Novel Pyranopyrazoles: Synthesis and Theoretical Studies
Abstract
:1. Introduction
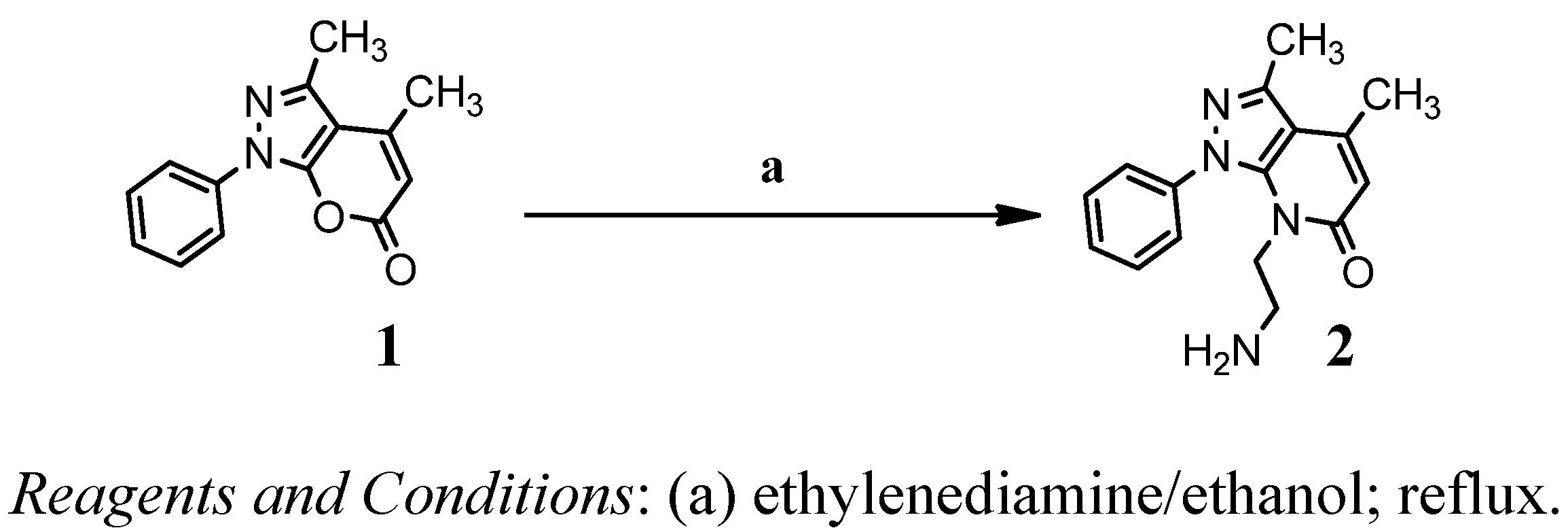
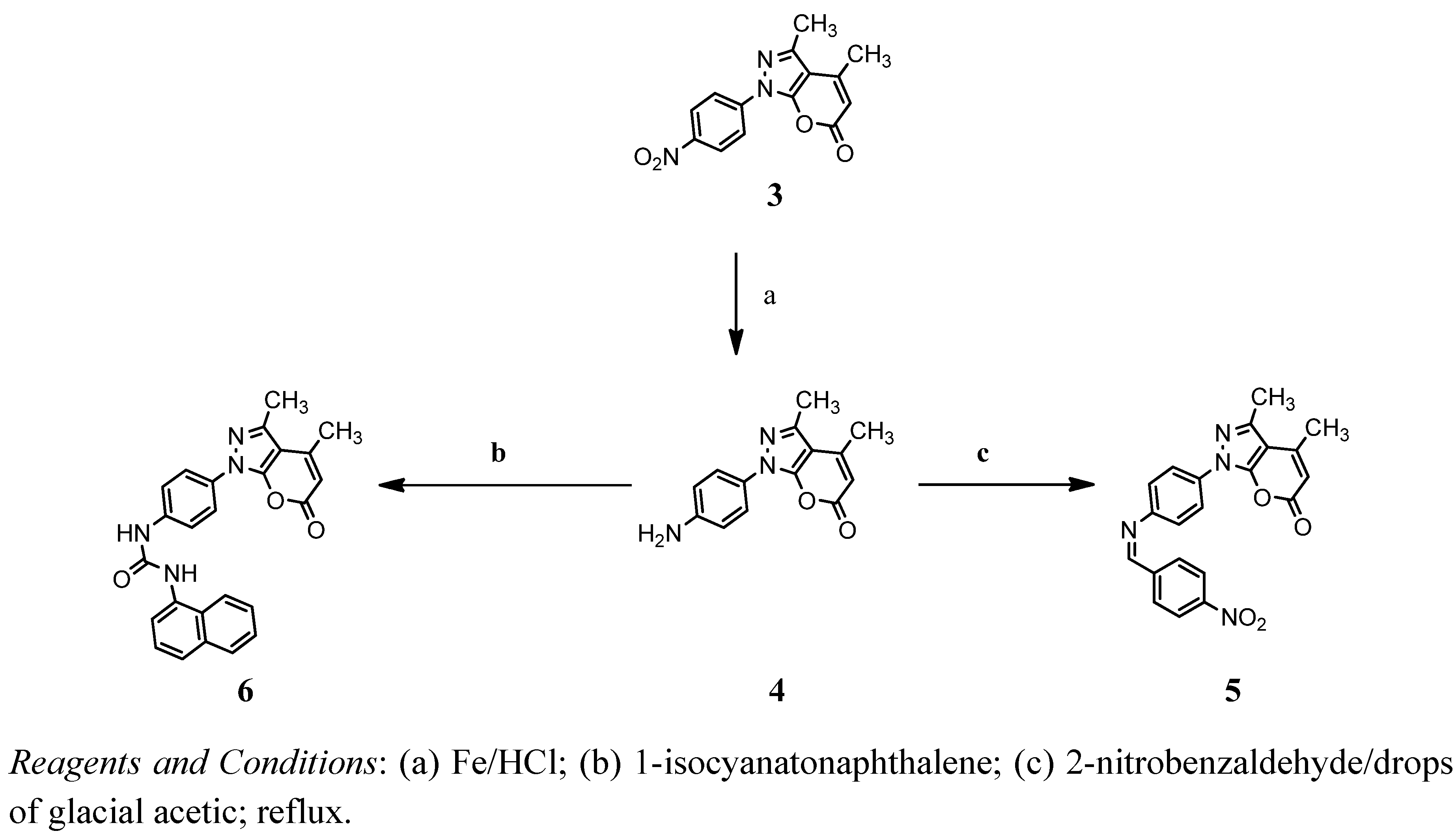

2. Results and Discussion
2.1. Chemistry
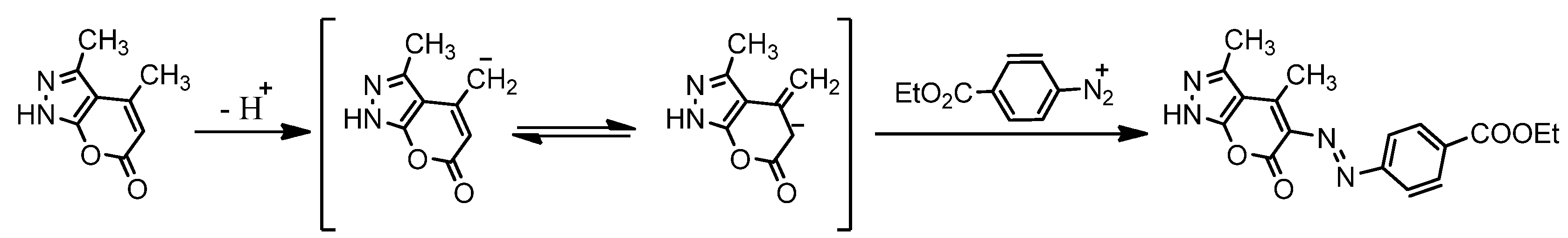
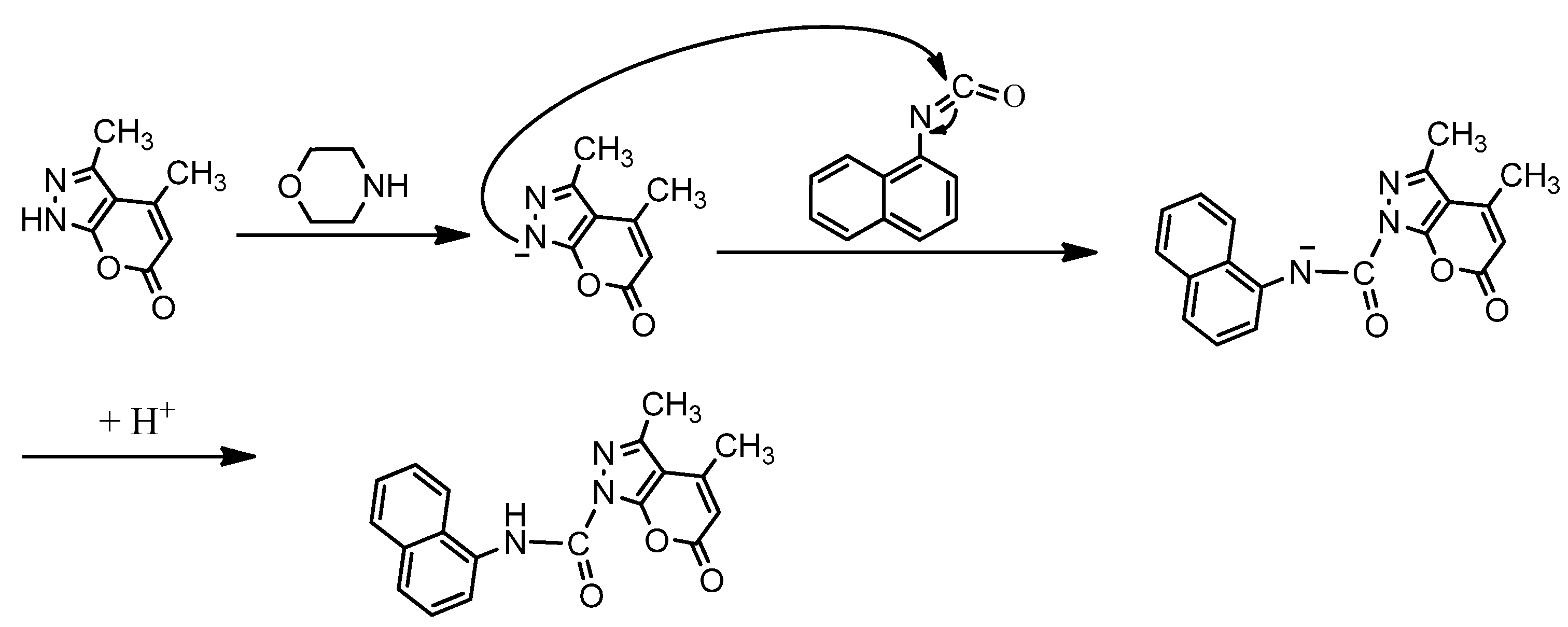
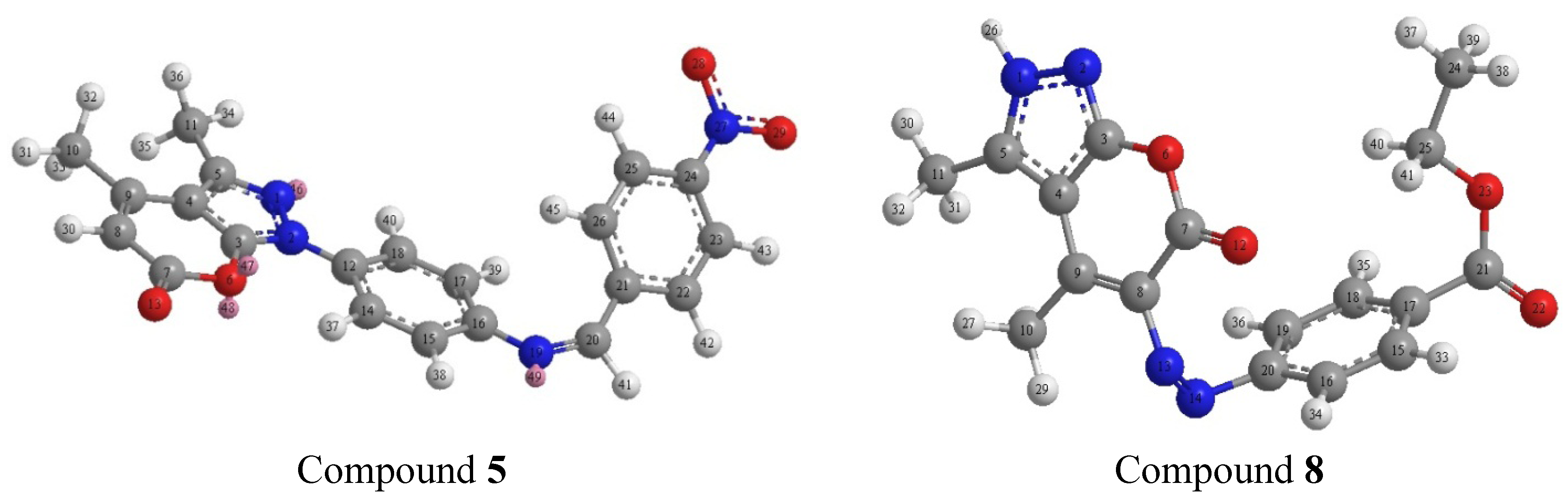
2.2. Compound Characterizations
2.3. Computational Studies
2.3.1. Atomic Charges
2.3.2. Density Function Theory (DFT)
| Compound | Total Energy | Dipole Moments |
|---|---|---|
| 5 | 56.0882 kcal/mol | 7.1113 |
| 8 | 167.4106 kcal/mol | 15.3621 |
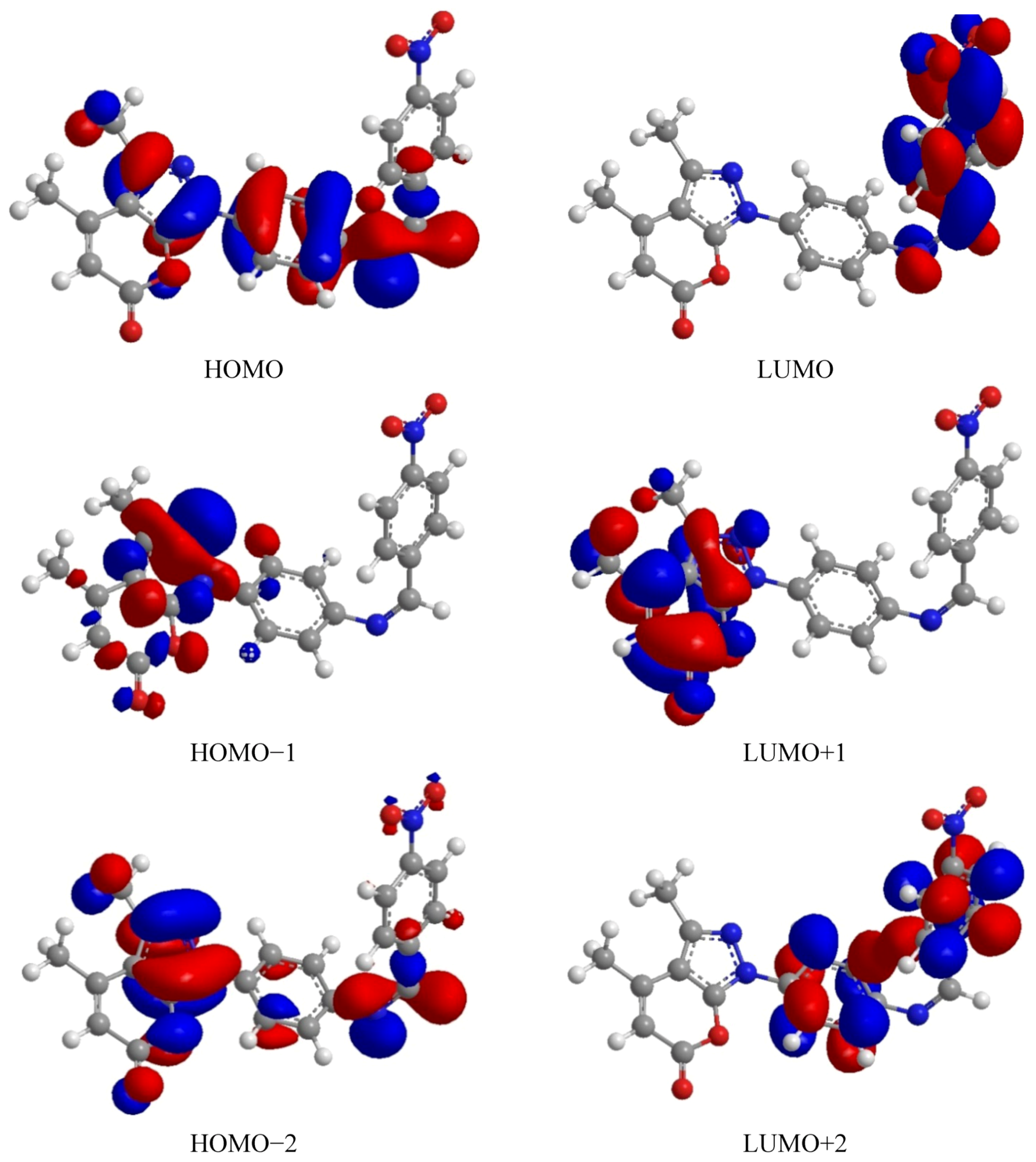
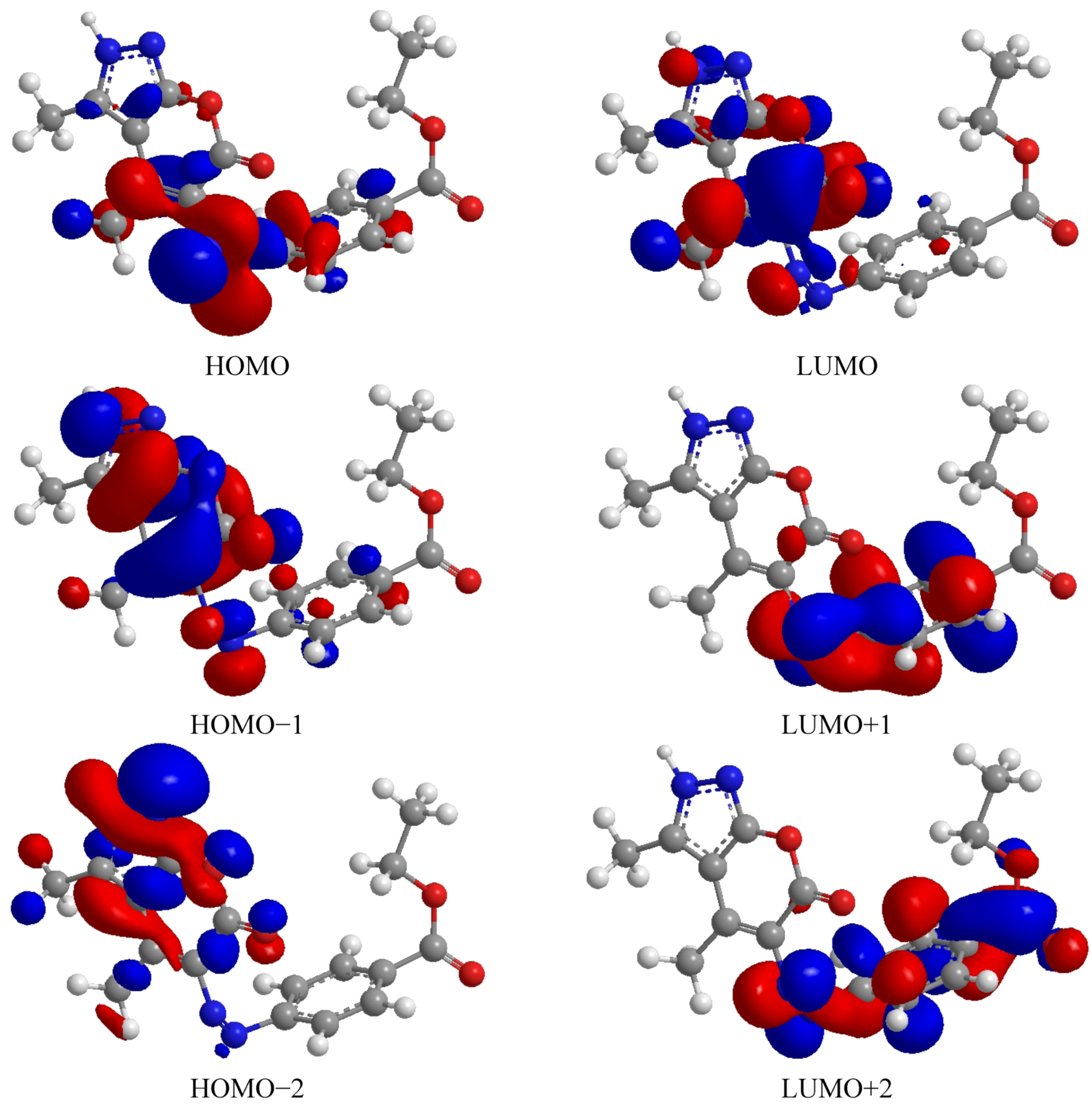
| Comp. | HOMO | LUMO | ∆E | HOMO−1 | LUMO+1 | ∆E | HOMO−2 | LUMO+2 | ∆E |
|---|---|---|---|---|---|---|---|---|---|
| 5 | −9.383 | −4.732 | −4.651 | −10.833 | −2.578 | −8.255 | −11.552 | −1.326 | −10.226 |
| 8 | −4.715 | −2.094 | −2.666 | −10.597 | −1.252 | −9.345 | −11.314 | −0.917 | −10.398 |
3. Experimental
3.1. General
3.2. Synthesis of Compounds 1, 3 and 7
3.3. Synthesis of Compounds 2, 4, 5, 6, 8 and 9
3.4. DFT
4. Conclusions
Acknowledgments
References
- El-Gazzar, A.B.A.; El-Enany, M.M.; Mahmoud, M.N. Synthesis, analgesic, anti-inflammatory, and antimicrobial activity of some novel pyrimido[4,5-b]quinolin-4-ones. Bioorg. Med. Chem. 2008, 16, 3261–3273. [Google Scholar] [CrossRef]
