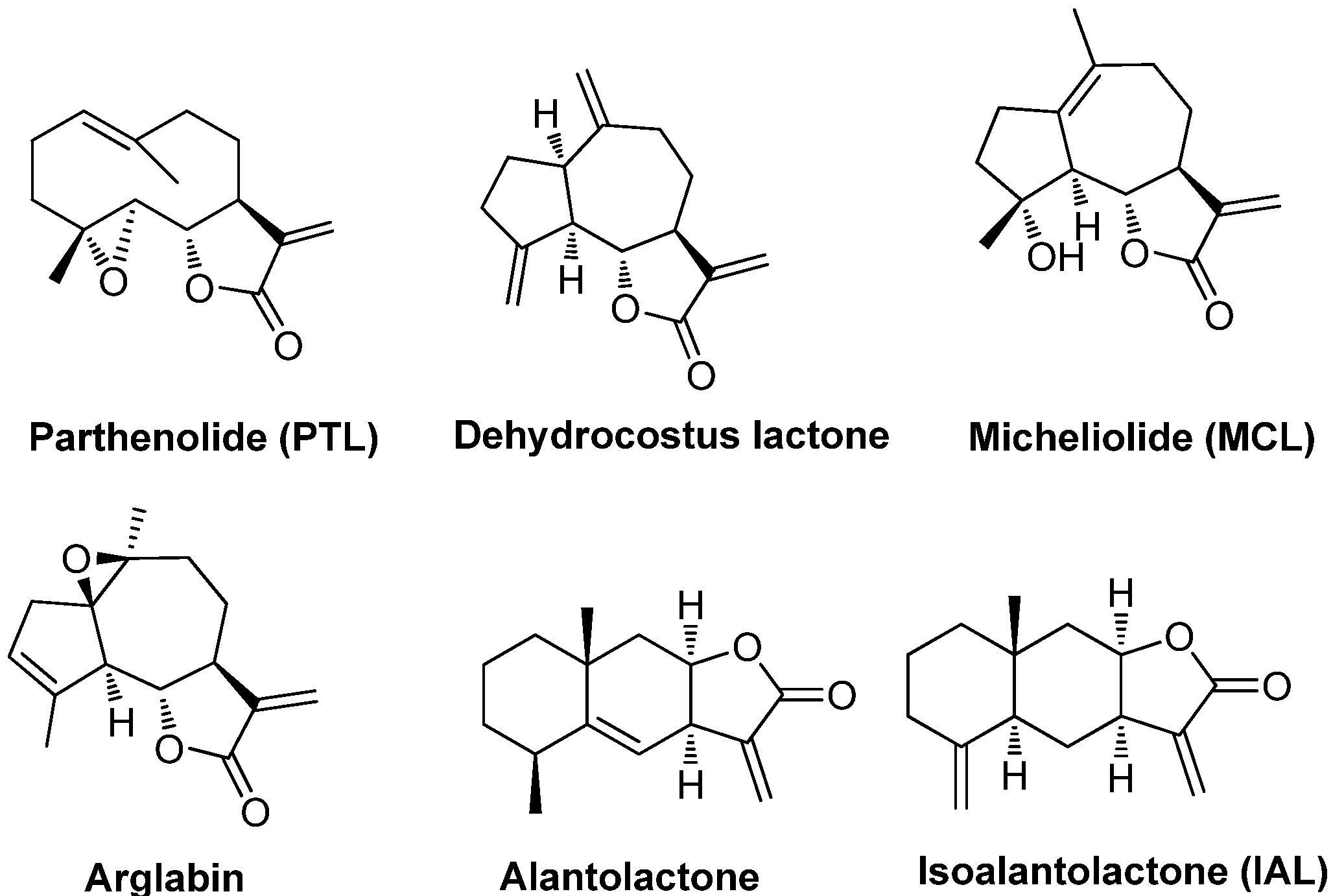
2. Results and Discussion
2.1. Chemistry
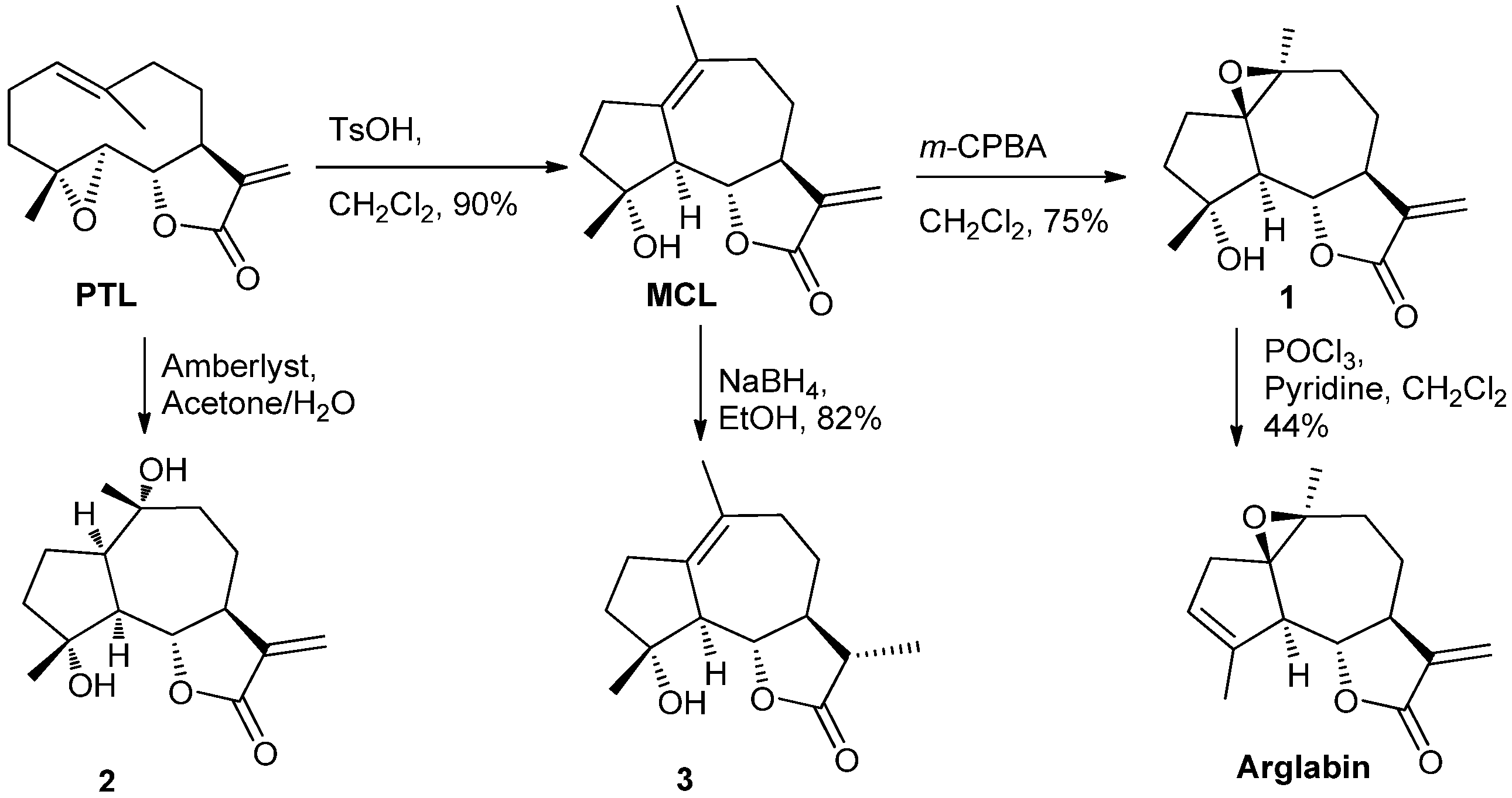
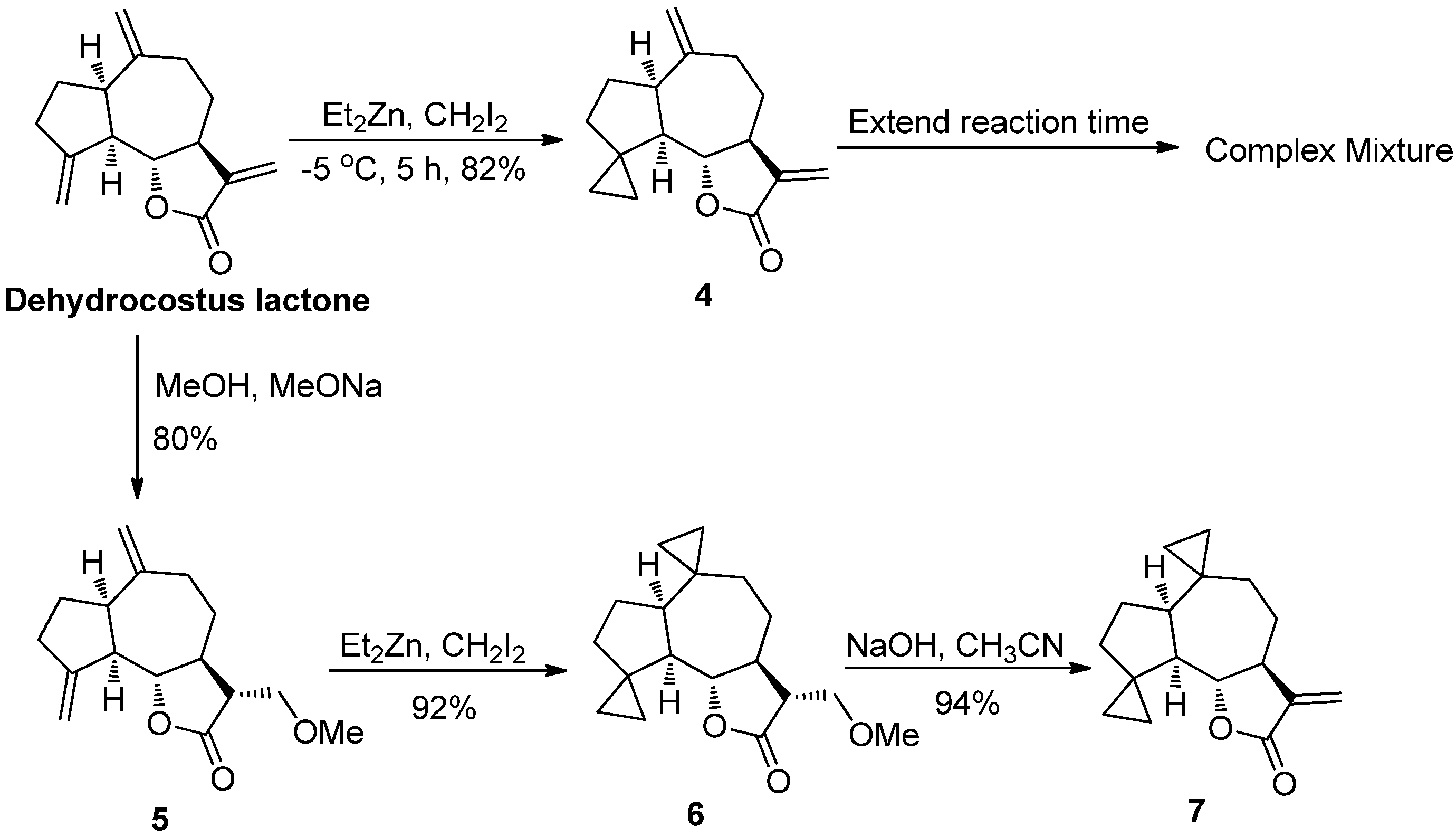
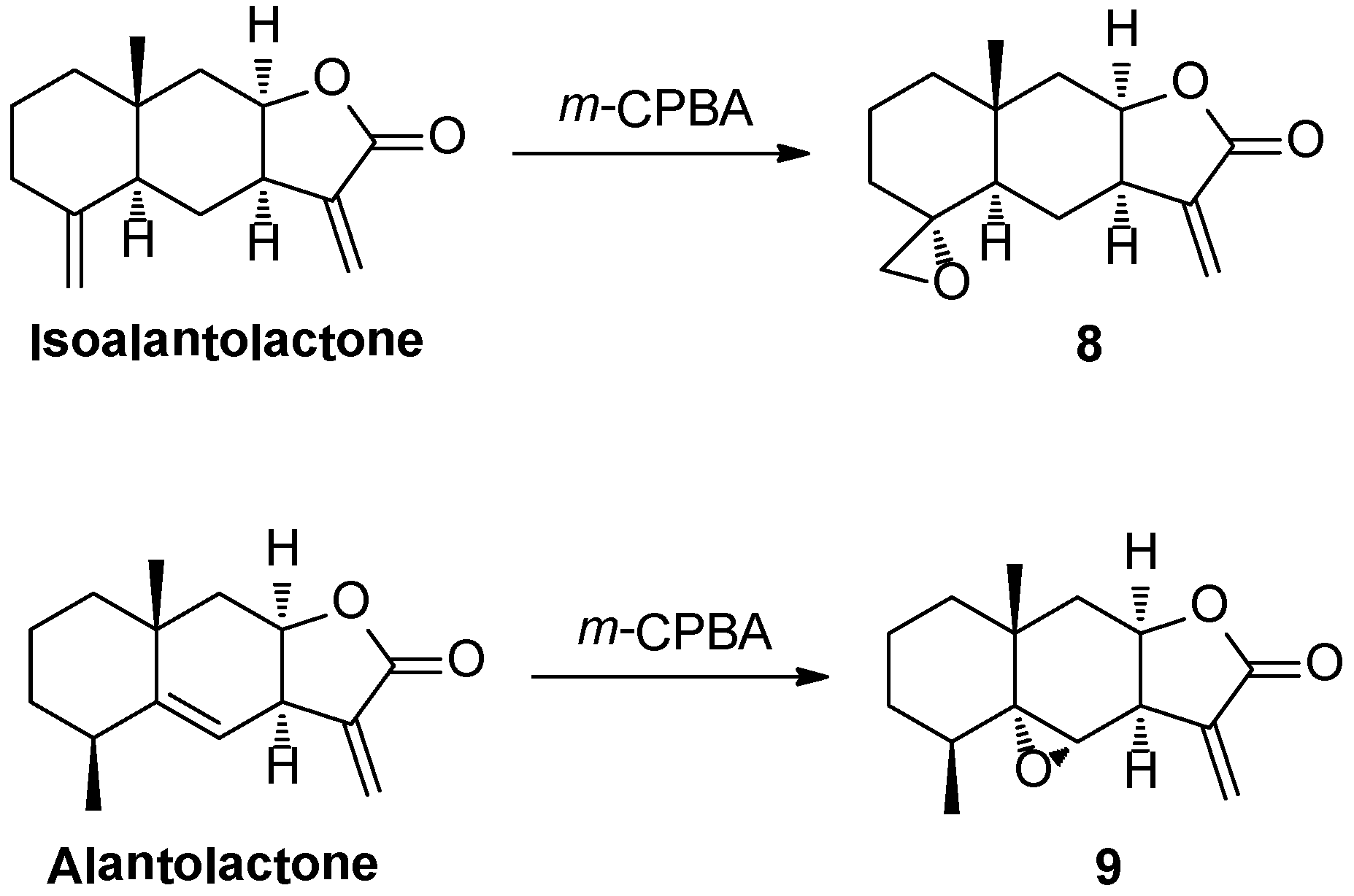
2.2. Bioactivities
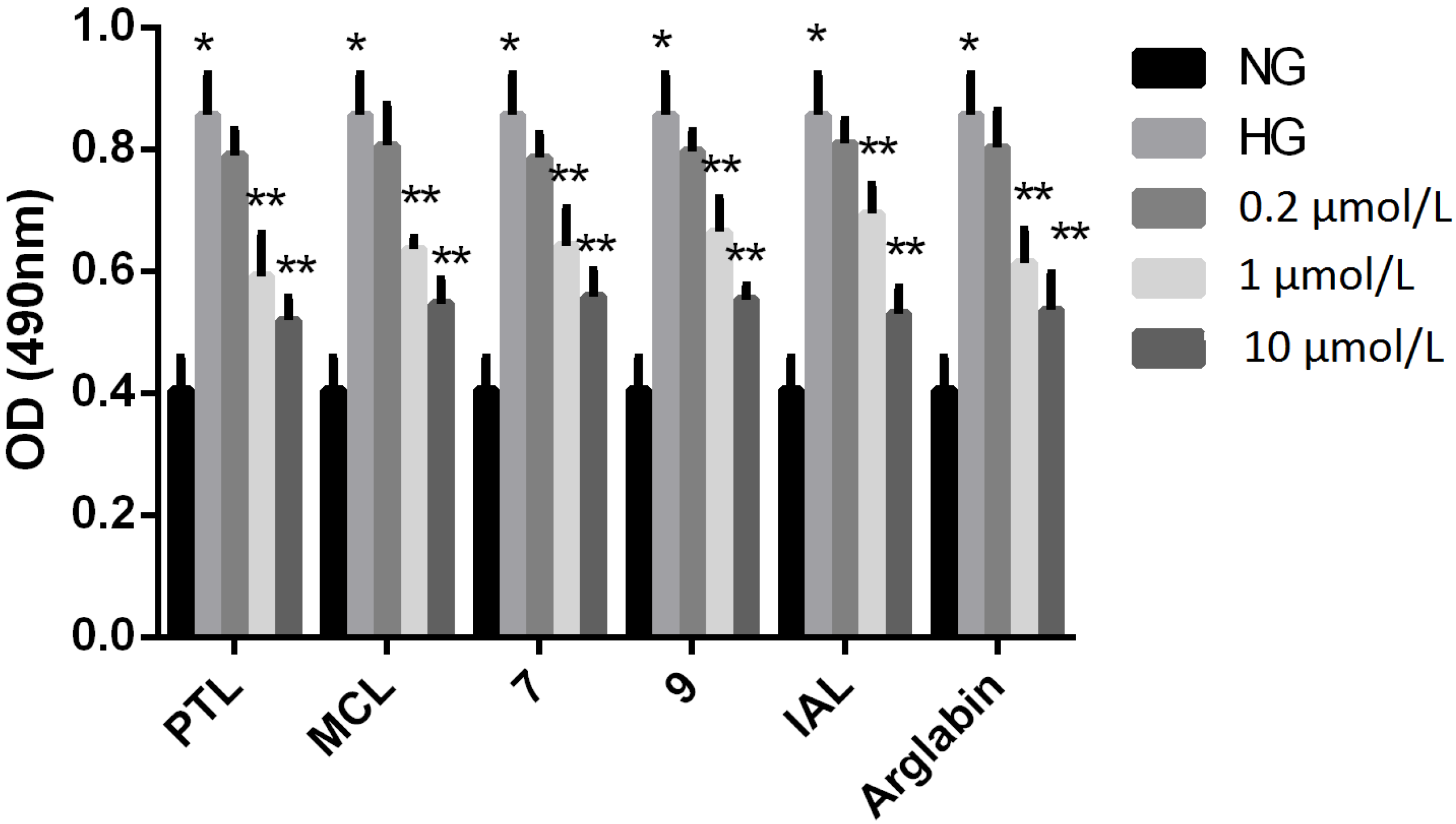
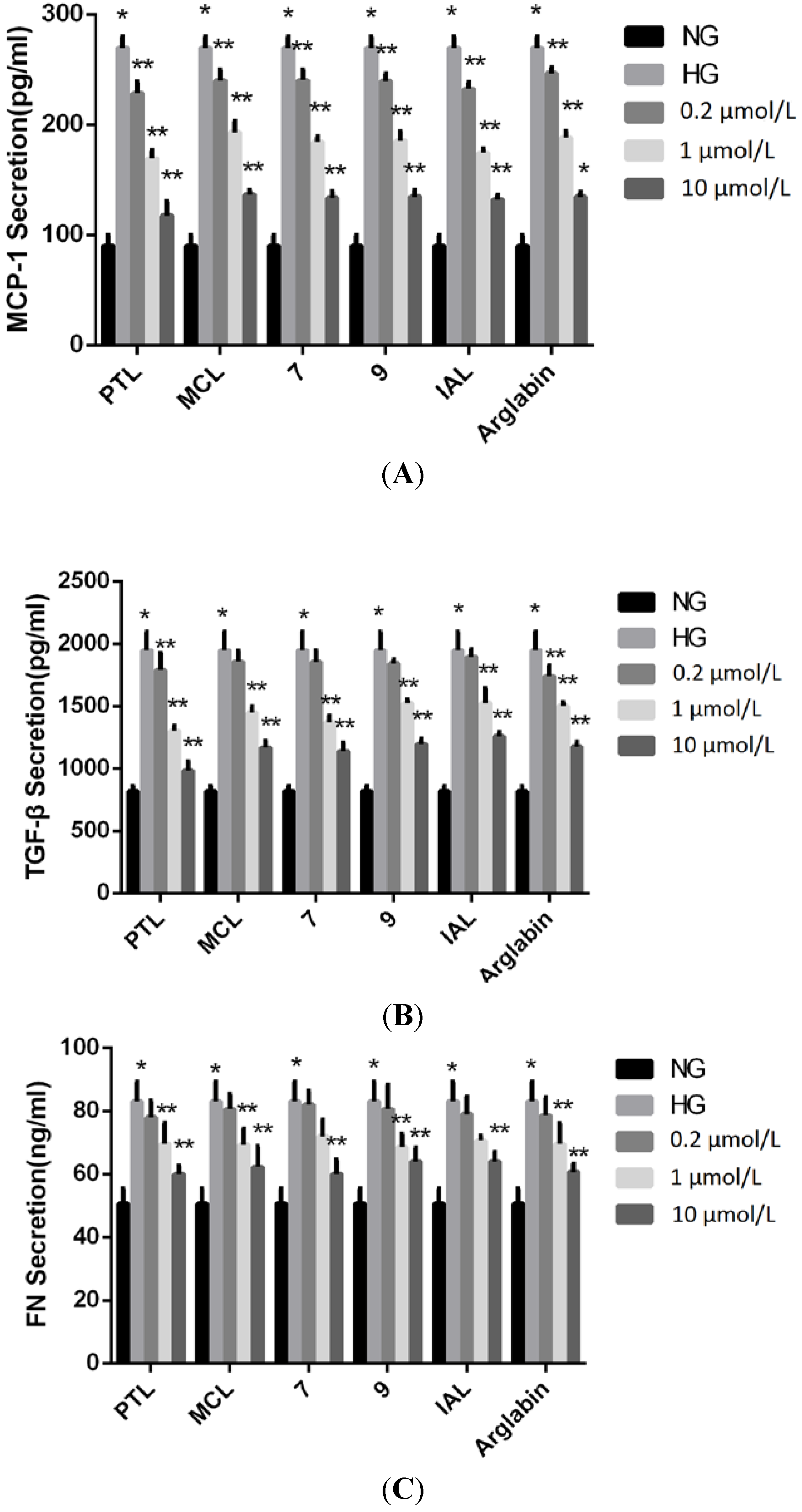
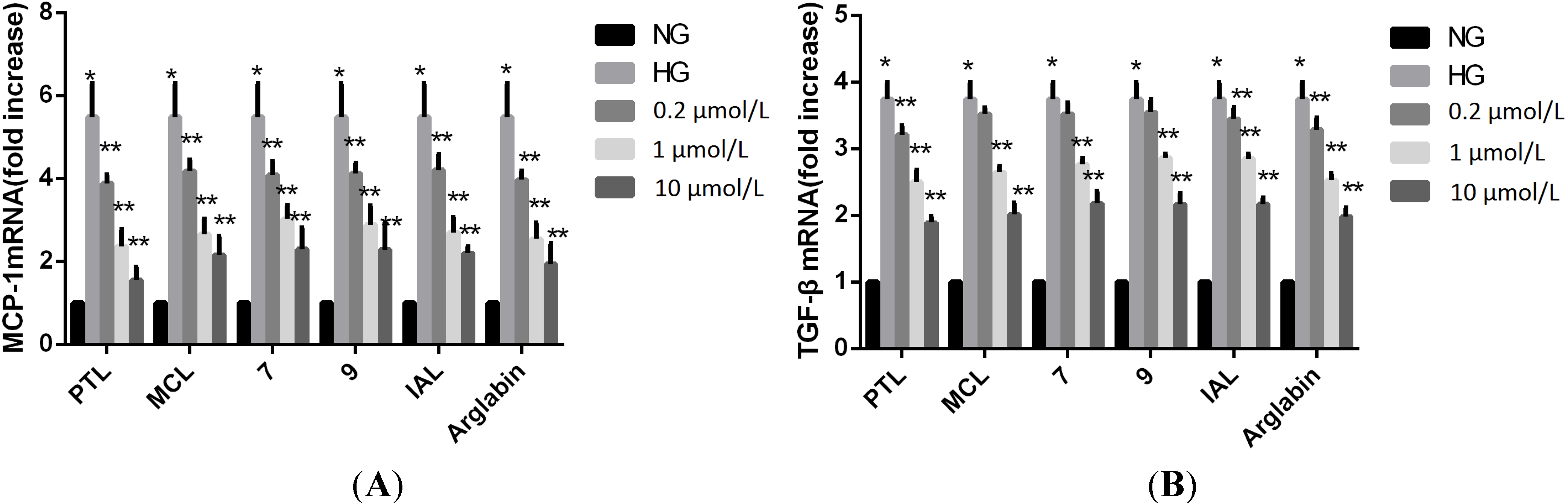
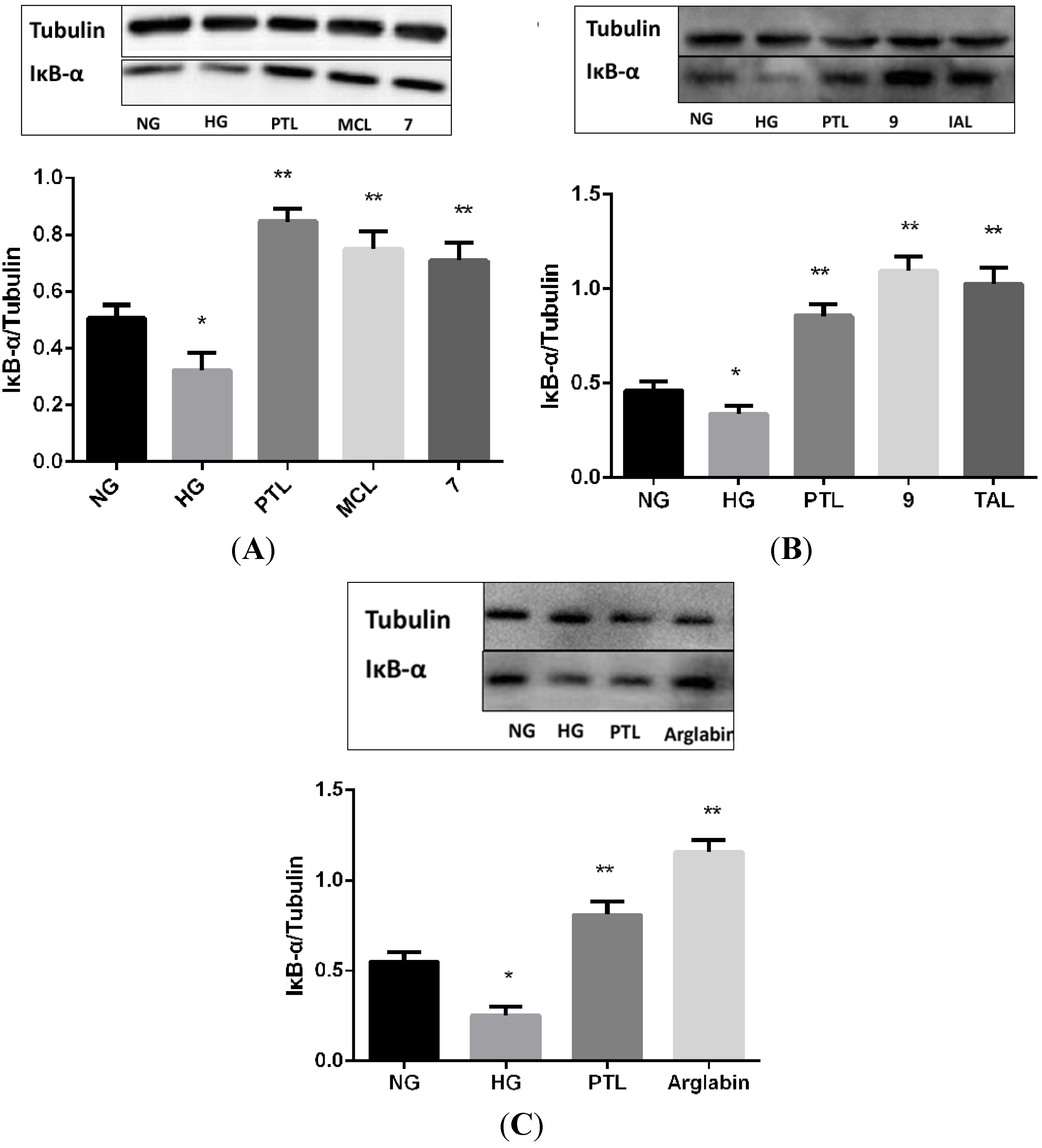
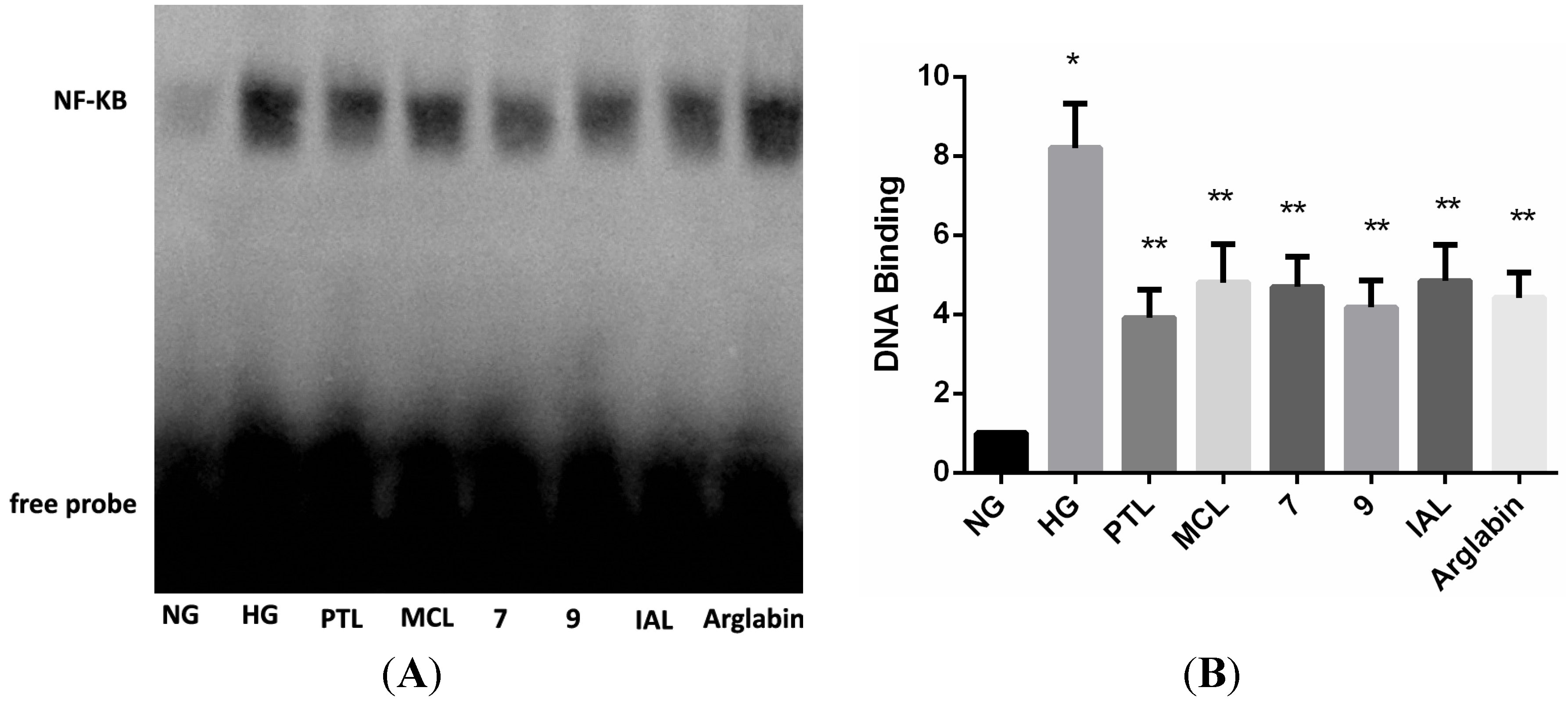
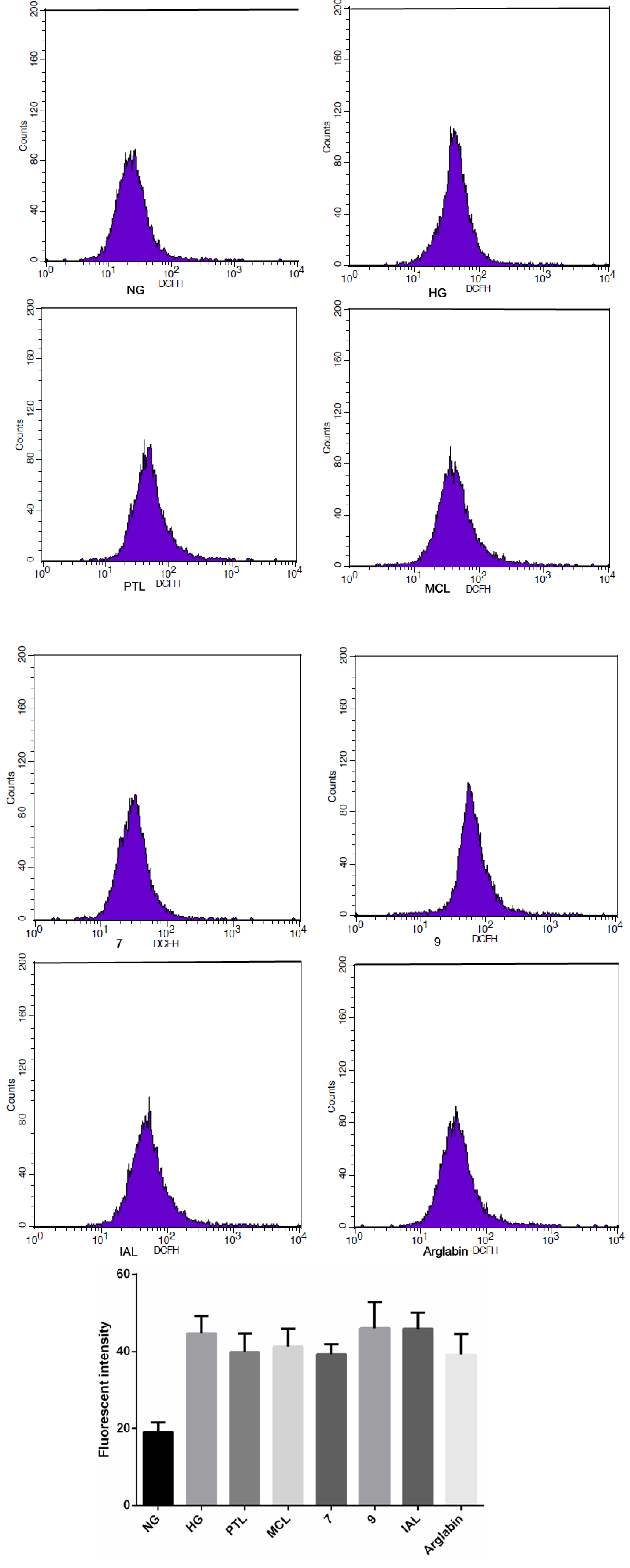
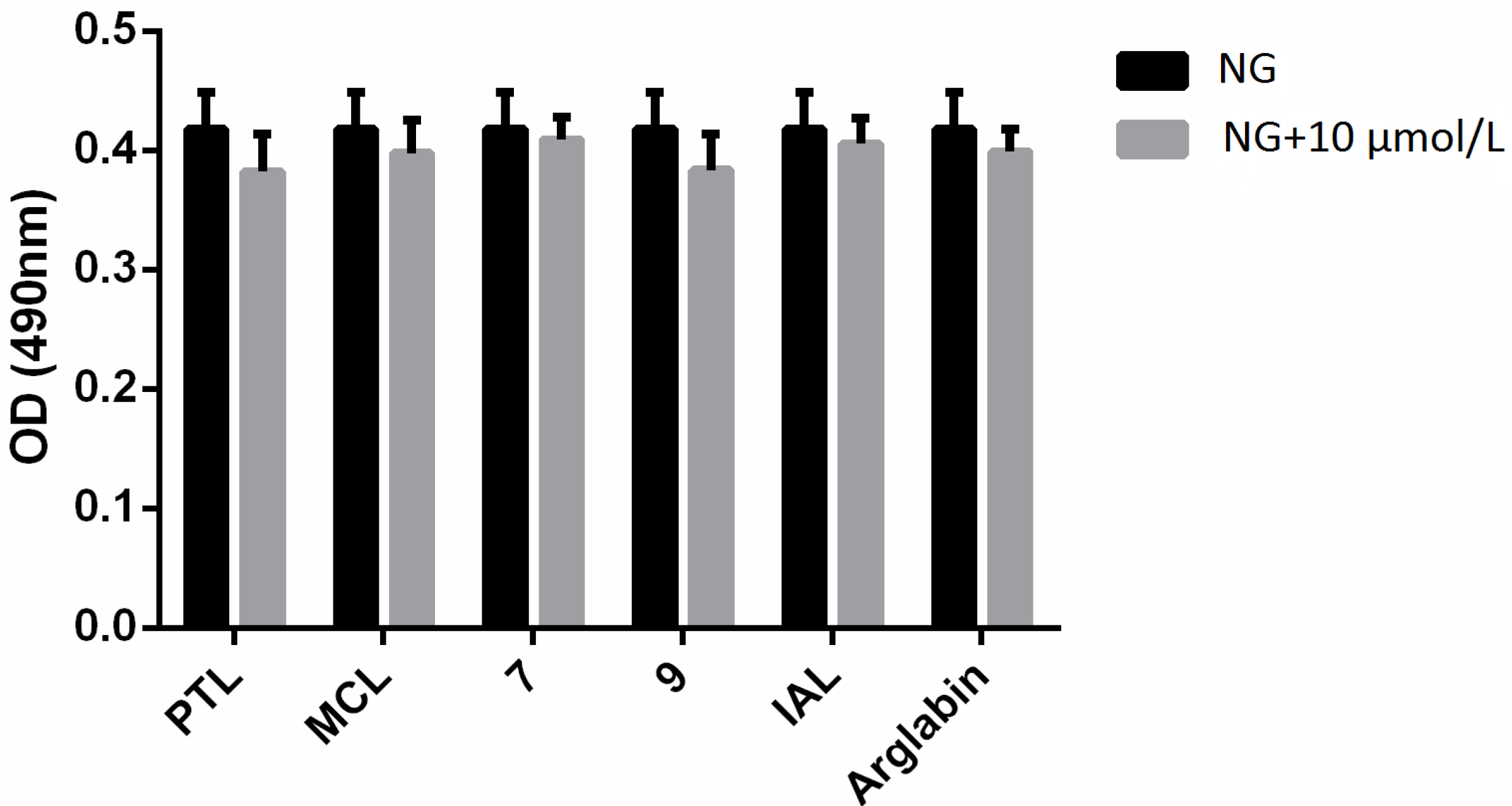
3. Experimental
3.1. Materials
3.2. Methods
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Packham, D.K.; Alves, T.P.; Dwyer, J.P.; Atkins, R.; de Zeeuw, D.; Cooper, M.; Shahinfar, S.; Lewis, J.B.; Lambers Heerspink, H.J. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am. J. Kidney Dis. 2012, 59, 75–83. [Google Scholar] [CrossRef]
- The ONTARGET Investigators; Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef]
- Kanwar, Y.S.; Wada, J.; Sun, L.; Xie, P.; Wallner, E.I.; Chen, S.; Chugh, S.; Danesh, F.R. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Bio. Med. 2008, 233, 4–11. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; de Fuentes, M.M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Min, D.; Lyons, J.G.; Bonner, J.; Twigg, S.M.; Yue, D.K.; McLennan, S.V. Mesangial cell-derivedfactors alter monocyte activation and function through inflammatory pathways: Possible pathogenic role in diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2009, 297, 1229–1237. