A Structural Study of Epoxidized Natural Rubber (ENR-50) and Its Cyclic Dithiocarbonate Derivative Using NMR Spectroscopy Techniques
Abstract
:1. Introduction
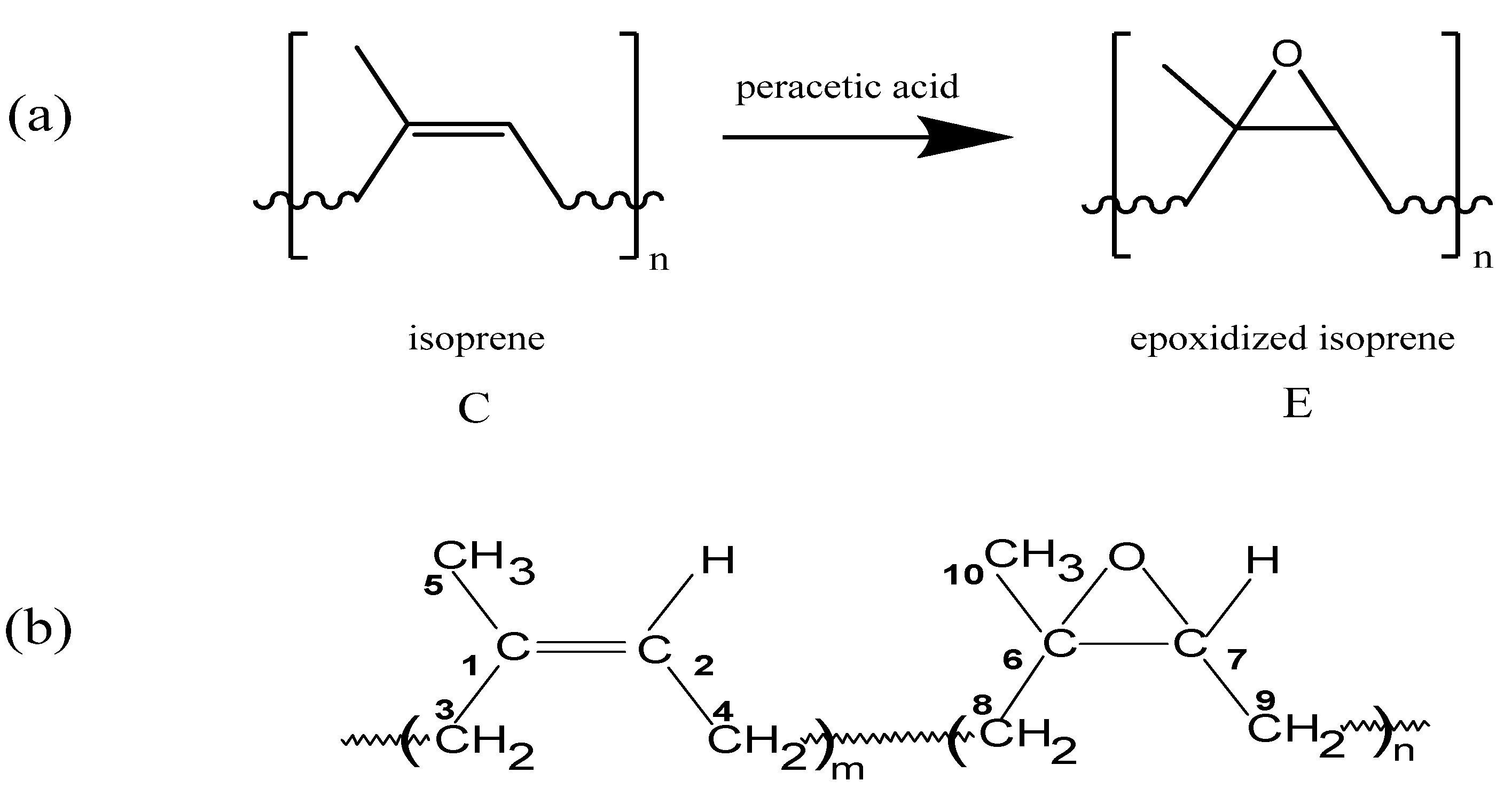
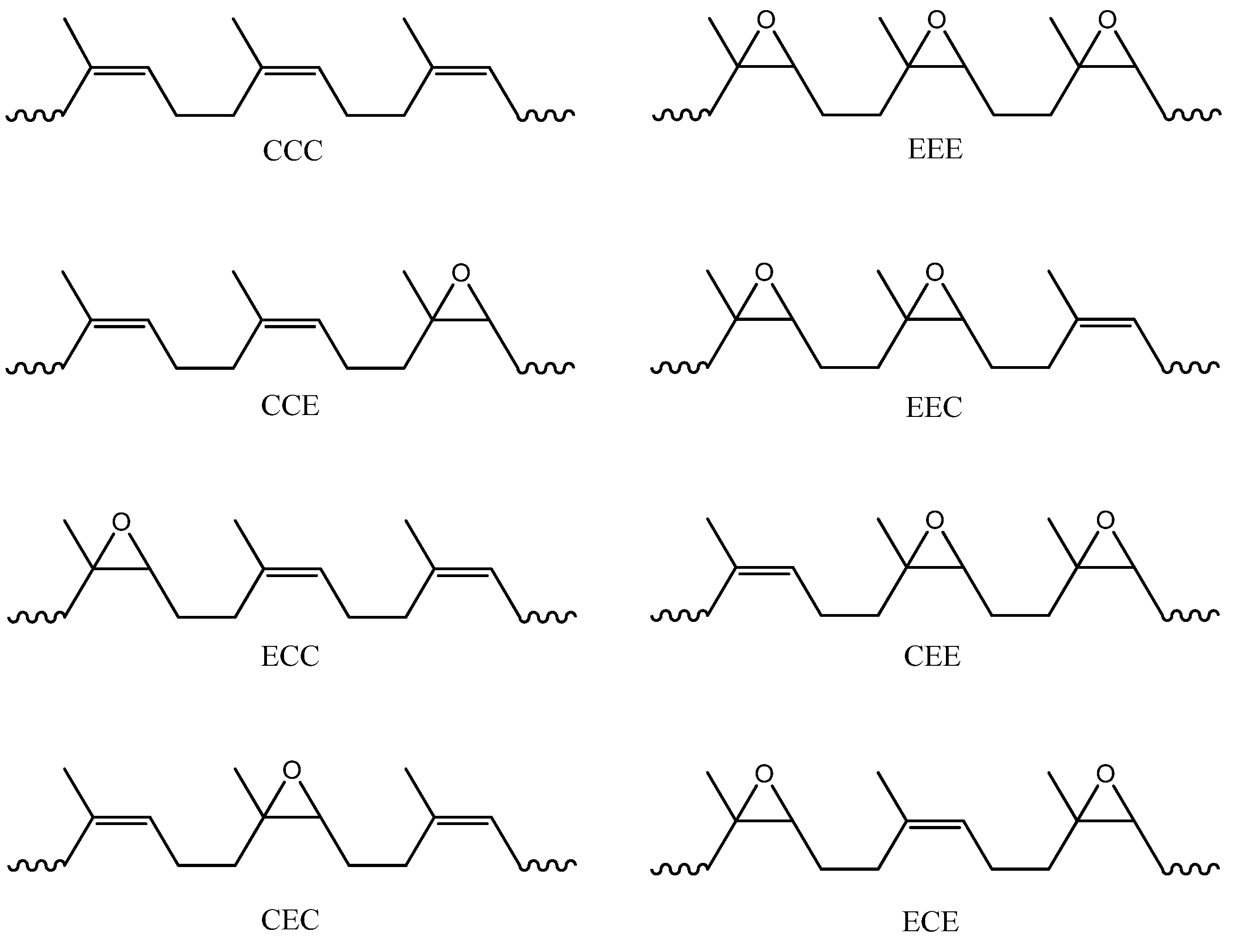
| 1H chemical shift δ (ppm) | 13C chemical shiftδ (ppm) | HMQC | HMBC Coupling correlation | COSY Coupling correlation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ref: 14–15 | Ref: 11 | This work | Ref: 11 | This work | Triad assignment | Middle unit | Within same unit | Middle unit | Within same unit |
| δ (ppm) | δ (ppm) | δ (ppm) | δ (ppm) | ||||||
| 1.31 | 1.29 | 1.30 | 22.1 | 22.3 | E10 | 29.7 (E8), 60.7 (E6), 64.5 (E7) | None | None | None |
| 1.38 | 1.55 | 1.56 | 29.5 | 29.7 | EE8C, E8 | 22.3 (E10), 60.7 (E6), 64.5 (E7) | 24.7 (E9), 64.5 (E7) | None | 1.68–1.70 (E9) |
| 33.0 | 33.1 | CE8C, CE8E | 22.3 (E10), 60.7 (E6), 64.5 (E7) | 23.9 (C4), 125.0 (C2) | None | 2.15–2.19 (C4) | |||
| 26.9 | 27.0 | CE9C, EE9C | 60.7 (E6), 64.5 (E7) | 28.7 (C3), 134.7 (C1) | 2.72 (E7) | 2.15–2.19 (C3) | |||
| 1.42 | 1.68 | 1.68–1.70 | 23.3 | 23.4 | C5 | 32.0 (C3), 125.0 (C2), 134.7 (C1) | None | None | None |
| 1.38 | 24.6 | 24.7 | CE9E, E9 | 60.7 (E6), 64.5 (E7) | 29.7 (E8), 60.7 (E6) | 2.72 (E7) | 1.56 (E8) | ||
| 2.00 | 2.05 | 2.06 | 26.2 | 26.3 | C4, EC4C | 125.0 (C2), 134.7 (C1) | 32.0 (C3), 134.7 (C1) | 5.12–5.17 (C2) | 2.06 (C3) |