- Fayed, A.A.; Hosni, H.M.; Flefel, E.M.; Amr, A.E.E. Synthesis and pharmacological activity of some new thieno[2,3-d]pyrimidine and pyrimidopyrazolothienopyrimidine derivatives. World J. Chem. 2009, 4, 58–65. [Google Scholar]
- Sondhi, S.M.; Singh, N.; Johar, M.; Kumar, A. Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg. Med. Chem. 2005, 13, 6158–6166. [Google Scholar] [CrossRef]
- Hafez, H.S.; Abbas, H.N.; El-Gazzar, A.B.A. Antimicrobial activity of some synthesized glucopyranosyl-pyrimidine carbonitrile and fused pyrimidine systems. Acta Pharm. 2008, 58, 359–378. [Google Scholar] [CrossRef]
- Fathalla, O.A.; Zeid, I.F.; Haiba, M.E.; Soliman, A.M.; Abd-Elmoez, S.I.; Serwy, W.S. Synthesis, Antibacterial and Anticancer Evaluation of Some Pyrimidine Derivatives. World J. Chem. 2009, 4, 127–132. [Google Scholar]
- El-Assiery, S.A.; Sayed, G.H.; Fouda, A. Synthesis of some new annulated pyrazolo-pyrido (or pyrano) pyrimidine, pyrazolopyridine and pyranopyrazole derivatives. Acta Pharm. 2004, 54, 143–150. [Google Scholar]
- Vaghasiya, S.J.; Dodiya, D.K.; Trivedi, A.R.; Shah, V.H. Synthesis and biological screening of some novel pyrazolo[3',4':4,5]thieno[2,3d]pyrimidin-8-ones via Gewald Reaction. ARKIVOC 2008, xii, 1–8. [Google Scholar]
