Schiff Bases: A Short Survey on an Evergreen Chemistry Tool
Abstract
:1. Introduction
1.1. Ugo Schiff (Frankfurt, 26 April 1834-Florence, 8 September 1915): A Brief Biography



1.2. Schiff Bases: Physical-Chemical Properties
2. Preparations of Imines
2.1. Preparation of N-Aryl or Alkyl Substituted Imines
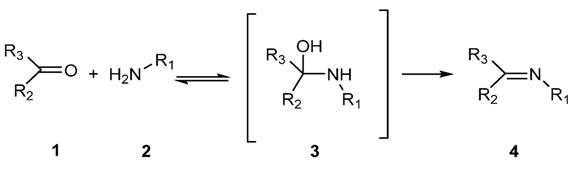
2.1.1. Reaction of Aldehydes and Ketones with Amines
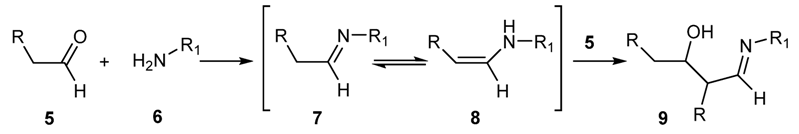
2.1.2. Aerobic Oxidative Synthesis in the Preparation of Schiff’s Bases
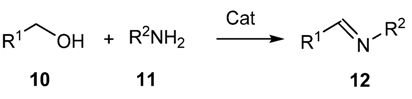
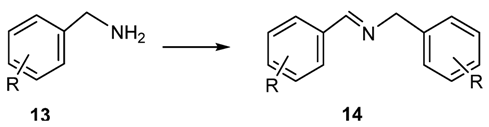
2.1.3. Addition of Organometallic Reagents to Cyanides
2.1.4. Reaction of Phenols and Phenol-Ethers with Nitriles
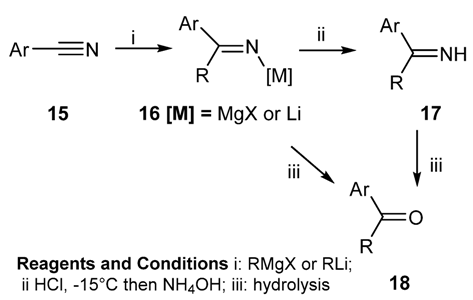
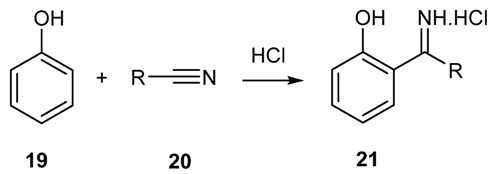
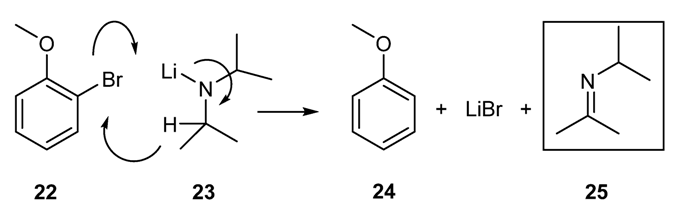
2.1.5. Reaction of Metal Amides
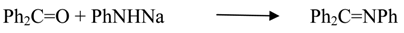
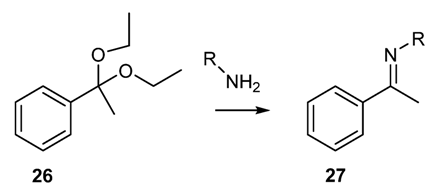
2.1.6. Other Methodologies
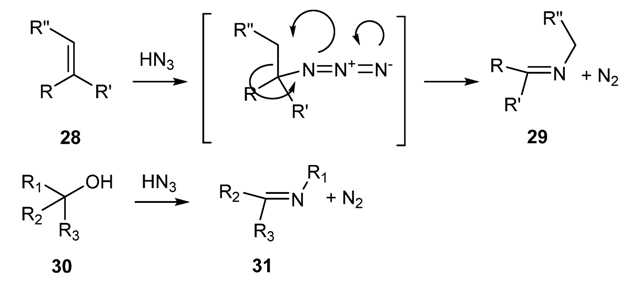
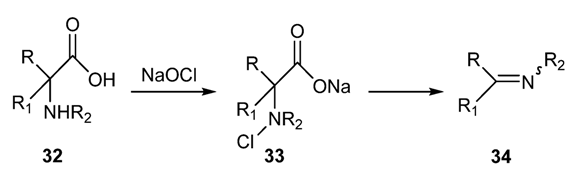
2.2. Preparation of N-Metallo-Imines as Stable Synthetic Equivalents of N-Unsubstituted Schiff Bases [58]