| 32.0 | 32.0 | C3, CC3E | 23.4 (C5), 125.0 (C2), 134.7 (C1) | 26.3 (C4), 125.0 (C2) | None | 2.06 (C4) | |||
| 1.96 | 2.15 | 2.15-2.19 | 23.7 | 23.9 | CC4E, EC4E | 125.0 (C2), 134.7 (C1) | 33.1 (E8), 60.7 (E6) | 5.12–5.17 (C2) | 1.56 (E8) |
| 28.5 | 28.7 | EC3C, EC3E | 23.4 (C5), 125.0 (C2), 134.7 (C1) | 27.0 (E9), 64.5 (E7) | None | 1.56 (E9) | |||
| - | - | - | 60.3 | 60.7 | E6 | - | - | - | - |
| 2.51 | 2.70 | 2.72 | 64.0 | 64.5 | E7 | 22.3 (E10), 24.7 (E9), 29.7 (E8), 60.7 (E6) | 29.7 (E8), (None) | 1.68-1.70 (E9) | None |
| 5.20 | 5.10 | 5.12–5.17 | 125.0 | 125.0 | C2 | 23.4 (C5), 26.3 (C4), 32.0 (C3), 134.7 (C1) (NOT DETECTED) | 32.0 (C3) | 2.06 (C4) | None |
| - | - | - | 135.0 | 134.7 | C1 | - | - | - | - |
2. Results and Discussion
2.1. Epoxidized Natural Rubber
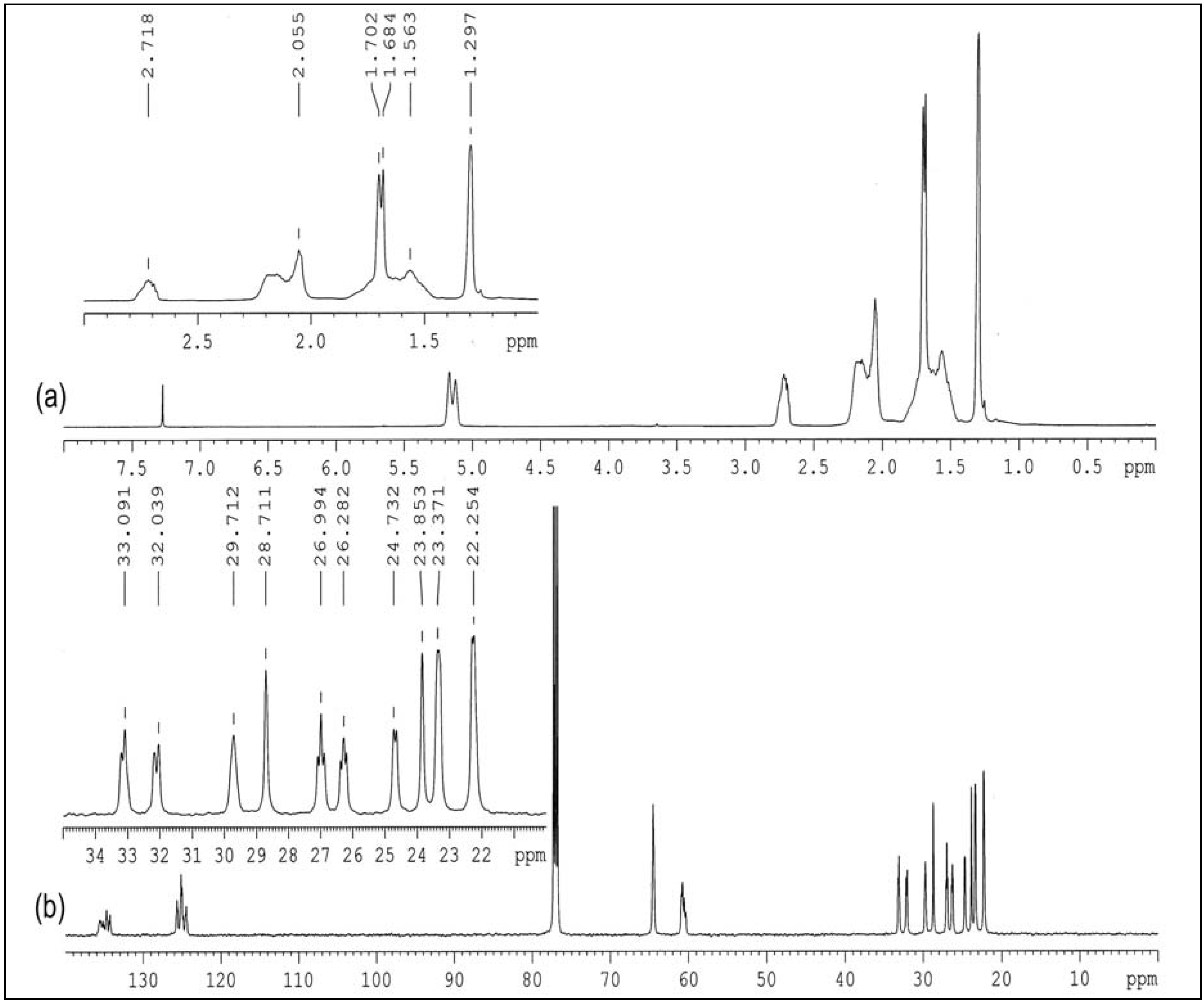
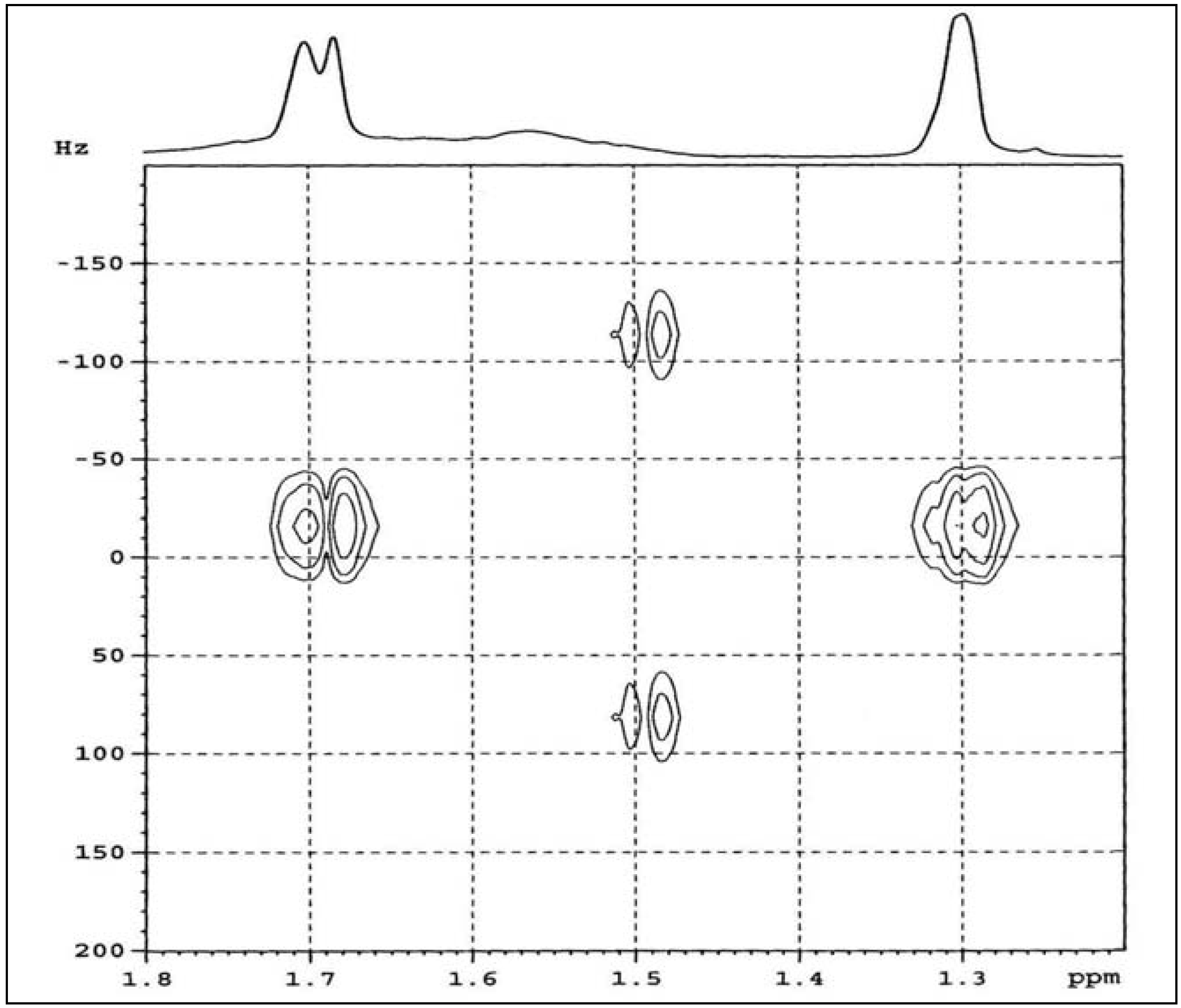
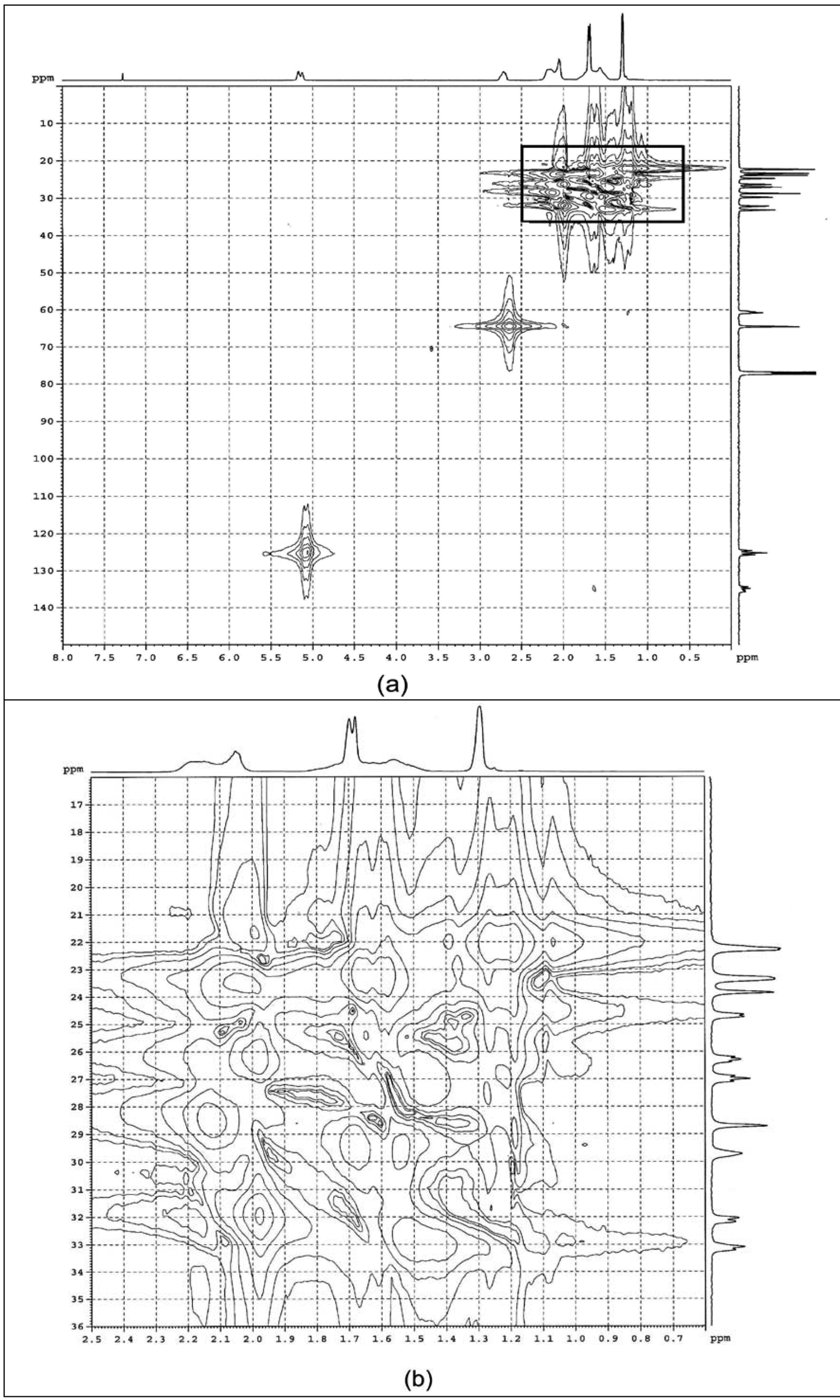
2.1.2. HMQC
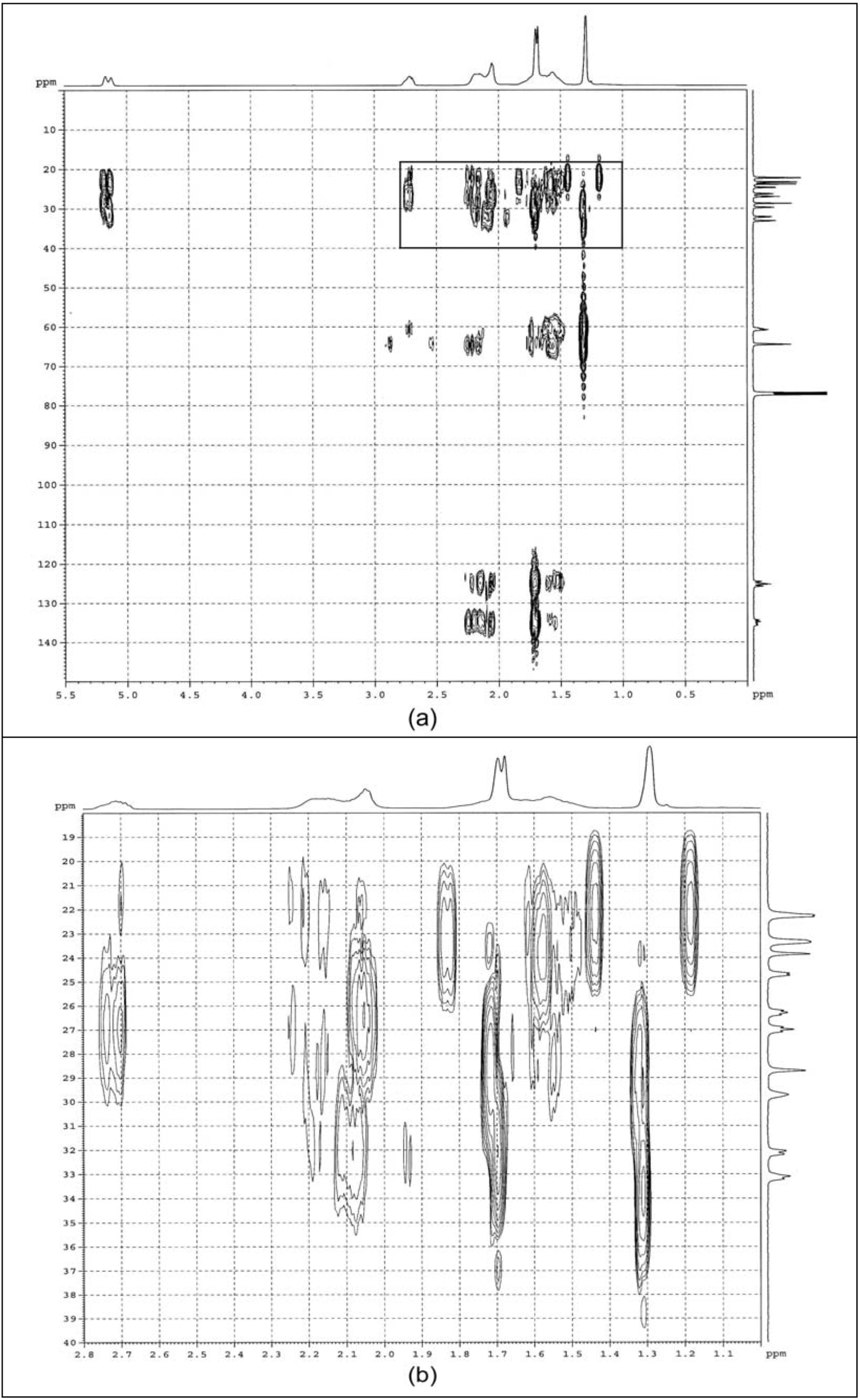
2.1.3. HMBC
2.1.4. COSY
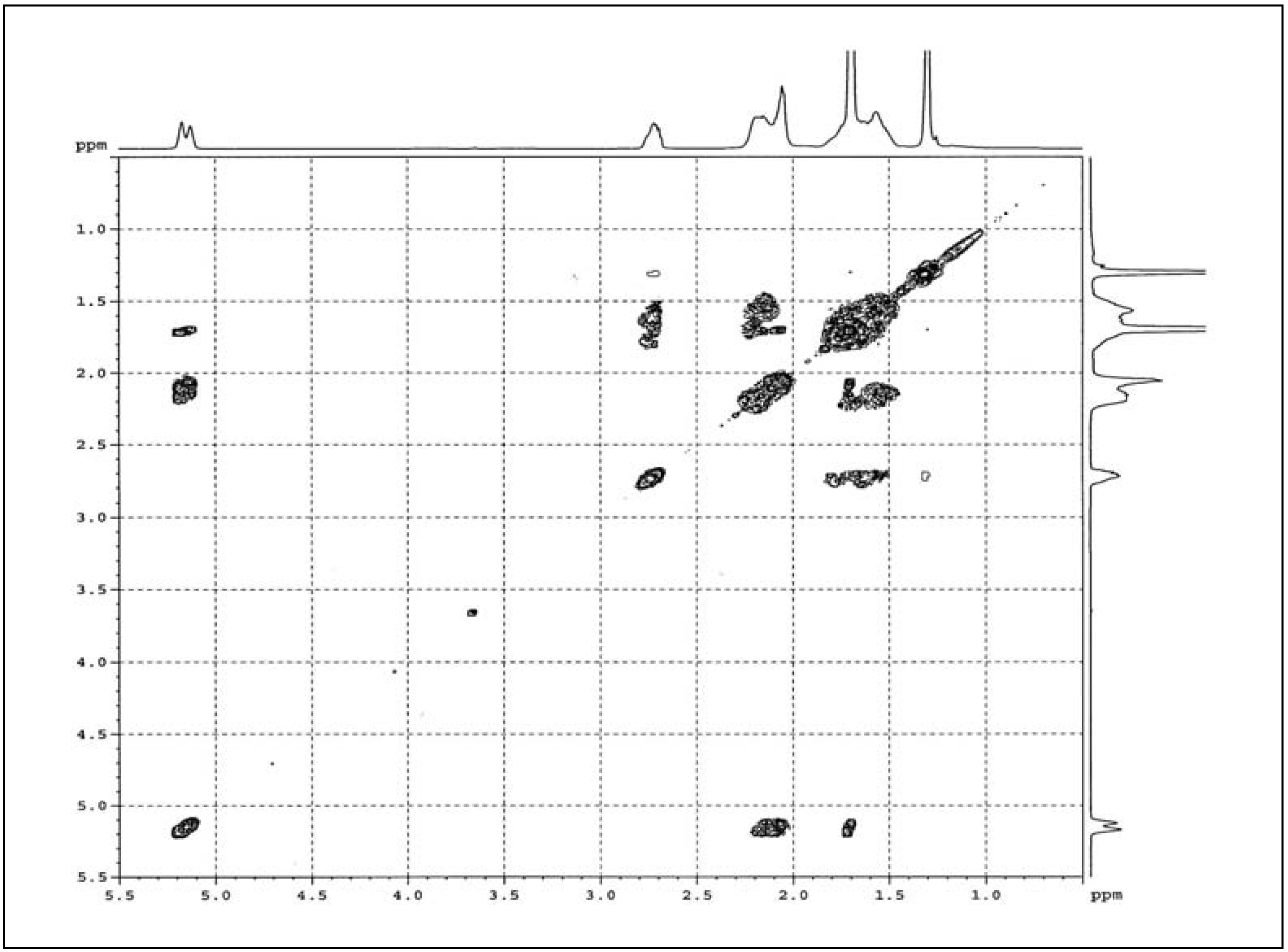
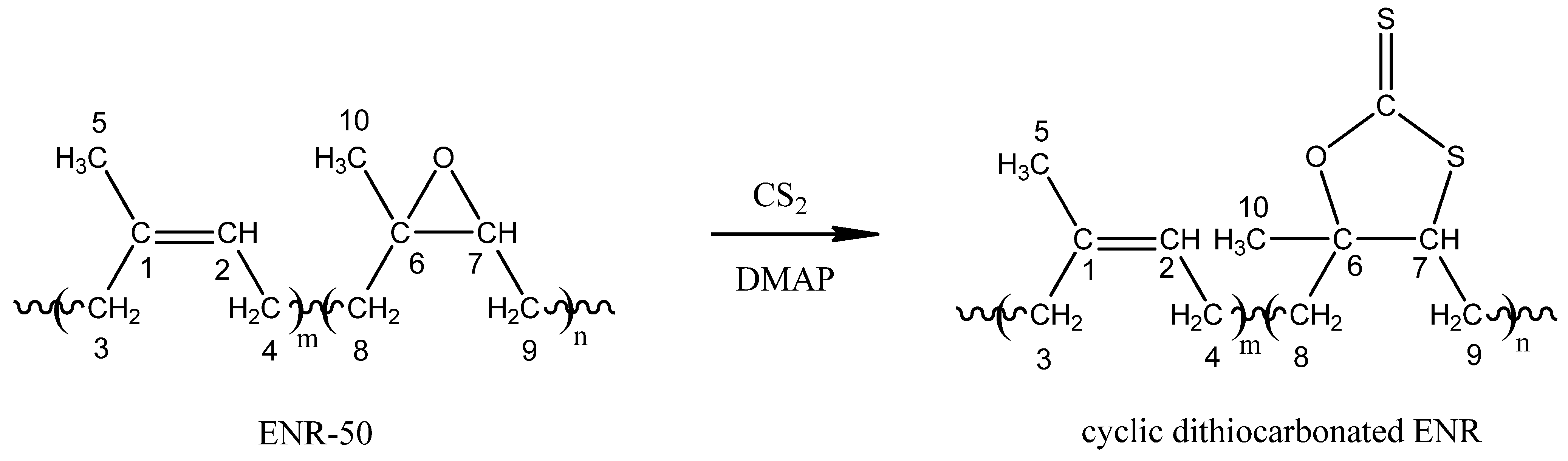
2.2. Cyclic Dithiocarbonate Derivative of ENR-50
2.2.1. FTIR Spectroscopy
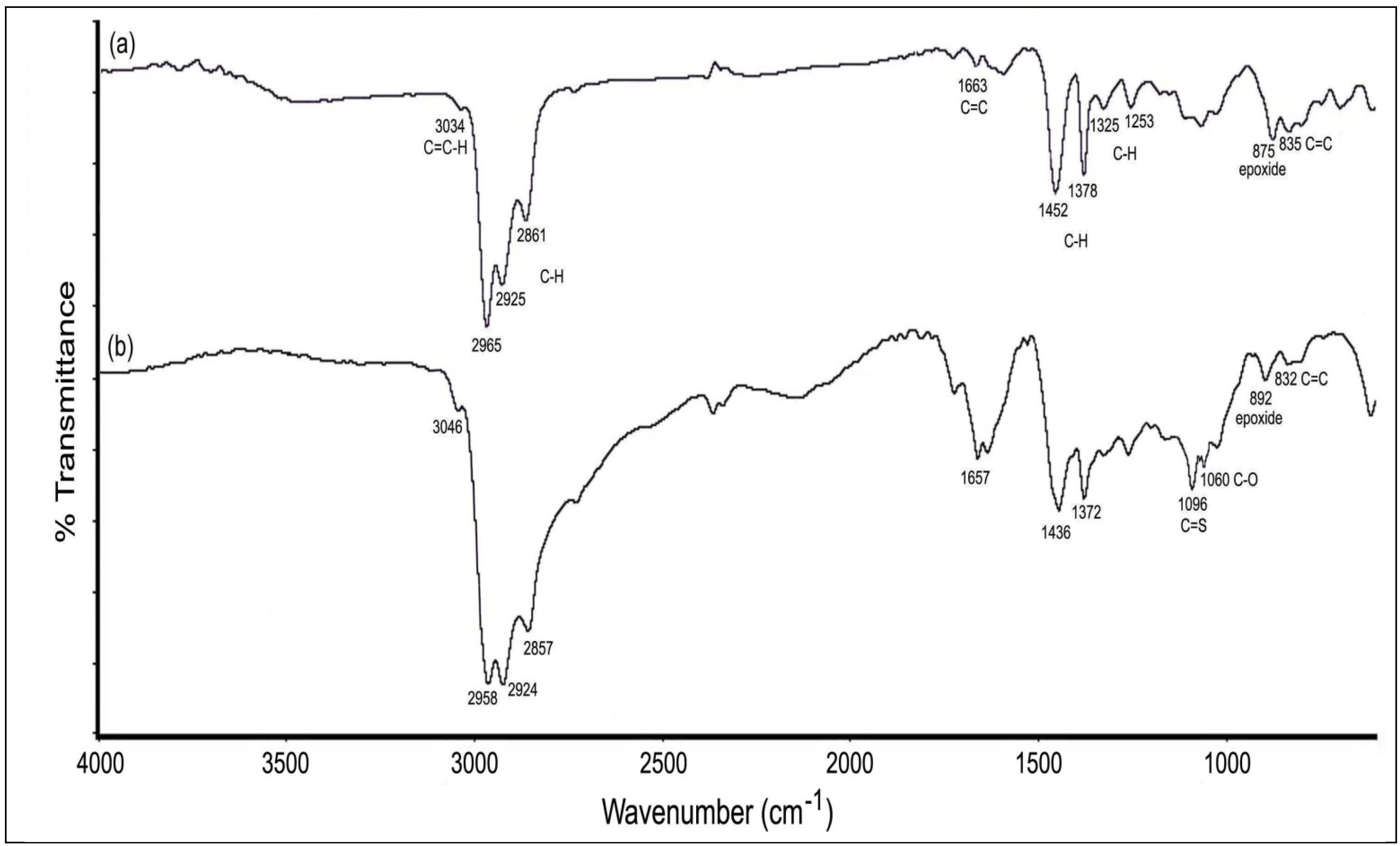
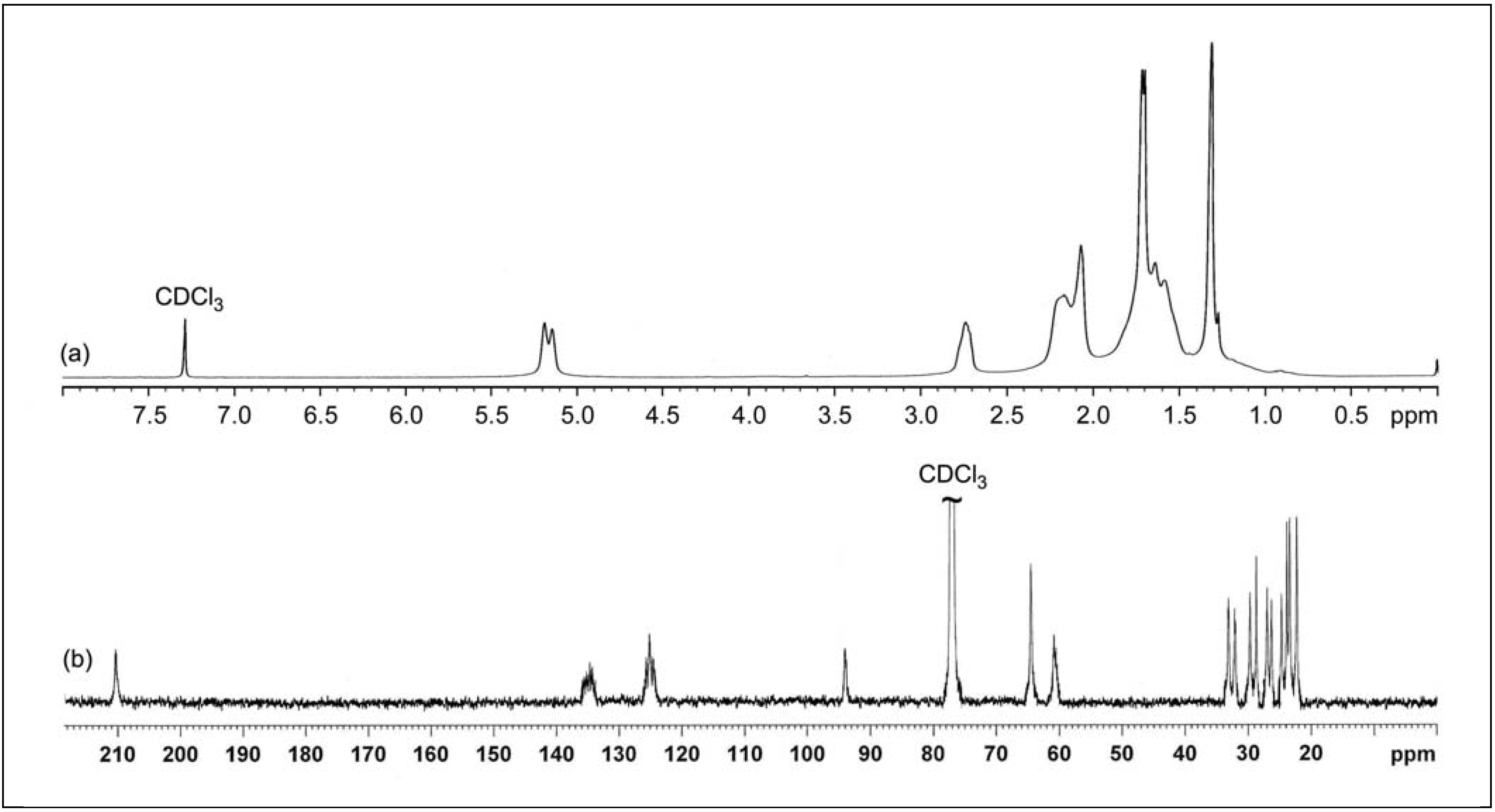
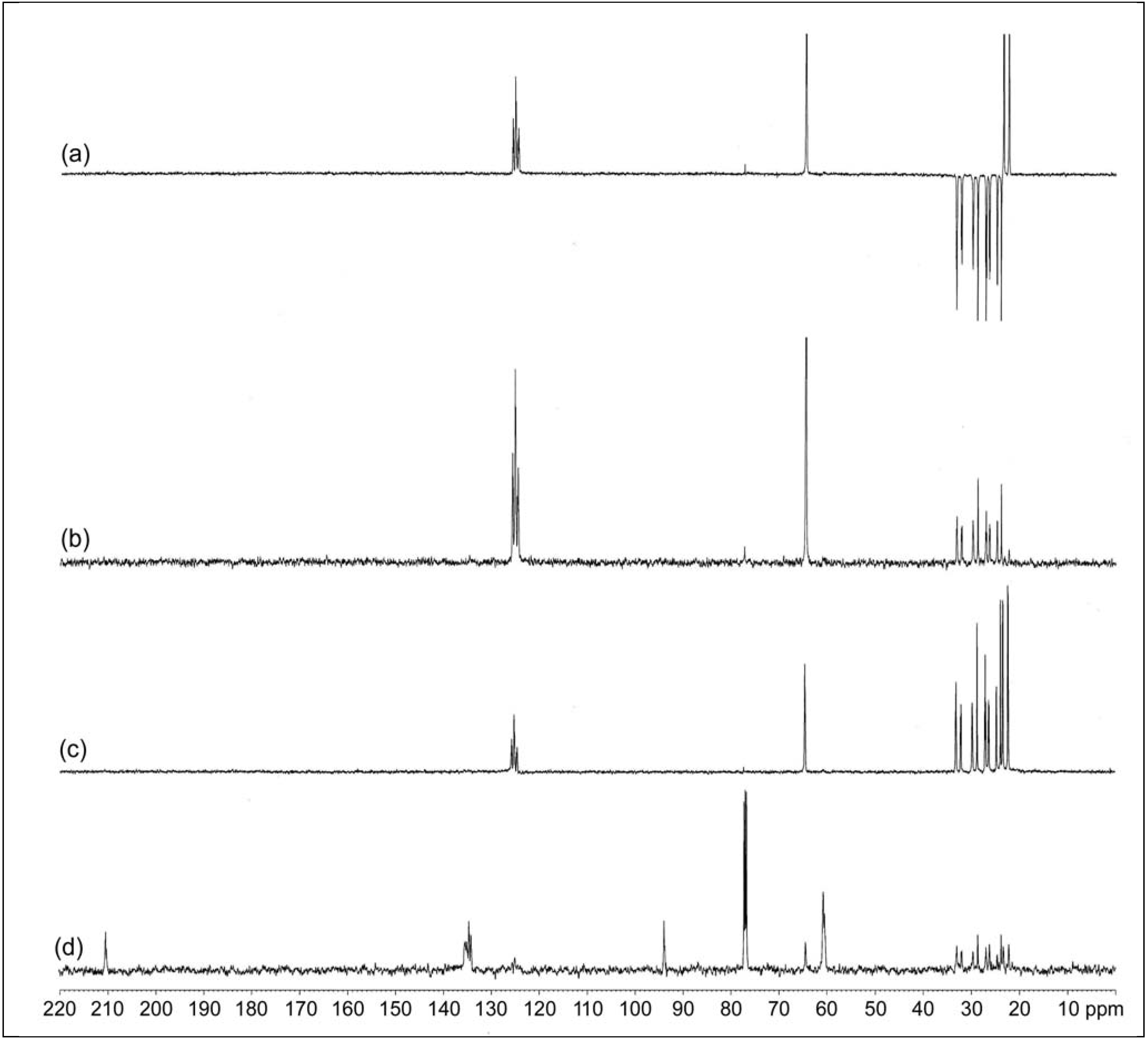
2.2.2. 1H and 13C-NMR Spectroscopy
| Chemical shift δ (ppm) | Triad assignment | |||
|---|---|---|---|---|
| 1H | 13C | |||
| ENR-50 * | Cyclic dithiocarbonate derivative | ENR-50 * | Cyclic dithiocarbonate derivative | |