- Klein, M.; Diner, P.; Dorin-Semblat, D.; Doerig, C.; Grøtli, M. Synthesis of 3-(1,2,3-triazol-1-yl)- and 3-(1,2,3-triazol-4-yl)-substituted pyrazolo[3,4-d]pyrimidin-4-amines via click chemistry: Potential inhibitors of the Plasmodium falciparum PfPK7 protein kinase. Org. Biomol. Chem. 2009, 7, 3421–3429. [Google Scholar] [CrossRef]
- Naito, H.; Sugimori, M.; Mitsui, I.; Nakamura, Y.; Iwahana, M.; Ishii, M.; Hirotani, K.; Kumazawa, E.; Ejima, A. Synthesis and antitumor activity of novel pyrimidinyl pyrazole derivatives. Chem. Pharm. Bull. 1999, 47, 1679–1684. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; El-Shehry, M.F.; Badria, F.A. Design and synthesis of novel thiophenecarbohydrazide, thienopyrazole and thienopyrimidine derivatives as antioxidant and antitumor agents. Acta Pharm. 2010, 60, 311–323. [Google Scholar] [CrossRef]
- Porba, K.; Wietrzyk, J.; Opolski, A. Synthesis and antiproliferative activity in vitro of new 2-, 3- or 4-substituted pyrido[2',3':3,4]pyrazolo[1,5-a]pyrimidines. Acta Pol. Pharm. Drug Res. 2006, 63, 189–194. [Google Scholar]
- Svetlik, J.; Veizerova, L.; Liptaj, T.; Kubista, J. Transformation of oxygen-bridged pyrimidines with nitrogen nucleophiles and characterization of resulting products. ARKIVOC 2009, 2009, 79–86. [Google Scholar]