2.2.1. Preparation of Certain N-Metallo Imines (Metallo = B, Al, Si, Sn) [70]
2.2.1.1. Preparation of N-Boryl [70,71,72,73] and N-Aluminium Imines [74,75,76,77,78]: Addition of an Organometallic Reagents or a Metallo Hydride to a Nitrile [59,79,80,81,82]
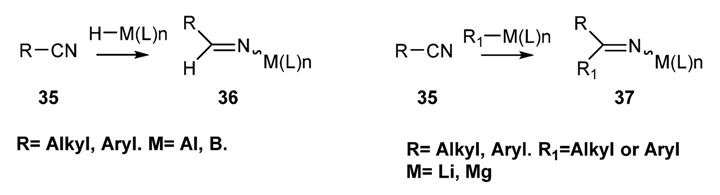
2.2.1.2. Preparation of N-Silylimines
2.2.1.2.1. Via Reaction of the Hexalkyldisilylamide of Group I Metals (Li, Na, K) with an Aldehyde or a Nonenolizable Ketones [83,84,85,86]
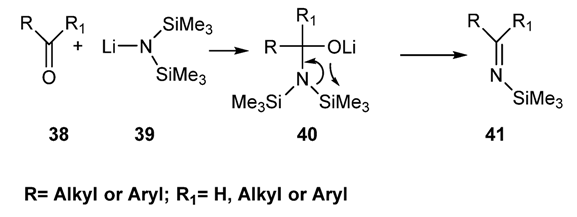
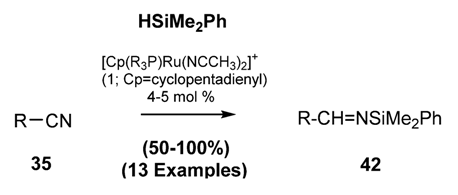
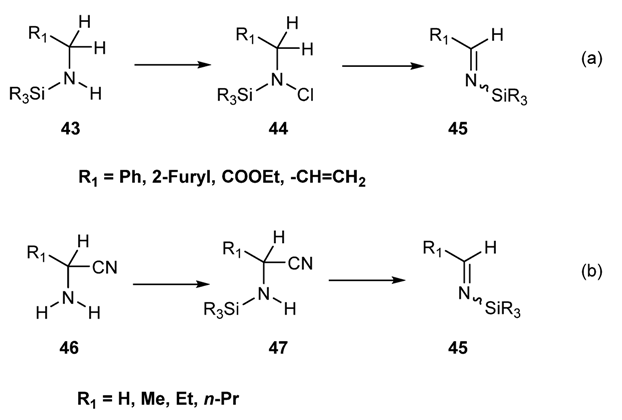
2.2.1.2.2. Preparation of N-Silylimines via Base-Induced Elimination of Vicinal Substituent from N-Silyl Amines [89,90]
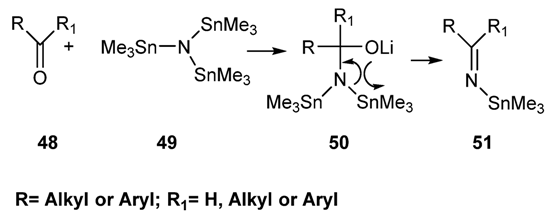
2.2.1.3. Preparation of N-tin-Imines via Reaction of Carbonyl Compounds with Tris(trimethylstannyl)amine [91]
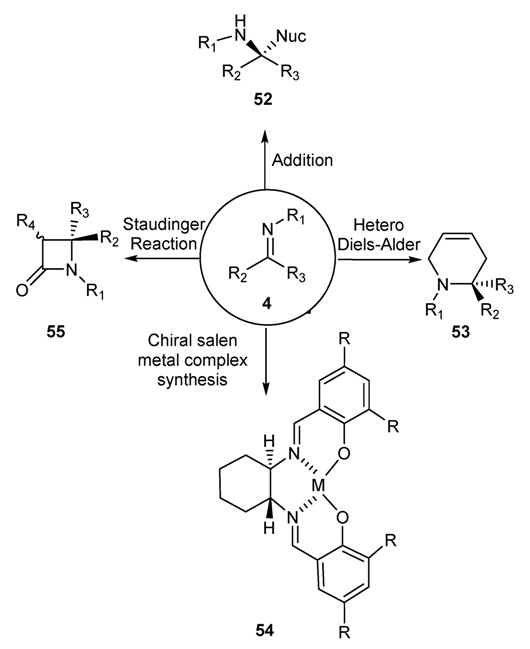
3. Importance of Schiff Bases in Organic Synthesis, Bio-Processes and Pharmaceutical Chemistry
3.1. Schiff Bases as Precursors of Countless Versatile Organic Processes for the Production of Intermediates/Products