| 1.30 | 1.31 | 22.3 | 22.4 | E10 |
| 1.56 | 1.58–1.63 | 29.7 | 29.8 | EE8C, E8 |
| 33.1 | 33.1 | CE8C, CE8E | ||
| 27.0 | 27.0 | CE9C, EE9C | ||
| 1.68–1.70 | 1.70–1.71 | 23.4 | 23.5 | C5 |
| 24.7 | 24.8 | CE9E, E9 | ||
| 2.06 | 2.08 | 26.3 | 26.4 | C4, EC4C |
| 32.0 | 32.2 | C3, CC3E | ||
| 2.15–2.19 | 2.12–2.19 | 23.9 | 24.0 | CC4E, EC4E |
| 28.7 | 28.7 | EC3C, EC3E | ||
| - | - | 60.7 | 61.0 | E6 |
| 2.72 | 2.73 | 64.5 | 64.8 | E7, E7of five-membered ring |
| - | - | - | 94.0 | E6 of five-membered ring |
| 5.12–5.17 | 5.15–5.19 | 125.0 | 125.1 | C2 |
| - | - | 134.7 | 134.9 | C1 |
| - | - | - | 210.5 | CS2 carbon of five-membered ring |
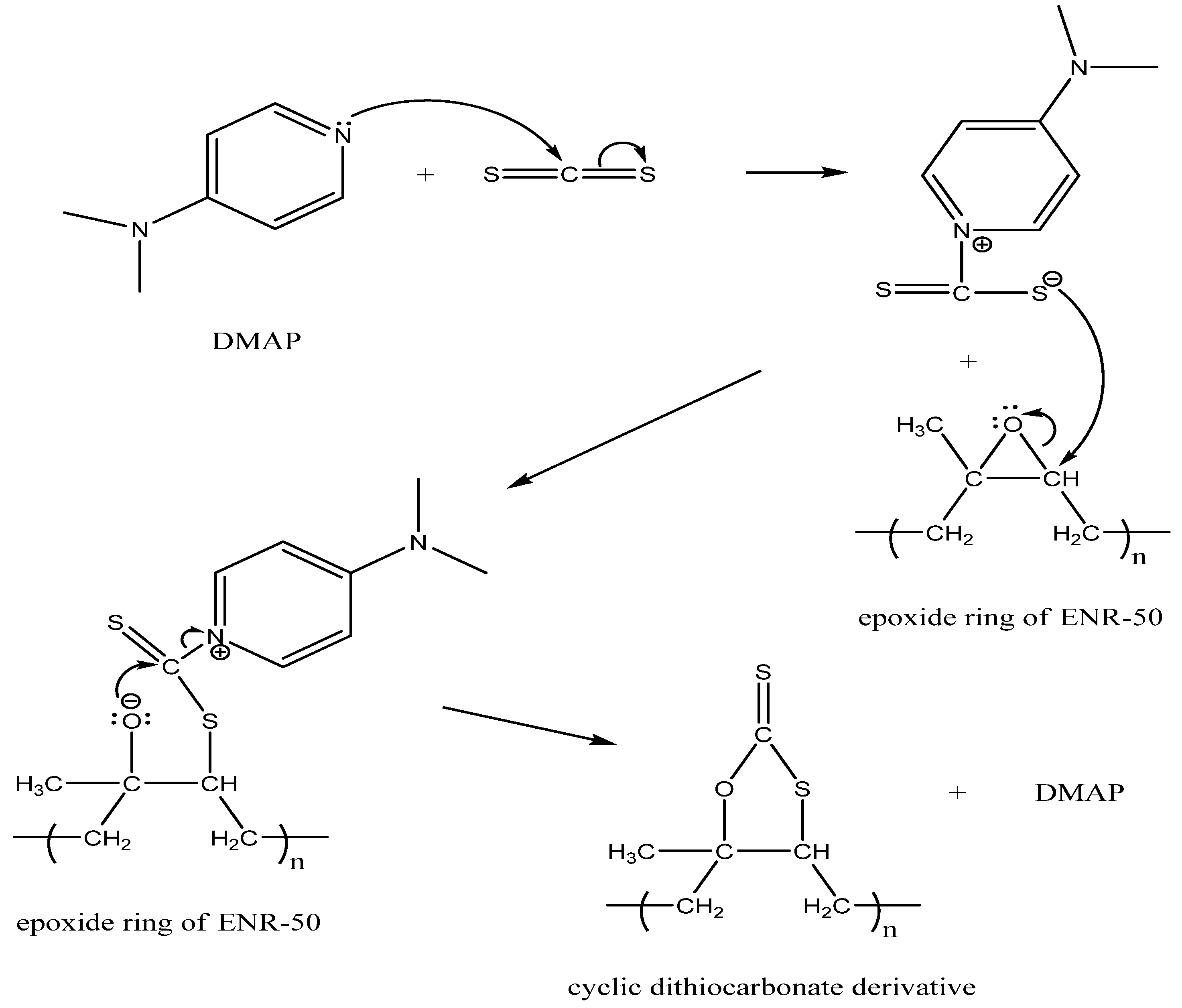
2.2.3. Proposed Mechanism for the Formation of Cyclic Dithiocarbonate
3. Experimental
3.1. Materials
3.2. Preparative Procedure
3.2.1. Purification of ENR-50 [19]
3.2.2. Reaction of Purified ENR-50 with CS2 [24]
3.3. Measurements and Characterization Techniques
3.4. Theoretical Treatments
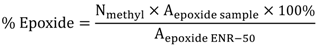
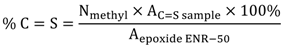
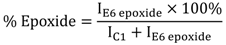
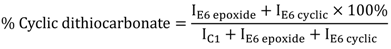
4. Conclusions
Acknowledgments
References
- Baker, C.S.L.; Gelling, I.R. Epoxidized Natural Rubber. In Development of Rubber Technology; Gelling, I.R., Ed.; Elsevier Applied Science: London, UK, 1987; Volume 4, p. 87. [Google Scholar]