- Wang, S.Q.; Fang, L.; Liu, X.J.; Zhao, K. Design, synthesis, and hypnotic activity of pyrazolo[1,5a]pyrimidine derivatives. Chin. Chem. Lett. 2004, 15, 885–888. [Google Scholar]
- Li, M.; Wang, S.W.; Wen, L.R.; Qi, W.Y.; Yang, H.Z. Synthesis and Crystal Structure of 1-[(1'-Phenyl-5'-methyl-4'-pyrazolcarbonyl)-3-methylthio-5-amino-1H-yl-1,2,4-triazole. Chin. J. Struct. Chem. 2005, 24, 64–68. [Google Scholar]
- Xia, Y.; Dong, Z.W.; Zhao, B.X.; Ge, X.; Meng, N.; Shin, D.S.; Miao, J.Y. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg. Med. Chem. 2007, 15, 6893–6899. [Google Scholar]
- Ohsumi, K.; Matsueda, H.; Hatanaka, T.; Hirama, R.; Umemura, T.; Oonuki, A.; Ishida, N.; Kageyama, Y.; Maezono, K.; Kondo, N. Pyrazole-O-glucosides as novel Na(+)-glucose cotransporter (SGLT) inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 2269–2272. [Google Scholar] [CrossRef]
- Kett, W.C.; Batley, M.; Redmond, J.W. Heterocyclic derivatives of sugars: The formation of 1-glycosyl-3-methylpyrazol-5-ones from hydrazones. Carbohydr. Res. 2000, 329, 169–177. [Google Scholar] [CrossRef]
- Nishimura, N.; Banno, M.; Maki, A.; Nishiyama, Y.; Maeba, I. Synthesis of 3-β-d-ribofuranosylpyrazole-1-carboxamide. Carbohydr. Res. 1998, 307, 211–215. [Google Scholar] [CrossRef]