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, G.; Sharma, S.S. JSH-23 targets nuclear factor-kappa B and reverses various deficits in experimental diabetic neuropathy: Effect on neuroinflammation and antioxidant defence. Diabetes Obes. Metab. 2011, 13, 750–758. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, G.; Chen, G.; Huang, B.; Zhang, X.; Shen, K.; Wu, S. Microcystin-LR induces apoptosis via NF-κB/iNOS pathway in INS-1 cells. Int. J. Mol. Sci. 2011, 12, 4722–3474. [Google Scholar] [CrossRef]
- Pai, S.; Thomas, R. Immune deficiency or hyperactivity-Nf-kb illuminates autoimmunity. J. Autoimmun. 2008, 31, 245–251. [Google Scholar] [CrossRef]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biophys. Acta 2011, 1812, 719–731. [Google Scholar] [CrossRef]
- Wu, J.; Guan, T.J.; Zheng, S.; Grosjean, F.; Liu, W.; Xiong, H.; Gordon, R.; Vlassara, H.; Striker, G.E.; Zheng, F. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice. Lab. Invest. 2011, 91, 1459–1471. [Google Scholar] [CrossRef]
- Nogueira-Machado, J.A.; Volpe, C.M.; Veloso, C.A.; Chaves, M.M. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert. Opin. Ther. Targets 2011, 15, 1023–1035. [Google Scholar] [CrossRef]