- Gelling, I.R. Modification of Natural Rubber with Peracetic Acid. Rubber Chem. Technol. 1985, 58, 86–96. [Google Scholar] [CrossRef]
- Jeerupun, J.; Wootthikanokkhan, J.; Phinyocheep, P. Effects of Epoxidation Content of ENR on Morphology and Mechanical Properties of Natural Rubber Blended PVC. Macromol. Symp. 2004, 216, 281–292. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hassan, A.; Tan, I.K.P.; Terakawa, K.; Ichikawa, N.; Gan, S.N. Reaction of Palm Oil Based mcl-PHAs with Epoxidized Natural Rubber. J. Appl. Polym. Sci. 2010, 115, 2039–2043. [Google Scholar] [CrossRef]
- Gelling, I.R. Epoxidised Natural Rubber. J. Nat. Rubb. Res. 1991, 6, 184–205. [Google Scholar]
- Burfield, D.R.; Lim, K.L.; Law, K.S.; Ng, S. Analysis of Epoxidized Natural Rubber: A Comparative Study of D.S.C., N.M.R., Elemental Analysis and Direct Titration Methods. Polymer 1984, 25, 995–998. [Google Scholar]
- Gelling, I.R. Epoxidized Natural Rubber. In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1996; Volume 3 (D-E), p. 2192. [Google Scholar]
- Bhattacharjee, S.; Bhowmick, A.K.; Avasthi, B.N. Hydrogenation of Epoxidized Natural Rubber in the Presence of Palladium Acetate Catalyst. Polymer 1993, 34, 5168–5173. [Google Scholar] [CrossRef]
- Gan, S.N.; Ziana, A.H. Partial Conversion of Epoxide Groups to Diols in Epoxidized Natural Rubber. Polymer 1997, 38, 1953–1956. [Google Scholar]