- Michalik, D.; Feist, H.; Peseke, K. Synthesis of derivatives of C-nucleoside analogues using “push-pull” functionalized monosaccharides. Carbohydr. Res. 2001, 333, 197–201. [Google Scholar] [CrossRef]
- Zsoldos-Mády, V.; Csámpai, A.; Szabó, R.; Mészáros-Alapi, E.; Pásztor, J.; Hudecz, F.; Sohár, P. Synthesis, Structure, and in vitro Antitumor Activity of Some Glycoside Derivatives of Ferrocenyl-Chalcones and Ferrocenyl-Pyrazolines. ChemMedChem 2006, 1, 1119–1125. [Google Scholar] [CrossRef]
- Zoorob, H.; Elsherbini, M.; Wafaa, S. Utility of Cyclododecanone as Synthon to Synthesize Fused Heterocycles. Am. J. Org. Chem. 2012, 2, 63–68. [Google Scholar]
- Zhang, J.; Chang, C.W.T. Divergent Synthesis of Three Classes of Aryl N-Glycosides by Solvent Control. J. Org. Chem. 2009, 74, 685–695. [Google Scholar]
- Popsavin, M.; Torovic, V.; Kolic, G.; Bogdanovic, G.; Spaic, S.; Popsavin, V. De novo synthesis of two new cytotoxictiazofurin analogues with Modified sugar moieties. Bioorg. Med. Chem. Lett. 2003, 13, 3167–3170. [Google Scholar] [CrossRef]
- Popsavin, M.; Torovic, L.; Spaic, S.; Stankov, S.; Popsavin, V. ChemInform Abstract: Stereospecific Synthesis of Two Novel Cytotoxic Pyrazole C-Nucleosides from D-Glucose. ChemInform 2000, 31. [Google Scholar]
- Samb, I.; Pellegrini-Moïse, P.; Chapleur, Y. Synthesis of annelated pyranosides: A rapid and efficient entry to highly functionalized optically pure branched-pyrazoles. TetrahedronLett. 2007, 48, 7978. [Google Scholar]