- Ingaramo, P.I.; Ronco, M.T.; Francés, D.E.; Monti, J.A.; Pisani, G.B.; Ceballos, M.P.; Galleano, M.; Carrillo, M.C.; Carnovale, C.E. Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes Mellitus. Mol. Immunol. 2011, 48, 1397–1407. [Google Scholar] [CrossRef]
- Giunti, S.; Tesch, G.H.; Pinach, S.; Burt, D.J.; Cooper, M.E.; Cavallo-Perin, P.; Camussi, G.; Gruden, G. Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 2008, 51, 198–207. [Google Scholar]
- Chow, F.Y.; Nikolic-Paterson, D.J.; Ma, F.Y.; Ozols, E.; Rollins, B.J.; Tesch, G.H. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 2007, 50, 471–480. [Google Scholar] [CrossRef]
- Murphy, M.; Docherty, N.G.; Griffin, B.; Howlin, J.; McArdle, E.; McMahon, R.; Schmid, H.; Kretzler, M.; Droguett, A.; Mezzano, S.; et al. IHG-1 amplifies TGF-beta 1 signaling and is increased in renal fibrosis. J. Am. Soc. Nephrol. 2008, 19, 1672–1680. [Google Scholar] [CrossRef]
- Lan, Y.; Zhou, Q.; Wu, Z.L. NF-kappa B involved in transcription enhancement of TGF-beta 1 induced by Ox-LDL in rat mesangial cells. Chin. Med. J. (Engl.) 2004, 117, 225–230. [Google Scholar]
- Chen, K.H.; Cheng, M.L.; Jing, Y.H.; Chiu, D.T.; Shiao, M.S.; Chen, J.K. Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 853–863. [Google Scholar] [CrossRef]
- Ting, A.Y.; Endy, D. Decoding NF-KB signaling. Science 2002, 298, 1189–1190. [Google Scholar] [CrossRef]