- Derouet, D.; Brosse, J.C.; Challioui, A. Alcoholysis of Epoxidized Polyisoprene by Direct Opening of Oxirane Rings with Alcohol Derivatives. 2. Study on Epoxidized 1,4-Polyisoprene. Eur. Polym. J. 2001, 37, 1327–1337. [Google Scholar]
- Saito, T.; Klinklai, W.; Kawahara, S. Characterization of Epoxidized Natural Rubber by 2D NMR Spectroscopy. Polymer 2007, 48, 750–757. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Perera, M.C.S. Epoxidation of Natural Rubber Studied by NMR Spectroscopy. J. Appl. Polym. Sci. 1985, 30, 3347–3364. [Google Scholar] [CrossRef]
- Thames, S.F.; Gupta, S. Synthesis and Characterization of Pendent Hydroxy Fluoroesters of Secondary High Molecular Weight Guayule Rubber. J. Appl. Polym. Sci. 1997, 63, 1077–1089. [Google Scholar] [CrossRef]
- Furst, A.; Pretsch, E. A Computer Program for the Prediction of 13C-NMR Chemical Shifts of Organic Compounds. Anal. Chim. Acta 1990, 229, 17–25. [Google Scholar] [CrossRef]
- Pretsch, E.; Furst, A.; Badertscher, M.; Burgin, R. C13Shift: A Computer Program for the Prediction of 13C-NMR Spectra Based on an Open Set of Additivity Rules. J. Chem. Inf. Comput. Sci. 1992, 32, 291–295. [Google Scholar]
- Mohamad, Z.; Ismail, H.; Chantara Thevy, R. Characterization of Epoxidized Natural Rubber/Ethylene Vinyl Acetate (ENR-50/EVA) Blend: Effect of Blend Ratio. J. Appl. Polym. Sci. 2006, 99, 1504–1515. [Google Scholar] [CrossRef]