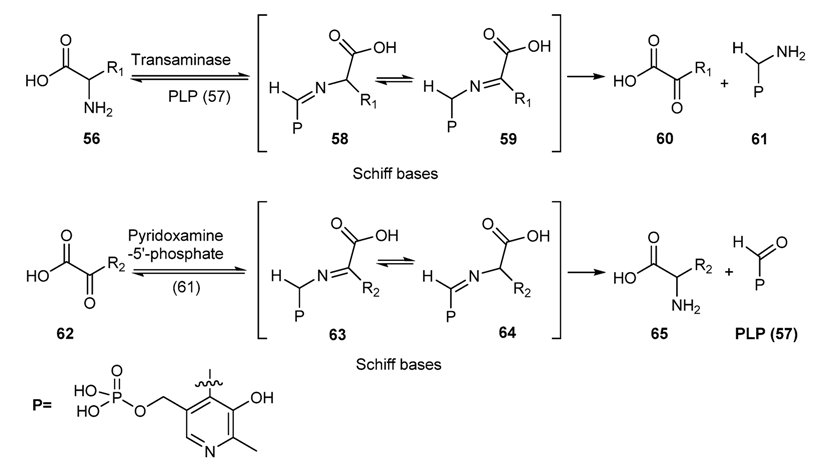
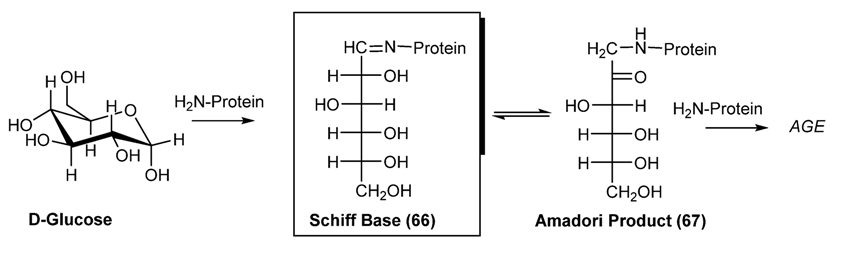
3.2. Schiff Bases as Intermediate of Bio-Processes
3.3. Some Application of Schiff Bases in Pharmaceutical Research
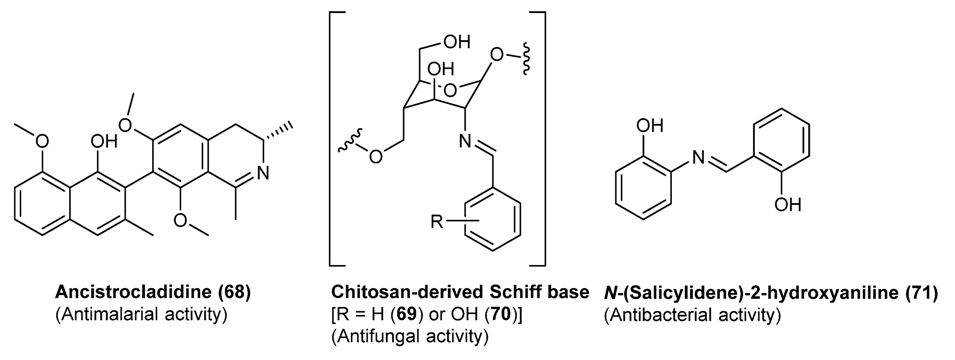
3.3.1. Antiparassitic Schiff Bases

3.3.2. Salicylidene Amines as Bioactive Compounds
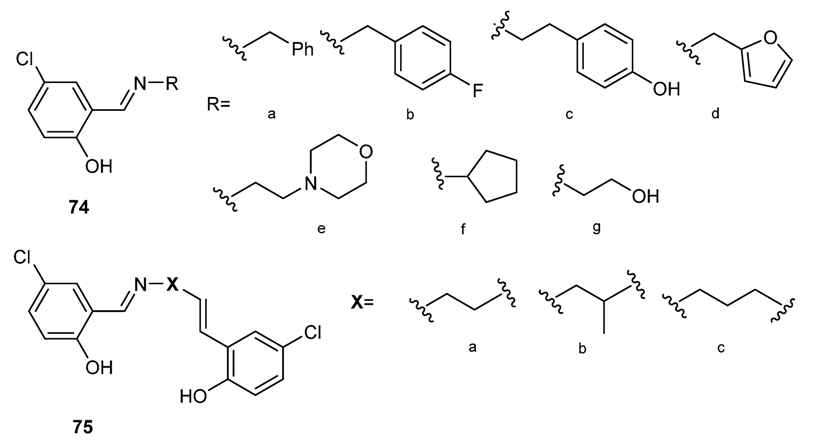
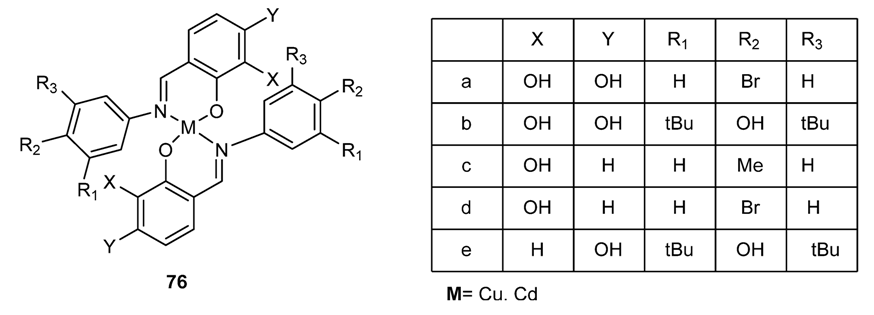
3.3.3. Other Antibacterial Schiff Bases
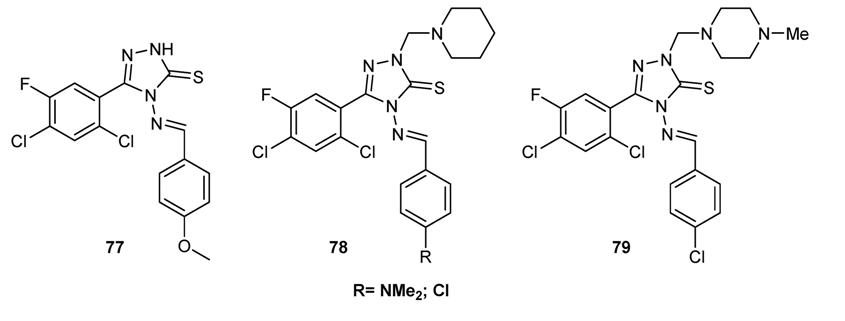
3.3.4. Antifungal Schiff Bases
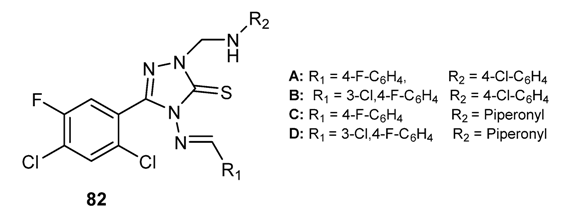
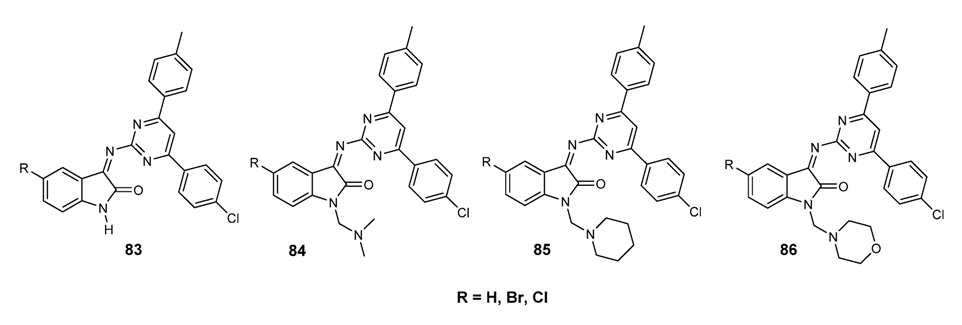
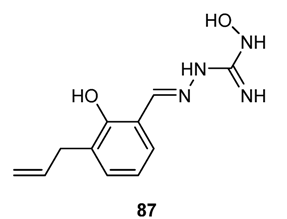
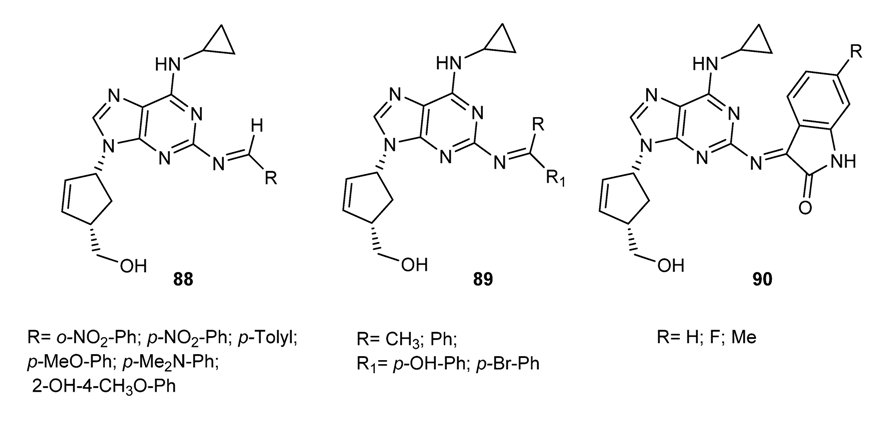
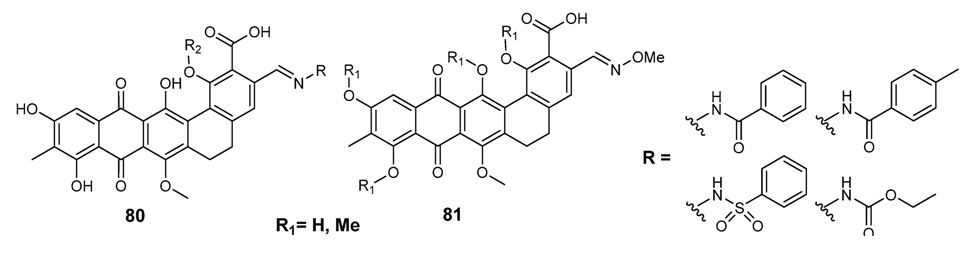
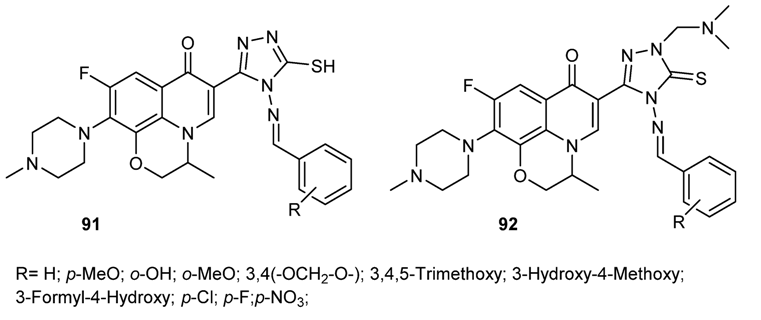
3.3.6. Hybrid Structures
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Fontani, M.; Costa, M. La Dinastia degli Schiff e l’Italia. (in Italian). Chimica e Industria 2011, 93, 106–110. [Google Scholar]
- Chemical Heritage Foundation. Available online: http://www.chemheritage.org/discover/online-resources/chemistry-inhistory/themes/electrochemistry/berzelius.aspx (accessed on 27 September 2013).