- Lawrence, T.; Bebien, M.; Liu, G.Y.; Nizet, V.; Karin, M. IKKalpha limits macrophage NF-kappaB activation and contribution of inflammation. Nature 2005, 434, 1138–1143. [Google Scholar] [CrossRef]
- Giarratana, N.; Penna, G.; Amuchastegui, S.; Mariani, R.; Daniel, K.C.; Adorini, L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J. Immunol. 2004, 173, 2280–2287. [Google Scholar]
- Vaughan, S.; Jat, P.S. Deciphering the role of nuclear factor kappaB in cellular senescence. Aging (Albany N.Y.) 2011, 3, 913–919. [Google Scholar]
- Janecka, A.; Wyrębska, A.; Gach, K.; Fichna, J.; Janecki, T. Natural and synthetic α-methylenelactones and α-methylenelactams with anticancer potential. Drug Discov. Today 2012, 17, 561–572. [Google Scholar] [CrossRef]
- Knight, D.W. Feverfew: chemistry and biological activity. Nat. Prod. Rep. 1995, 12, 271–276. [Google Scholar] [CrossRef]
- Wang, W.J.; Wu, F.; Qian, Y.; Cheng, L.F.; Shao, X.T.; Tong, X.M.; Li, H. Inhibition of inflammatory factors by parthenolide in human renal mesangial cells under hyperglycemic condition. Afr. J. Biotechnol. 2010, 9, 3458–3463. [Google Scholar]
- Oka, D.; Nishimura, K.; Shiba, M.; Nakai, Y.; Arai, Y.; Nakayama, M.; Takayama, H.; Inoue, H.; Okuyama, A.; Nonomura, N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappa B. Int. J. Cancer 2007, 120, 2576–2581. [Google Scholar] [CrossRef]