- Noriman, N.Z.; Ismail, H.; Rashid, A.A. Characterization of Styrene Butadiene Rubber/Recycled Acrylonitrile-butadiene Rubber (SBR/NBRr) Blends: The Effects of Epoxidized Natural Rubber (ENR-50) As a Compatibilizer. Polym. Test. 2010, 29, 200–208. [Google Scholar] [CrossRef]
- Abu Bakar, M.; Ismail, J.; Teoh, C.H.; Tan, W.L.; Abu Bakar, N.H.H. Modified Natural Rubber Induced Aqueous to Toluene Phase Transfer of Gold and Platinum Colloids. J. Nanomater. 2008, 2008, 1–8. [Google Scholar]
- Lee, H.K.; Ismail, J.; Kammer, H.W.; Bakar, M.A. Melt Reaction in Blends of Poly(3-Hydroxybutyrate) (PHB) and Epoxidized Natural Rubber (ENR-50). J. Appl. Polym. Sci. 2005, 95, 113–129. [Google Scholar] [CrossRef]
- Han, C.C.; Ismail, J.; Kammer, H.W. Melt Reaction in Blends of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Epoxidized Natural Rubber. Polym. Degrad. Stab. 2004, 85, 947–955. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.W.; Kim, D.K. Green Thermoplastic Elastomer Based on Polycaprolactone/Epoxidized Natural Rubber Blend as a Heat Shrinkable Materials. Mater. Lett. 2007, 61, 3551–3554. [Google Scholar]
- Nghia, P.T.; Siripitakchai, N.; Klinklai, W.; Saito, T.; Yamamoto, Y.; Kawahara, S. Compatibility of Liquid Deproteinized Natural Rubber Having Epoxy Group (LEDPNR)/Poly (L-Lactide) Blend. J. Appl. Polym. Sci. 2008, 108, 393–399. [Google Scholar]