- Schiff, H. Mitteilungen aus dem universitatslaboratorium in Pisa: Eineneue reihe organischer basen. (in German). Justus Liebigs Ann. Chem. 1984, 131, 118–119. [Google Scholar] [CrossRef]
- Tidwell, T.T. Hugo (ugo) schiff, schiff bases, and a century of b-lactam synthesis. Angew. Chem. Int. Ed. 2008, 47, 1016–1020. [Google Scholar] [CrossRef]
- Schiff, H. Eine neue Reihe organischer Diamine. (in German). Justus Liebigs Ann. Chem. 1866, 140, 92–137. [Google Scholar] [CrossRef]
- Shriner, R.L.; Hermann, C.K.F.; Morrill, T.C.; Fuson, R.C. The Systematic Identification of Organic Compounds; Wiley: New York, NY, USA, 2004. (in German) [Google Scholar]
- Schiff, H. Ueber die Einwirkung des Phosphorsuperchlorids auf einige anorganische Säuren. (in German). Justus Liebigs Ann. Chem. 1857, 102, 111–118. [Google Scholar] [CrossRef]
- Patai, S. The Chemistry of the Carbon-Nitrogen Double Bond; Wiley: New York, NY, USA, 1970. [Google Scholar]
- Tennant, G. Comprehensive organic chemistry. In Comprehensive Organic Chemistry; Sutherland, I.O., Ed.; Pergamon: Oxford, UK, 1979; Volume 2, pp. 385–590. [Google Scholar]
- Whitesell, J.K. Comprehensive organic synthesis. In Comprehensive Organic Synthesis; Winterfeldt, E., Ed.; Pergamon: Oxford, UK, 1991; Volume 6, pp. 703–732. [Google Scholar]
- Robertson, G.M. Imines and their N-substituted derivatives: NH, NR and N-haloimines. In Comprehensive Organic Functional Group Transformations, 1st ed.; Katritzky, A.R., Meth-Cohn, O., Rees, C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 3, pp. 403–423. [Google Scholar]
- Pawlenko, S. Methoden der Organische Chemie (Houben-Weyl). In Methoden der Organische Chemie (Houben-Weyl); (in German). Klamann, D., Hagemann, H., Eds.; Thieme: Stuttgart, Germany, 1980; Volume E14b, Part 1, pp. 222–281. [Google Scholar]
- Holm, R.H.; Everett, J.G.W.; Chakravorty, A. Metal complexes of schiff bases and o-ketoamines. Prog. Inorg. Chem. 1966, 7, 83–214. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Layer, R.W. The chemistry of imines. Chem. Rev. 1963, 63, 489–510. [Google Scholar] [CrossRef]
- Schiff, U. Giornale di scienze naturali ed economiche. (in Italian). Palermo 1867, II, 1–59. [Google Scholar]
- Dobbs, A.P.; Rossiter, S. Imines and their N-substituted derivatives: NH, NR, and N-Haloimines. In Comprehensive Organic Functional Group Transformations II; Alan, R.K., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2005; pp. 419–450. [Google Scholar]
- Westheimer, F.H.; Taguchi, K. Catalysis by molecular sieves in the preparation of ketimines and enamines. J. Org. Chem. 1971, 36, 1570–1572. [Google Scholar] [CrossRef]
- Love, B.E.; Ren, J. Synthesis of sterically hindered imines. J. Org. Chem. 1993, 58, 5556–5557. [Google Scholar] [CrossRef]
- Look, G.C.; Murphy, M.M.; Campbell, D.A.; Gallop, M.A. Trimethylorthoformate: A mild and effective dehydrating reagent for solution and solid phase imine formation. Tetrahedron Lett. 1995, 36, 2937–2940. [Google Scholar] [CrossRef]
- Billman, J.H.; Tai, K.M. Reduction of schiff bases. II. Benzhydrylamines and structurally related compounds1a,b. J. Org. Chem. 1958, 23, 535–539. [Google Scholar] [CrossRef]
- White, W.A.; Weingarten, H. A versatile new enamine synthesis. J. Org. Chem. 1967, 32, 213–214. [Google Scholar] [CrossRef]
- Liu, G.; Cogan, D.A.; Owens, T.D.; Tang, T.P.; Ellman, J.A. Synthesis of enantiomerically Pure N-tert-butanesulfinyl imines (tert-butanesulfinimines) by the direct condensation of tert-butanesulfinamide with aldehydes and ketones. J. Org. Chem. 1999, 64, 1278–1284. [Google Scholar]
- Chakraborti, A.K.; Bhagat, S.; Rudrawar, S. Magnesium perchlorate as an efficient catalyst for the synthesis of imines and phenylhydrazones. Tetrahedron Lett. 2004, 45, 7641–7644. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Nair, R.R.; Vijayalakshmi, G.; Subramanian, E.H.; Sridhar, S.K. Synthesis of Schiff bases of 4-(4aminophenyl)-morpholine as potential antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 225–229. [Google Scholar] [CrossRef]
- Dalpozzo, R.; de Nino, A.; Nardi, M.; Russo, B.; Procopio, A. Erbium(III) triflate: A valuable catalyst for the synthesis of aldimines, ketimines and enaminones. Synthesis 2006, 7, 1127–1132. [Google Scholar]
- Naeimi, H.; Salimi, F.; Rabiei, K. Mild and convenient one pot synthesis of Schiff bases in the presence of P2O5/Al2O3 as new catalyst under solvent-free conditions. J. Mol. Catal. A Chem. 2006, 260, 100–104. [Google Scholar] [CrossRef]
- Reddelien, G. Über Selbstkondensation bei Anilen. (Studien über Zinkchlorid als Kondensationsmittel III). (in German). Berichte der deutschen chemischen Gesellschaft 1913, 46, 2172–2178. [Google Scholar] [CrossRef]
- Varma, R.S.; Dahiya, R.; Kumar, S. Clay catalyzed synthesis of imines and enamines under solvent-free conditions using microwave irradiation. Tetrahedron Lett. 1997, 38, 2039–2042. [Google Scholar] [CrossRef]
- Schmeyers, J.; Toda, F.; Boy, J.; Kaupp, G. Quantitative solid–solid synthesis of azomethines. J. Chem. Soc. Perkin Trans 2 1998, 4, 989–994. [Google Scholar] [CrossRef]
