Activity-Guided Isolation of Antioxidant Compounds from Rhizophora apiculata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activities of Crude Extract and Fractions
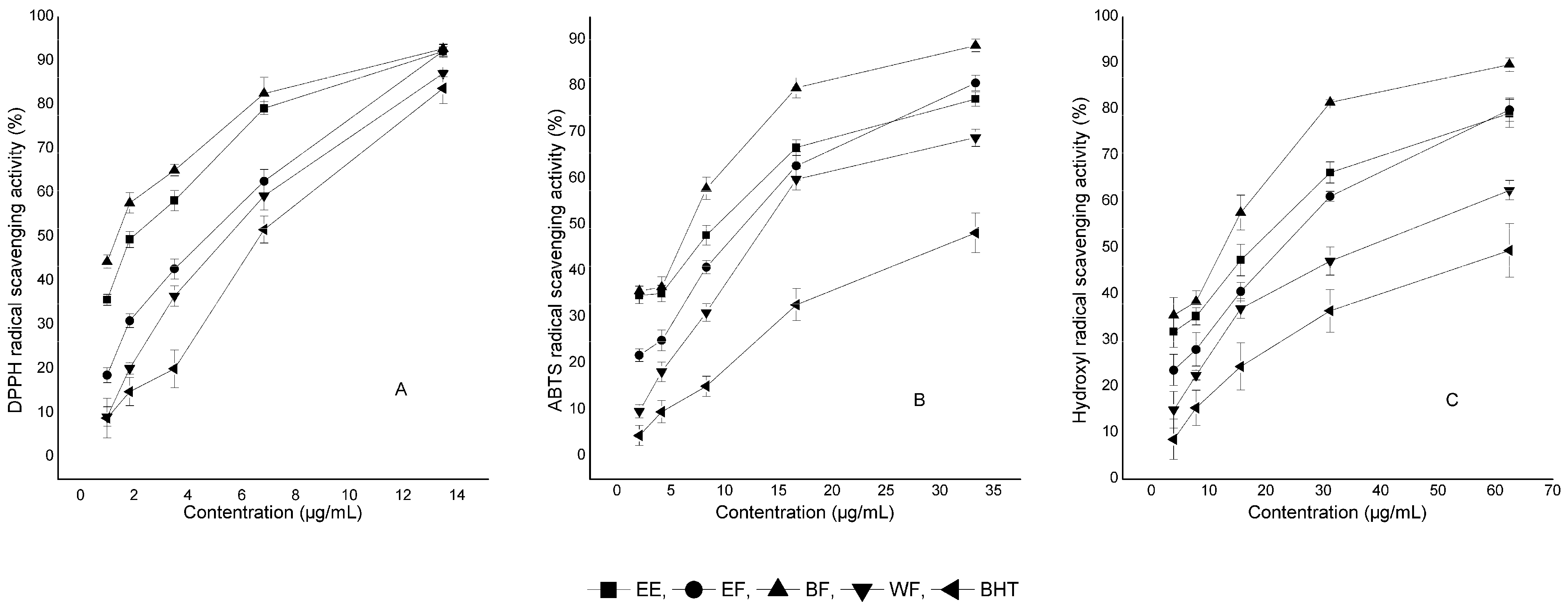
2.2. Total Phenolic Content
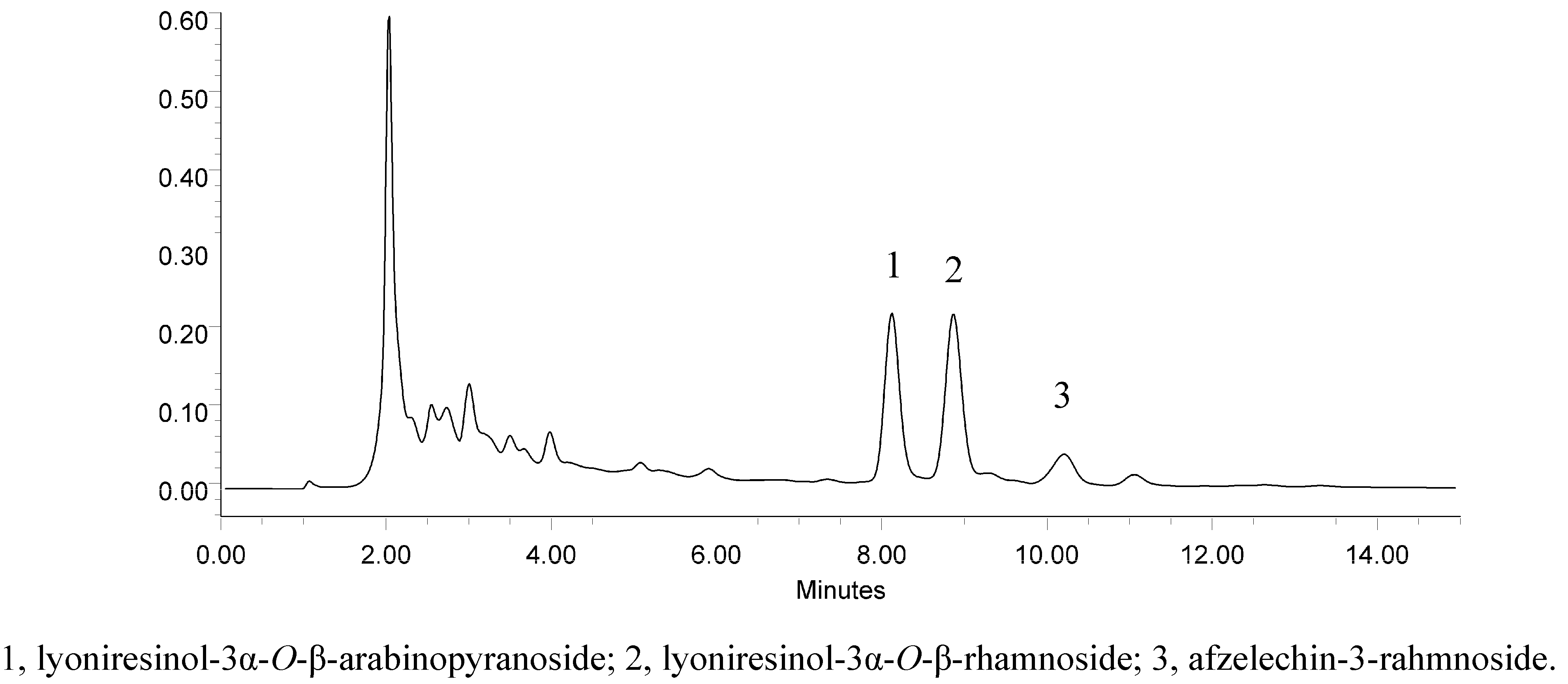
2.3. Separation of Compounds
2.4. Activity and Quantity Analysis of Isolated Compounds
| Compound | IC50 (μg/mL) | ||
|---|---|---|---|
| DPPH | ABTS•+ | OH | |
| Lyoniresinol-3α-O-β-arabinopyranoside | 2.06 | 1.64 | 5.83 |
| Lyoniresinol-3α-O-β-rhamnoside | 2.64 | 2.09 | 9.07 |
| Afzelechin-3-rahmnoside | 2.26 | 1.96 | 7.05 |
| BHT | 55.20 | 9.63 | 45.58 |
| Compound | Content (%) | ||
|---|---|---|---|
| twig | leaf | bark | |
| Lyoniresinol-3α-O-β-arabinopyranoside | 0.047 | 0.012 | 0.068 |
| Lyoniresinol-3α-O-β-rhamnoside | 0.059 | 0.016 | 0.066 |
| Afzelechin-3-rahmnoside | 0.010 | 0.006 | 0.011 |
3. Experimental
3.1. Plant Material and Chemicals
3.2. Extraction and Chemical Isolation
3.3. Antioxidant Activity
3.3.1. DPPH free Radical Scavenging Assay Activity
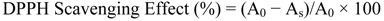
3.3.2. ABTS Scavenging Activity
3.3.3. Hydroxyl Radical Scavenging Ability Assay
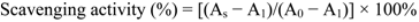
3.4. Total Phenolic Content
3.5. HPLC Analysis of Lignans
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the three compounds are available from the authors.