- Nghia, P.T.; Onoe, H.; Yamamoto, Y.; Kawahara, S. Hydrogenation of Natural Rubber Having Epoxy Group. Colloid Polym. Sci. 2008, 286, 993–998. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Ebrahimi, F.; Azizi, N.; Saidi, M.R. A Simple and Novel Eco-Friendly Process for the Synthesis of Cyclic Dithiocarbonates from Epoxides and Carbon Disulfide in Water. J. Heterocycl. Chem. 2009, 46, 347–350. [Google Scholar]
- Giraudeau, P.; Akoka, S. Resolution and Sensitivity Aspects of Ultrafast J-resolved 2D NMR Spectra. J. Magn. Reson. 2008, 190, 339–345. [Google Scholar] [CrossRef]
- Viant, M.R. Improved Methods for the Acquisition and Interpretation of NMR Metabolomic Data. Biochem. Biophys. Res. Commun. 2003, 310, 943–948. [Google Scholar] [CrossRef]
- Macomber, R.S. A Complete Introduction to Modern NMR Spectroscopy; John Wiley & Sons Inc.: New York, NY, USA, 1998; p. 77. [Google Scholar]
- Shamsuzzaman; Salim, A. Synthesis of 1,3-Oxathiolane-2-Thiones by the Reaction of Steroidal Oxiranes with Carbon Disulfide. Tetrahedron Lett. 1997, 32, 5705–5708. [Google Scholar] [CrossRef]
- Conley, R.T. Infrared Spectroscopy, 2nd ed; Allyn and Bacon Inc.: Boston, MA, USA, 1972. [Google Scholar]
- Kawahara, S.; Saito, T. Preparation of Carbonated Natural Rubber. J. Polym. Sci. A Polym. Chem. 2006, 44, 1561–1567. [Google Scholar] [CrossRef]
- Kawahara, S.; Chaikumpollert, O.; Sakurai, S.; Yamamoto, Y.; Akabori, K. Crosslinking Junctions of Vulcanized Natural Rubber Analyzed by Solid-state NMR Spectroscopy Equipped with Field-gradient-magic Angle Spin Probe. Polymer 2009, 50, 1626–1631. [Google Scholar] [CrossRef]