- Schall, A.; Reiser, O. Synthesis of biologically active guaianolides with a trans-annulated lactone moiety. Eur. J. Org. Chem. 2008, 2353–2364. [Google Scholar] [CrossRef]
- Hussain, R.F.; Nouti, A.M.; Oliver, R.T. A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods 1993, 160, 89–96. [Google Scholar] [CrossRef]
- Koch, E.; Klaas, C.A.; Rüngeler, P.; Castro, V.; Mora, G.; Vichnewski, W.; Merfort, I. Anti-inflammatory effects of sesquiterpene lactones by inhibition of cytokine production and lymphocyte proliferation. Biochem. Pharm. 2001, 62, 795–801. [Google Scholar] [CrossRef]
- Corona, D.; Díaz, E.; Nava, J.L.; Guzmán, A.; Barrios, H.; Fuentes, A.; Hernandez-Plata, S.A.; Allard, J.; Jankowski, C.K. 1H, 13C-NMR and X-ray crystallographic studies of highly polyhalogenated derivatives of costunolide lactone. Spectrochim. Acta A 2005, 62, 604–613. [Google Scholar] [CrossRef]
- Shaikenov, T.E.; Adekenov, S.M.; Williams, R.M.; Prashad, N.; Baker, F.L.; Madden, T.L.; Newman, R. Arglabin-DMA, a plant derived sesquiterpene, inhibits farnesyltransferase. Oncol. Rep. 2001, 8, 173–179. [Google Scholar]
- Zhangabylov, N.S.; Dederer, L.Y.; Gorbacheva, L.B.; VasilLeva, S.V.; Terekhov, A.S.; Adekenov, S.M. Sesquiterpene lactone arglabin influences DNA synthesis in P388 leukemia cells in vivo. Pharm. Chem. J. 2004, 38, 651–653. [Google Scholar] [CrossRef]