- Vass, A.; Duda, S.J.; Varma, R.S. Solvent-free synthesis of Nsulfonylimines using microwave irradiation. Tetrahedron Lett. 1999, 40, 4951–4954. [Google Scholar] [CrossRef]
- Tanaka, K.; Shiraishi, R. Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chem. 2000, 2, 272–273. [Google Scholar] [CrossRef]
- Andrade, C.K.Z.; Takada, S.C.S.; Alves, L.M.; Rodrigues, J.P.; Suarez, P.A.Z.; Brandão, R.F.; Rodrigo, F.; Soares, V.C.D. Molecular sieves in ionic liquids as an efficient and recyclable medium for the synthesis of imines. Synlett 2004, 12, 2135–2138. [Google Scholar]
- Vázquez, M.Á.; Landa, M.; Reyes, L.; Miranda, R.; Tamariz, J.; Delgado, F. Infrared irradiation: Effective promoter in the formation of N-benzylideneanilines in the absence of solvent. Synth. Commun. 2004, 34, 2705–2718. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Sureshkumar, P.; Kanagarajan, V.; Thanusu, J. New environmentally-friendly solvent-free synthesis of imines using calcium oxide under microwave irradiation. Res. Chem. Intermed. 2007, 33, 541–548. [Google Scholar] [CrossRef]
- Guzen, K.P.; Guarezemini, A.S.; Órfão, A.T.G.; Cella, R.; Pereira, C.M.P.; Stefani, H.A. Eco-friendly synthesis of imines by ultrasound irradiation. Tetrahedron Lett. 2007, 48, 1845–1848. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Ikeda, M.; Tsukamoto, D.; Tanaka, S.; Hirai, T. One-pot synthesis of imines from alcohols and amines with TiO2 loading Pt nanoparticles under UV irradiation. Chem. Commun. 2011, 47, 4811–4813. [Google Scholar]
- Jiang, L.; Jin, L.; Tian, H.; Yuan, X.; Yu, X.; Xu, Q. Direct and mild palladium-catalyzed aerobic oxidative synthesis of imines from alcohols and amines under ambient conditions. Chem. Commun. 2011, 47, 10833–10835. [Google Scholar]
- Huang, B.; Tian, H.; Lin, S.; Xie, M.; Yu, X.; Xu, Q. Cu(I)/TEMPO-catalyzed aerobic oxidative synthesis of imines directly from primary and secondary amines under ambient and neat conditions. Tetrahedron Lett. 2013, 54, 2861–2864. [Google Scholar] [CrossRef]
- Soule, J.-F.; Miyamura, H.; Kobayashi, S. Selective imine formation from alcohols and amines catalyzed by polymer incarcerated gold/palladium alloy nanoparticles with molecular oxygen as an oxidant. Chem. Commun. 2013, 49, 355–357. [Google Scholar]
- Gnanaprakasam, B.; Zhang, J.; Milstein, D. Direct synthesis of imines from alcohols and amines with liberation of H2. Angew. Chem. Int. Ed. 2010, 49, 1468–1471. [Google Scholar] [CrossRef]
- Yuan, H.; Yoo, W.-J.; Miyamura, H.; Kobayashi, S. Discovery of a metalloenzyme-like cooperative catalytic system of metal nanoclusters and catechol derivatives for the aerobic oxidation of amines. J. Am. Chem. Soc. 2012, 134, 13970–13973. [Google Scholar]
- Largeron, M.; Fleury, M.-B. Bioinspired oxidation catalysts. Science 2013, 339, 43–44. [Google Scholar] [CrossRef]
- Lan, Y.-S.; Liao, B.-S.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Preparation of imines by oxidative coupling of benzyl alcohols with amines catalysed by dicopper complexes. Eur. J. Org. Chem. 2013, 2013, 5160–5164. [Google Scholar]
- Largeron, M. Protocols for the catalytic oxidation of primary amines to imines. Eur. J. Org. Chem. 2013. [Google Scholar] [CrossRef]
- Pickard, P.L.; Young, C.W. Ketimines. III. ι-cyclohexylalkyl alkyl type1. J. Am. Chem. Soc. 1951, 73, 42–43. [Google Scholar] [CrossRef]
- Pickard, P.L.; Tolbert, T.L. An improved method of ketimine synthesis. J. Org. Chem. 1961, 26, 4886–4888. [Google Scholar] [CrossRef]
- Porai-Koshits, B.A.; Remizov, A.L. Probl. mekhanizma org. reaktsii. Chem. Abstr. 1956, 50, 16686. [Google Scholar]
- Hoesch, K. Eine neue Synthese aromatischer Ketone. I. Darstellung einiger Phenol-ketone. (in German). Ber. Dtsch. Chem. Ges. 1915, 48, 1122–1133. [Google Scholar] [CrossRef]
- Hoesch, K. Eine neue Synthese aromatischer Ketone. II. Künstliche Darstellung des Maclurins und ihm verwandter Ketone. (in German). Ber. Dtsch. Chem. Ges. 1917, 50, 462–468. [Google Scholar] [CrossRef]
- Houben, J.; Fischer, W. Formation of aromatic nitriles by basic hydrolysis of trichloromethyl aryl ketimines. Acidic hydrolysis yields ketones. J. Prakt. Chem. 1929, 123, 262–313. [Google Scholar] [CrossRef]
- Britton, E.C.; Mich, M.; Bryner, F. Method of making imides of ketones. U.S. Patent 1,938,890, 9 April 1932. [Google Scholar]
- Mosher, H.; Blanz, J.E. Notes—Reduction of o-bromoanisole by lithium dineopentylamide. J. Org. Chem. 1957, 22, 445–446. [Google Scholar] [CrossRef]
- Claisen, L. Ueber eine eigenthümliche Umlagerung. (in German). Ber. Dtsch. Chem. Ges. 1896, 29, 2931–2933. [Google Scholar] [CrossRef]
- Reddelien, G. Über die Zersetzung von Anilen. (Über die katalytische Wirkungsweise von Halogenwasserstoffsäuren bei Kondensationen, II). (in German). Ber. Dtsch. Chem. Ges. 1920, 53, 355–358. [Google Scholar] [CrossRef]
- Boyer, J.H.; Canter, F.C. Alkyl and aryl azides. Chem. Rev. 1954, 54, 1–57. [Google Scholar] [CrossRef]
- Langheld, K. Über das Verhalten von α-Aminosäuren gegen Natriumhypochlorit. (in German). Ber. Dtsch. Chem. Ges. 1909, 42, 2360–2374. [Google Scholar] [CrossRef]
- In this Review other substitution to the imine-nitrogen will not be treated since they are outside of the scope of this work.