- Abdel-Wahab, B.; Khidre, R.; Farahat, A. Pyrazole-3(4)-carbaldehyde: Synthesis, reactions and biological Activity. ARKIVOC 2011, 196–245. [Google Scholar]
- Al-Amiery, A.A.; Al-Majedy, Y.; Abdulreazak, H.; Abood, H. Synthesis, Characterization, Theoretical Crystal Structure, and Antibacterial Activities of Some Transition Metal Complexes of the Thiosemicarbazone. Bioinorg. Chem. Appl. 2011, 2011, 1–5. [Google Scholar]
- Kadhum, A.A.H.; Mohamad, A.; Al-Amiery, A.A. Antimicrobial and anti-oxidant activities of new metal complexes derived from 3-aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Bayati, R.; Saour, K.; Radi, M. Cytotoxicity, Antioxidant and Antimicrobial activities of novel 2-quinolone derivatives derived from coumarins. Res. Chem. Intermediat. 2012, 38, 559–569. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A. The Antioxidant Activity of New Coumarin Derivatives. Int. J. Mol. Sci. 2011, 12, 5757–5761. [Google Scholar]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Shikara, M.; Mohamad, A.; Al-Bayati, R. Synthesis, Structure elucidation and DFT studies of New thiadiazoles. Int. J. Phys. Sci. 2012, 6, 6692–6697. [Google Scholar]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Ibrahim, H.H.; Al-Tamimi, A.A. Antioxidant, antimicrobial, and