- Ogura, M.; Cordell, G.A.; Farnsworth, N.R. Anticancer sesquiterpene lactones of Michelia Compressa (Magoliaceae). Phytochemistry 1978, 17, 957–961. [Google Scholar] [CrossRef]
- Jacobsson, U.; Kumar, V.; Saminathan, S. Sequiterpene lactones from Michelia Champaca. Phytochemistry 1995, 39, 839–843. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpenelactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 7285–7289. [Google Scholar] [CrossRef]
- Wang, K.T.; Liu, H.T.; Zhao, Y.K.; Chen, X.G.; Hu, Z.D.; Song, Y.C.; Ma, X. Separation and determination of alantolactone and isoalantolactone in traditional Chinese herbs by capillary electrophoresis. Talanta 2000, 52, 1001–1005. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4227–4435. [Google Scholar]
- Zhai, J.-D.; Li, D.; Long, J.; Zhang, H.-L.; Lin, J.-P.; Qiu, C.-J.; Zhang, Q.; Chen, Y. Biomimetic semisynthesis of arglabin from parthenolide. J. Org. Chem. 2012, 77, 7103–7107. [Google Scholar] [CrossRef]
- Charette, A.B.; Juteau, H.; Lebel, H.; Molinaro, C. Enantioselective cyclopropanation of allylic alcohols with dioxaborolane ligands: scope and synthetic applications. J. Am. Chem. Soc. 1998, 120, 11943–11952. [Google Scholar] [CrossRef]
- Matsuda, H.; Kageura, T.; Inoue, Y.; Morikawa, T.; Yoshikawa, M. Absolute stereostructures and syntheses of saussureamines A, B, C, D and E, amino acid-sesquiterpene conjugates with gastroprotective effect, from the roots of Saussurea lappa. Tetrahedron 2000, 56, 7763–7777. [Google Scholar] [CrossRef]