- Cainelli, G.; Panunzio, M.; Andreoli, P.; Martelli, G.; Spunta, G.; Giacomini, D.; Bandini, E. Metallo-imines: Useful reagents in organic chemistry. Pure Appl. Chem. 1990, 62, 605–612. [Google Scholar] [CrossRef]
- Barluenga, J.; Aznar, F.; Valdes, C. N-trialkylsilylimines as coupling partners for Pd-catalyzed C-N bond-forming reactions: One-step synthesis of imines and azadienes from aryl and alkenyl bromides. Angew. Chem. Int. Ed. 2004, 43, 343–345. [Google Scholar] [CrossRef]
- Barluenga, J.; Suarez-Sobrino, A.; Lopez, L.A. Chiral heterosubstituted 1,3-butadienes: Synthesis and 4+2 cycloaddition reactions. Aldrichim. Acta 1999, 32, 4–15. [Google Scholar]
- Barluenga, J.; Jimenez-Aquino, A.; Fernandez, M.A.; Aznar, F.; Valdes, C. Multicomponent and one-pot synthesis of trisubstituted pyridines through a Pd-catalyzed cross-coupling/cross-coupling/cycloaddition sequence. Tetrahedron 2007, 64, 778–786. [Google Scholar]
- Zhu, W.; Mena, M.; Jnoff, E.; Sun, N.; Pasau, P.; Ghosez, L. Multicomponent reactions for the synthesis of complex piperidine scaffolds. Angew. Chem. Int. Ed. 2009, 48, 5880–5883. [Google Scholar] [CrossRef]
- Jnoff, E.; Ghosez, L. Asymmetric diels—alder reactions of 2-azadienes catalyzed by a chiral copper(II) complex. A general route to enantiomerically pure piperidones. J. Am. Chem. Soc. 1999, 121, 2617–2618. [Google Scholar] [CrossRef]
- Long, S.; Monari, M.; Panunzio, M.; Bandini, E.; D’Aurizio, A.; Venturini, A. Hetero-Diels-Alder (HDA) strategy for the preparation of 6-aryl- and heteroaryl-substituted piperidin-2-one scaffolds: Experimental and theoretical studies. Eur. J. Org. Chem. 2011, 31, 6218–6225. [Google Scholar]
- Bongini, A.; Panunzio, M. A hetero Diels-Alder concerted vs. aldol stepwise mechanism in the cyclization of silyloxyazadienes with aldehydes: A theoretical study. Eur. J. Org. Chem. 2006, 4, 972–977. [Google Scholar]
- Panunzio, M.; Vicennati, P. From 3-Trialkylsilyloxy-2-Aza-1,3-dienes to biological interesting molecules through cyclization reactions. In Recent Research Development in Organic Chemistry, Part II; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2002; Volume 6, pp. 683–707. [Google Scholar]
- Panunzio, M.; Bandini, E.; D’Aurizio, A.; Xia, Z.; Mu, X. Synthesis of Venlafaxine from azadiene via a Hetero-Diels-Alder approach: New microwave-assisted transketalization and hydroxymethylation reactions. Synthesis 2008, 11, 1753–1756. [Google Scholar]
- Bandini, E.; Corda, G.; D’Aurizio, A.; Panunzio, M. A straightforward synthesis of conhydrine by hetero Diels-Alder strategy mediated by microwaves. Tetrahedron Lett. 2010, 51, 933–934. [Google Scholar] [CrossRef]
- We have limited our report to these metallo-imines because they have found applications in organic synthesis as Schiff-bases analogues.
- Lavrinovich, L.I.; Ignatenko, A.V.; Bubnov, Y.N. Synthesis of functional derivatives of 20methylenecyclopentane and 2-methylenecyclohexane based in the allylborylation of imines, nitriles, isocyanates, and isothiocyanates by cycloalkenylmethyl(dipropyl)boranes. Bull. Russ. Acad. Sci. Div. Chem. Sci. (Engl. Transl.) 1992, 41, 2051–2057. [Google Scholar] [CrossRef]
- Meller, A.; Maringgele, W. Monomere und dimere hochhalogenierte Iminoborane. Monatsh. Chem. 1968, 99, 2504–2513. [Google Scholar] [CrossRef]
- Evers, E.C.; Freitag, W.O.; Kriner, W.A.; MacDiarmid, A.G. The preparation of di-n-butylboron cyanide by the interaction of di-n-butylboron chloride with trimethylsilyl cyanide1. J. Am. Chem. Soc. 1959, 81, 5106–5108. [Google Scholar] [CrossRef]
- Hoberg, H.; Barluenga-Mur, J. Das verhalten von dialkyl-aluminium-amiden gegenüber benzonitril. (in German). J. Organometall. Chem. 1969, 17, 30–32. [Google Scholar] [CrossRef]
- Hoberg, H.; Barluenga, J. Addition der Cα-H-bindung von N-aluminium-iminen und iminen an nitrile. (in German). Synthesis 1970, 3, 142–144. [Google Scholar] [CrossRef]
- Hoberg, H.; Barluenga, J. Addition von Al[BOND]Namin-verbindungen an benzonitril. (in German). Justus Liebigs Ann. Chem. 1970, 733, 141–151. [Google Scholar] [CrossRef]
- Piotrowski, A.; Kunicki, A.; Pasynkiewicz, S. The reactions of tetraalkylaluminoxanes with benzonitrile. J. Organometall. Chem. 1980, 201, 105–112. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Itoh, K.; Sakai, S.; Ishii, Y. Insertion reactions of diethylaluminium derivatives II. reaction of diethylaluminium dimethylamide and diethylaluminium ethanethiolate with nitriles. J. Organometall. Chem. 1970, 21, 273–280. [Google Scholar] [CrossRef]
- Andreoli, P.; Cainelli, G.; Giacomini, D.; Martelli, G.; Panunzio, M. A synthetic approach to azetidinones from nitriles and lithium-triethoxy aluminium hydride. Tetrahedron Lett. 1986, 27, 1695–1698. [Google Scholar] [CrossRef]
- Andreoli, P.; Cainelli, G.; Contento, M.; Giacomini, D.; Martelli, G.; Panunzio, M. Reaction of silylimines with ester enolates. Synthesis of N-unsubstituted azetidinones starting from nitriles. J. Chem. Soc. Perkin Trans. 1 1998, 4, 945–948. [Google Scholar]
- Cainelli, G.; Giacomini, D.; Mezzina, E.; Panunzio, M.; Zarantonello, P. N-metallo imines–a new approach to alpha-amino alcohols from aldehydes. Tetrahedron Lett. 1991, 32, 2967–2970. [Google Scholar] [CrossRef]
- Cainelli, G.; Panunzio, M.; Contento, M.; Giacomini, D.; Mezzina, E.; Giovagnoli, D. Preparation of 1,2 aminols from cyanohydrins via N-diisobutylaluminium imines. Tetrahedron 1993, 49, 3809–3826. [Google Scholar]
- Chan, L.-H.; Roschow, E.G. Syntheses and ultraviolet spectra of N-organosilyl ketimines. J. Organometall. Chem. 1967, 9, 231–250. [Google Scholar] [CrossRef]
- Kruger, C.; Rochow, G.; Wannagat, U. ber die einwirkung von natrium-bis-trimethylsilyl-amid auf benzophenon, benzaldehyd und benzochinon. (in German). Chem. Ber. 1963, 96, 2132–2137. [Google Scholar] [CrossRef]
- Hart, D.J.; Kanai, K.; Thomas, D.G.; Yang, T.K. Preparation of primary amines and 2-azetidinones via N-(trimethylsilyl)imines. J. Org. Chem. 1983, 48, 289–294. [Google Scholar] [CrossRef]
- Panunzio, M.; Zarantonello, P. Synthesis and use of N-(trimethylsilyl)imines. Org. Process Res. Dev. 1998, 2, 49–59. [Google Scholar] [CrossRef]