theoretical studies of the thiosemicarbazone derivative Schiff base 2-(2-imino-1-methylimidazolidin-4-ylidene) hydrazinecarbothioamide (IMHC). Org. Med. Chem. Lett. 2012, 2, 4. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A. Antifungal and Antioxidant Activities of Pyrrolidonethiosemicarbazone Complexes. Bioinorg. Chem. Appl. 2012, 2012, 1–5. [Google Scholar]
- Al-Amiery, A.A. Synthesis and antioxidant, antimicrobial evaluation, DFT studies of novel metal complexes derivate from Schiff base. Res. Chem. Intermediat. 2012, 38, 745–759. [Google Scholar] [CrossRef]
- Al-Amiery, A.A. Antimicrobial and Antioxidant Activities of New Metal Complexes Derived from (E)-3-((5-phenyl-1,3,4-oxadiazol-2-ylimino)methyl)naphthalen-2-ol. Med. Chem. Res. 2011. [Google Scholar]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A. Antifungal Activities of New Coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar]
- Junaedi, S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.; Takriff, M. Synthesis and Characterization of Novel Corrosion Inhibitor Derived from Oleic Acid: 2-Amino 5-Oleyl-1,3,4-Thiadiazol (AOT). Int. J. Electrochem. Sci. 2012, 7, 3543–3554. [Google Scholar]