- Alves, J.C.F.; Fantini, E.C. Study of the inversion reaction of the lactonic fusion on eremanthine derivatives. J. Braz. Chem. Soc. 2007, 18, 643–664. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Pridgeon, J.W.; Fronczek, F.R.; Becnelb, J.J. Structure–activity relationship studies on derivatives of eudesmanolides from Inula helenium as toxicants against Aedes aegypti Larvae and adults. Chem. Biodiv. 2010, 7, 1681–1697. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Brandt, W.; Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev. 2003, 103, 1625–1648. [Google Scholar] [CrossRef]
- Nocquet, P.-A.; Hazelard, D.; Compain, P. Synthesis of spirocyclopropyl γ-lactams by tandem intramolecular azetidine ring-opening/closing cascade reaction: synthetic and mechanistic aspects. Tetrahedron 2012, 68, 4117. [Google Scholar] [CrossRef]
- Day, B.W.; Magarian, R.A.; Pento, J.T.; Jain, P.T.; Mousissian, G.K.; Meyer, K.L. Synthesis and biological evaluation of a series of 1,1-dichloro-2,2,3-triarylcyclopropanes as pure antiestrogens. J. Med. Chem. 1991, 34, 842–851. [Google Scholar]
- Sample Availability: Samples of all compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jia, Q.-Q.; Wang, J.-C.; Long, J.; Zhao, Y.; Chen, S.-J.; Zhai, J.-D.; Wei, L.-B.; Zhang, Q.; Chen, Y.; Long, H.-B. Sesquiterpene Lactones and Their Derivatives Inhibit High Glucose-Induced NF-κB Activation and MCP-1 and TGF-β1 Expression in Rat Mesangial Cells. Molecules 2013, 18, 13061-13077. https://doi.org/10.3390/molecules181013061
Jia Q-Q, Wang J-C, Long J, Zhao Y, Chen S-J, Zhai J-D, Wei L-B, Zhang Q, Chen Y, Long H-B. Sesquiterpene Lactones and Their Derivatives Inhibit High Glucose-Induced NF-κB Activation and MCP-1 and TGF-β1 Expression in Rat Mesangial Cells. Molecules. 2013; 18(10):13061-13077. https://doi.org/10.3390/molecules181013061
Chicago/Turabian StyleJia, Qian-Qian, Jian-Cheng Wang, Jing Long, Yan Zhao, Si-Jia Chen, Jia-Dai Zhai, Lian-Bo Wei, Quan Zhang, Yue Chen, and Hai-Bo Long. 2013. "Sesquiterpene Lactones and Their Derivatives Inhibit High Glucose-Induced NF-κB Activation and MCP-1 and TGF-β1 Expression in Rat Mesangial Cells" Molecules 18, no. 10: 13061-13077. https://doi.org/10.3390/molecules181013061
APA StyleJia, Q.-Q., Wang, J.-C., Long, J., Zhao, Y., Chen, S.-J., Zhai, J.-D., Wei, L.-B., Zhang, Q., Chen, Y., & Long, H.-B. (2013). Sesquiterpene Lactones and Their Derivatives Inhibit High Glucose-Induced NF-κB Activation and MCP-1 and TGF-β1 Expression in Rat Mesangial Cells. Molecules, 18(10), 13061-13077. https://doi.org/10.3390/molecules181013061