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 1985. [Google Scholar]
- Moskovitz, J.; Yim, M.B.; Chock, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [CrossRef]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621–629. [Google Scholar]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef]
- Arbhabhirama, A.; Phantumvanit, D.; Elkington, J.; Ingkasuwan, P. Thailand: Natural Resources Profile; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Kokpol, U.; Chavasiri, W.; Chittawong, V.; Miles, D.H. Taraxeryl cis-p-hydroxycinnamate, a novel taraxeryl from Rhizophora apiculata. J. Nat. Prod. 1990, 53, 953–955. [Google Scholar] [CrossRef]
- Gao, M.Z.; Yuan, X.Y.; Cheng, M.C.; Xiao, H.B.; Bao, S.X. A new diterpenoid from Rhizophora apiculata. J. Asian Nat. Prod. Res. 2011, 13, 776–779. [Google Scholar] [CrossRef]
- Loo, A.; Jain, K.; Darah, I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem. 2008, 107, 1151–1160. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Ibrahim, M.S.; Osman, H. Antioxidant activities of mangrove Rhizophora apiculata bark extracts. Food Chem. 2008, 107, 200–207. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M. Free radical scavenging activity of the marine mangrove Rhizophora apiculata bark extract with reference to naphthalene induced mitochondrial dysfunction. Chem. Biol. Interact. 2006, 163, 170–175. [Google Scholar] [CrossRef]
- Premanathan, M.; Arakaki, R.; Izumi, H.; Kathiresan, K.; Nakano, M.; Yamamoto, N.; Nakashima, H. Antiviral properties of a mangrove plant, Rhizophora apiculata Blume, against human immunodeficiency virus. Antiviral Res. 1999, 44, 113–122. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Ronald, L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. J. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar]
- Cortez, D.A.G.; Fernandes, J.B.; Cass, Q.B.; Vieira, P.C.; Da Silva, M.F.G.F.; Ferreira, A.G.; Pirani, J.R. Lignan glycosides from Trichilia estipulata bark. Nat. Prod. Lett. 1998, 11, 255–262. [Google Scholar] [CrossRef]
- Fuchino, H.; Satoh, T.; Tanaka, N. Chemical evaluation of Betula species in Japan. I: Constituents of Betula ermanii. Chem. Pharm. Bull. 1995, 43, 1937–1942. [Google Scholar] [CrossRef]
- Drewes, S.E.; Taylor, C.W.; Cunningham, A.B. (+)-Afzelechin 3-rhamnoside from Cassipourea gerrardii. Phytochemistry 1992, 31, 1073–1075. [Google Scholar]
- Kaur, R.; Arora, S.; Singh, B. Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss. Bioresour.Technol. 2008, 99, 7692–7698. [Google Scholar] [CrossRef]
- Wenli, Y.; Yaping, Z.; Bo, S. The radical scavenging activities of radix puerariaeisoflavonoids: A chemiluminescence study. Food Chem. 2004, 86, 525–529. [Google Scholar] [CrossRef]
- Boligon, A.A.; Pereira, R.P.; Feltrin, A.C.; Machado, M.M.; Janovik, V.; Rocha, J.B.T.; Athayde, M.L. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss. Bioresour.Technol. 2009, 100, 6592–6598. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, M.; Xiao, H. Activity-Guided Isolation of Antioxidant Compounds from Rhizophora apiculata. Molecules 2012, 17, 10675-10682. https://doi.org/10.3390/molecules170910675
Gao M, Xiao H. Activity-Guided Isolation of Antioxidant Compounds from Rhizophora apiculata. Molecules. 2012; 17(9):10675-10682. https://doi.org/10.3390/molecules170910675
Chicago/Turabian StyleGao, Mingzhe, and Hongbin Xiao. 2012. "Activity-Guided Isolation of Antioxidant Compounds from Rhizophora apiculata" Molecules 17, no. 9: 10675-10682. https://doi.org/10.3390/molecules170910675
APA StyleGao, M., & Xiao, H. (2012). Activity-Guided Isolation of Antioxidant Compounds from Rhizophora apiculata. Molecules, 17(9), 10675-10682. https://doi.org/10.3390/molecules170910675