- Musa, A.Y.; Mohamad, A.; Al-Amiery, A.A.; Kadhum, A.A.H.; Tien, L.T. Galvanic corrosion of aluminum alloy (Al2024) and copper in 1.0 M hydrochloric acid solution. Korean J. Chem. Eng. 2012, 29, 818–822. [Google Scholar] [CrossRef]
- Dominguez, J.; Leon, C.; Gut, J.; Rossenthal, P. Synthesis and evaluation of new antimalarialphenylurenylchalcone derivatives. J. Med. Chem. 2005, 48, 3654–3658. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Amiery, A.A.; Al-Bayati, R.I.; Saed, F.M.; Ali, W.B.; Kadhum, A.A.H.; Mohamad, A.B. Novel Pyranopyrazoles: Synthesis and Theoretical Studies. Molecules 2012, 17, 10377-10389. https://doi.org/10.3390/molecules170910377
Al-Amiery AA, Al-Bayati RI, Saed FM, Ali WB, Kadhum AAH, Mohamad AB. Novel Pyranopyrazoles: Synthesis and Theoretical Studies. Molecules. 2012; 17(9):10377-10389. https://doi.org/10.3390/molecules170910377
Chicago/Turabian StyleAl-Amiery, Ahmed A., Redah I. Al-Bayati, Fouad M. Saed, Wassan B. Ali, Abdul Amir H. Kadhum, and Abu Bakar Mohamad. 2012. "Novel Pyranopyrazoles: Synthesis and Theoretical Studies" Molecules 17, no. 9: 10377-10389. https://doi.org/10.3390/molecules170910377
APA StyleAl-Amiery, A. A., Al-Bayati, R. I., Saed, F. M., Ali, W. B., Kadhum, A. A. H., & Mohamad, A. B. (2012). Novel Pyranopyrazoles: Synthesis and Theoretical Studies. Molecules, 17(9), 10377-10389. https://doi.org/10.3390/molecules170910377