- Cainelli, G.; Giacomini, D.; Panunzio, M.; Martelli, G.; Spunta, G. β-Lactam from esters and silylimines: A revaluation. Synthesis of N-unsubstituted 4-Alkyl-β-lactam. Tetrahedron Lett. 1987, 28, 5369–5372. [Google Scholar] [CrossRef]
- Gutsulyak, D.V.; Nikonov, G.I. Chemoselective catalytic hydrosilylation of nitriles. Angew. Chem. Int. Ed. 2010, 49, 7553–7556. [Google Scholar] [CrossRef]
- Colvin, E.W.; McGarry, D.; Nugent, M.J. Silicon-assisted synthesis of β-lactams. Tetrahedron 1988, 44, 4157–4172. [Google Scholar] [CrossRef]
- Guillemin, J.-C.; Ammi, L.; Denis, J.-M. A convenient synthesis of enolizable N-trialkylsilylimines using vacuum gas-solid reactions. Tetrahedron Lett. 1988, 29, 1287–1288. [Google Scholar] [CrossRef]
- Busato, S.; Cainelli, G.; Panunzio, M.; Bandini, E.; Martelli, G.; Spunta, G. Beta-lactams from ester enolates and metalloimines—synthesis and reactivity of tert-butyl-dimethylsilylimines. Synlett 1991, 4, 243–244. [Google Scholar]
- Yoon, T.P.; Jacobsen, E.N. Privileged chiral catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Katsuki, T. Unique asymmetric catalysis of cis-beta metal complexes of salen and its related Schiff-base ligands. Chem. Soc. Rev. 2004, 33, 437–444. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shibasaki, M. Multimetallic schiff base complexes as cooperative asymmetric catalysts. Synthesis 2013, 45, 421–437. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with pi-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef]
- Dalla Cort, A.; de Bernardin, P.; Forte, G.; Mihan, F.Y. Metal-salophen-based receptors for anions. Chem. Soc. Rev. 2010, 39, 3863–3874. [Google Scholar] [CrossRef]
- Szumna, A. Inherently chiral concave molecules-from synthesis to applications. Chem. Soc. Rev. 2010, 39, 4274–4285. [Google Scholar] [CrossRef]
- Das, M.C.; Xiang, S.C.; Zhang, Z.J.; Chen, B.L. Functional mixed metal-organic frameworks with metalloligands. Angew. Chem. Int. Ed. 2011, 50, 10510–10520. [Google Scholar] [CrossRef]
- Dhanaraj, C.J.; Johnson, J.; Joseph, J.; Joseyphus, R.S. Quinoxaline-based Schiff base transition metal complexes: Review. J. Coord. Chem. 2013, 66, 1416–1450. [Google Scholar] [CrossRef]
- Drozdzak, R.; Allaert, B.; Ledoux, N.; Dragutan, I.; Dragutan, V.; Verpoort, F. Synthesis of Schiff base-ruthenium complexes and their applications in catalytic processes. Adv. Synth. Catal. 2005, 347, 1721–1743. [Google Scholar] [CrossRef]
- Frischmann, P.D.; MacLachlan, M.J. Metallocavitands: An emerging class of functional multimetallic host molecules. Chem. Soc. Rev. 2013, 42, 871–890. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Kumar, S.; Dhar, D.N.; Saxena, P.N. Applications of metal complexes of Schiff bases-A review. J. Sci. Ind. Res. India 2009, 68, 181–187. [Google Scholar]
- Lim, S.; Choi, B.; Min, Y.S.; Kim, D.; Yoon, I.; Lee, S.S.; Lee, I.M. A study on the development of CVD precursors V—Syntheses and characterization of new N-alkoxy-beta-ketoiminate complexes of titanium. J. Organomet.Chem. 2004, 689, 224–237. [Google Scholar] [CrossRef]
- Kobayashi, S.; Mori, Y.; Fossey, J.S.; Salter, M.M. Catalytic enantioselective formation of C−C bonds by addition to imines and hydrazones: A ten-year update. Chem. Rev. 2011, 111, 2626–2704. [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M. Trichlorosilane-mediated stereoselective reduction of C=N bonds. Eur. J. Org. Chem. 2010, 29, 5529–5541. [Google Scholar] [CrossRef]
- Noble, A.; Anderson, J.C. Nitro-mannich reaction. Chem. Rev. 2013, 113, 2887–2939. [Google Scholar] [CrossRef]
- Verkade, J.M.M.; Hemert, L.J.C.V.; Quaedflieg, P.J.L.M.; Rutjes, F.P.J.T. Organocatalysed asymmetric Mannich reactions. Chem. Soc. Rev. 2008, 37, 29–41. [Google Scholar]
- Masson, G.; Lalli, C.; Benohoud, M.; Dagousset, G. Catalytic enantioselective [4 + 2]—cycloaddition: A strategy to access aza-hexacycles. Chem. Soc. Rev. 2013, 42, 902–923. [Google Scholar] [CrossRef]
- Ali, F.E.; Bondinell, W.E.; Dandridge, P.A.; Frazee, J.S.; Garvey, E.; Girard, G.R.; Kaiser, C.; Ku, T.W.; Lafferty, J.J.; Moonsammy, G.I.; et al. Orally active and potent inhibitors of γ-aminobutyric acid uptake. J. Med. Chem. 1985, 28, 653–660. [Google Scholar] [CrossRef]
- Smith, C.D.; Gavrilyuk, J.I.; Lough, A.J.; Batey, R.A. Lewis acid catalyzed three-component Hetero-Diels-Alder (Povarov) reaction of N-arylimines with strained norbornene-derived dienophiles. J. Org. Chem. 2010, 75, 702–715. [Google Scholar] [CrossRef]
- Ueno, S.; Ohtsubo, M.; Kuwano, R. [4 + 2] cycloaddition of o-xylylenes with imines using palladium catalyst. J. Am. Chem. Soc. 2009, 131, 12904–12905. [Google Scholar] [CrossRef]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Jørgensen, K.A. Catalytic asymmetric Hetero-Diels-Alder reactions of carbonyl compounds and imines. Angew. Chem. In. Ed. Engl. 2000, 39, 3558–3588. [Google Scholar] [CrossRef]
- Boger, D.L.; Weinreb, S.M. Hetero Diels-Alder Methodology in Organic Synthesis; Academic Press: New York, NY, USA, 1987; Volume 47. [Google Scholar]
- Haak, R.M.; Wezenberg, S.J.; Kleij, A.W. Cooperative multimetallic catalysis using metallosalens. Chem. Commun. 2010, 46, 2713–2723. [Google Scholar] [CrossRef]
- Jacobsen, E.N. Asymmetric catalysis of epoxide ring-opening reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef]
- Allen, A.D.; Tidwell, T.T. New directions in ketene chemistry: The land of opportunity. Eur. J. Org. Chem. 2012, 2012, 1081–1096. [Google Scholar] [CrossRef]
- D’hooghe, M.; van Brabandt, W.; Dekeukeleire, S.; Dejaegher, Y.; de Kimpe, N. Highly stereoselective synthesis of beta-lactams utilizing alpha-chloroimines as new and powerful chiral inductors. Chem. Eur. J. 2008, 14, 6336–6340. [Google Scholar] [CrossRef]
- David, O.; Meester, W.J.N.; Bieraugel, H.; Schoemaker, H.E.; Hiemstra, H.; van Maarseveen, J.H. Intramolecular staudinger ligation: A powerful ring-closure method to form medium-sized lactams. Angew. Chem. Int. Ed. 2003, 42, 4373–4375. [Google Scholar] [CrossRef]