- Bruice, P.Y. Organic Chemistry, 3rd ed; Prentice-Hall Inc: Upper Saddle River, NJ, USA, 2001; p. 448. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamzah, R.; Bakar, M.A.; Khairuddean, M.; Mohammed, I.A.; Adnan, R. A Structural Study of Epoxidized Natural Rubber (ENR-50) and Its Cyclic Dithiocarbonate Derivative Using NMR Spectroscopy Techniques. Molecules 2012, 17, 10974-10993. https://doi.org/10.3390/molecules170910974
Hamzah R, Bakar MA, Khairuddean M, Mohammed IA, Adnan R. A Structural Study of Epoxidized Natural Rubber (ENR-50) and Its Cyclic Dithiocarbonate Derivative Using NMR Spectroscopy Techniques. Molecules. 2012; 17(9):10974-10993. https://doi.org/10.3390/molecules170910974
Chicago/Turabian StyleHamzah, Rosniza, Mohamad Abu Bakar, Melati Khairuddean, Issam Ahmed Mohammed, and Rohana Adnan. 2012. "A Structural Study of Epoxidized Natural Rubber (ENR-50) and Its Cyclic Dithiocarbonate Derivative Using NMR Spectroscopy Techniques" Molecules 17, no. 9: 10974-10993. https://doi.org/10.3390/molecules170910974
APA StyleHamzah, R., Bakar, M. A., Khairuddean, M., Mohammed, I. A., & Adnan, R. (2012). A Structural Study of Epoxidized Natural Rubber (ENR-50) and Its Cyclic Dithiocarbonate Derivative Using NMR Spectroscopy Techniques. Molecules, 17(9), 10974-10993. https://doi.org/10.3390/molecules170910974