- Palomo, C.; Aizpurua, J.M.; Ganboa, I.; Oiarbide, M. Asymmetric synthesis of beta-lactams through the Staudinger reaction and their use as building blocks of natural and nonnatural products. Curr. Med. Chem. 2004, 11, 1837–1872. [Google Scholar] [CrossRef]
- Snell, E.E.; Jenkins, W.T. The mechanism of the transamination reaction. J. Cell. Comp. Phys. 1959, 54, 161–177. [Google Scholar] [CrossRef]
- Cohen, M.P. Clinical, pathophysiological and structure/function consequences of modification of albumin by Amadori-glucose adducts. Biochimi. Biophys. Acta (BBA) Gen. Subj. 2013. [Google Scholar] [CrossRef]
- Yamagishi, S.-I. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp. Geront. 2011, 46, 217–224. [Google Scholar] [CrossRef]
- Grillo, M.A.; Colombatto, S. Advanced glycation end-products (AGEs): Involvement in aging and in neurodegenerative diseases. Amino Acids 2008, 35, 29–36. [Google Scholar] [CrossRef]
- Desai, S.B.; Desai, P.B.; Desai, K.R. Synthesis of some Schiff bases, thiazolidinones and azetidinones derived from 2,6-diaminobenzo1,2-d: 4,5-d’ bisthiazole and their anticancer activities. Heterocycl. Commun. 2001, 7, 83–90. [Google Scholar]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of Schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; Salem, A.N.M.; Sayed, M.A.; Aboaly, M.M. Synthesis, structural characterization, thermal studies, catalytic efficiency and antimicrobial activity of some M(II) complexes with ONO tridentate Schiff base N-salicylideneO-aminophenol (saphH2). J. Mol. Struct. 2012, 1010, 130–138. [Google Scholar]
- Sinha, D.; Tiwari, A.K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A.K. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur. J. Med. Chem. 2008, 43, 160–165. [Google Scholar] [CrossRef]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: Synthesis and in vitro assessments. Food Chem. 2010, 120, 1011–1018. [Google Scholar] [CrossRef]
- Ronad, P.M.; Noolvi, M.N.; Sapkal, S.; Dharbhamulla, S.; Maddi, V.S. Synthesis and antimicrobial activity of 7-(2-substituted phenylthiazolidinyl)-benzopyran-2-one derivatives. Eur. J. Med. Chem. 2010, 45, 85–89. [Google Scholar] [CrossRef]
- Amin, R.; Krammer, B.; Abdel-Kader, N.; Verwanger, T.; El-Ansary, A. Antibacterial effect of some benzopyrone derivatives. Eur. J. Med. Chem. 2010, 45, 372–378. [Google Scholar] [CrossRef]
- Karthikeyan, M.S.; Prasad, D.J.; Poojary, B.; Bhat, K.S.; Holla, B.S.; Kumari, N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006, 14, 7482–7489. [Google Scholar] [CrossRef]
- Saravanan, G.; Pannerselvam, P.; Prakash, C.R. Synthesis and anti-microbial screening of novel Schiff bases of 3-amino-2-methyl quinazolin 4-(3H)-one. J. Adv. Pharm. Technol. Res. 2010, 1, 320–325. [Google Scholar] [CrossRef]
- De Souza, A.O.; Galetti, F.C.S.; Silva, C.L.; Bicalho, B.; Parma, M.M; Fonseca, S.F.; Marsaioli, A.J.; Trindade, A.C.L.B.; Freitas-Gil, R.P.; Bezerra, F.S.; et al. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Quim. Nova. 2007, 30, 1563–1566. [Google Scholar]
- Rathelot, P.; Vanelle, P.; Gasquet, M.; Delmas, F.; Crozet, M.P.; Timon-David, P.; Maldonado, J. Synthesis of novel functionalized 5-nitroisoquinolines and evaluation of in vitro antimalarial activity. Eur. J. Med. Chem. 1995, 30, 503–508. [Google Scholar] [CrossRef]
- Jarrahpour, A.; Khalili, D.; de Clercq, E.; Salmi, C.; Brunel, J.M. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules 2007, 12, 1720–1730. [Google Scholar] [CrossRef]
- Bringmann, G.; Dreyer, M.; Faber, J.H.; Dalsgaard, P.W.; Staerk, D.; Jaroszewski, J.W. Ancistrotanzanine C and related 5,1’ and 7,3’ -coupled naphthylisoquinoline alkaloids from Ancistrocladus tanzaniensis. J. Nat. Prod. 2004, 67, 743–748. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef]
- Wu, Z.S.; Lu, Z.P.; Yen, Z.H. Synthesis, characterization and antifungal activity of glycylglycine Schiff base complexes of 3d transition metal ions. Trans. Met. Chem. 1993, 18, 291–294. [Google Scholar]
- Shi, L.; Ge, H.M.; Tan, S.H.; Li, H.Q.; Song, Y.C.; Zhu, H.L. Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde. Eur. J. Med. Chem. 2007, 42, 558–564. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar]
- Paulus, E.F.; Dornberger, K.; Werner, W.; Fenske, D. Madurahydroxylactone. Acta Crystallogr 1994, 50, 2064–2067. [Google Scholar]
- Heinisch, L.; Roemer, E.; Jutten, P.; Haas, W.; Werner, W.; Mollmann, U. Semisynthetic derivatives of madurahydroxylactone and their antibacterial activities. J. Antibiot. 1999, 52, 1029–1041. [Google Scholar] [CrossRef]
- Pandeya, S.; Sriram, D.; Nath, G.; de Clercq, E. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. IL Farmaco 1999, 54, 624–628. [Google Scholar] [CrossRef]
- Wang, P.H.; Keck, J.G.; Lien, E.J.; Lai, M.M.C. Design, synthesis, testing and quantitative structure–activity relationship analysis of substituted salicylaldehyde Schiff bases of 1-amino-3hydroxyguanidine tosylate as new antiviral agents against coronavirus. J. Med. Chem. 1990, 33, 608–614. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Myneedu, N.S; Saraswat, V. Abacavir prodrugs: Microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg. Med. Chem. Lett. 2006, 16, 2127–2129. [Google Scholar] [CrossRef]
- Hu, G.; Wang, G.; Duan, N.; Wen, X.; Cao, T.; Xie, S.; Huang, W. Design, synthesis and antitumor activities of fluoroquinolone C-3 heterocycles (IV): S-triazole Schiff–Mannich bases derived from ofloxacin. Acta Pharm. Sinica B 2012, 2, 312–317. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264-12289. https://doi.org/10.3390/molecules181012264
Qin W, Long S, Panunzio M, Biondi S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules. 2013; 18(10):12264-12289. https://doi.org/10.3390/molecules181012264
Chicago/Turabian StyleQin, Wenling, Sha Long, Mauro Panunzio, and Stefano Biondi. 2013. "Schiff Bases: A Short Survey on an Evergreen Chemistry Tool" Molecules 18, no. 10: 12264-12289. https://doi.org/10.3390/molecules181012264
APA StyleQin, W., Long, S., Panunzio, M., & Biondi, S. (2013). Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules, 18(10), 12264-12289. https://doi.org/10.3390/molecules181012264