Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of Cosmos caudatus in Response to Organic and Mineral-Based Fertilizer Rates
Abstract
:1. Introduction
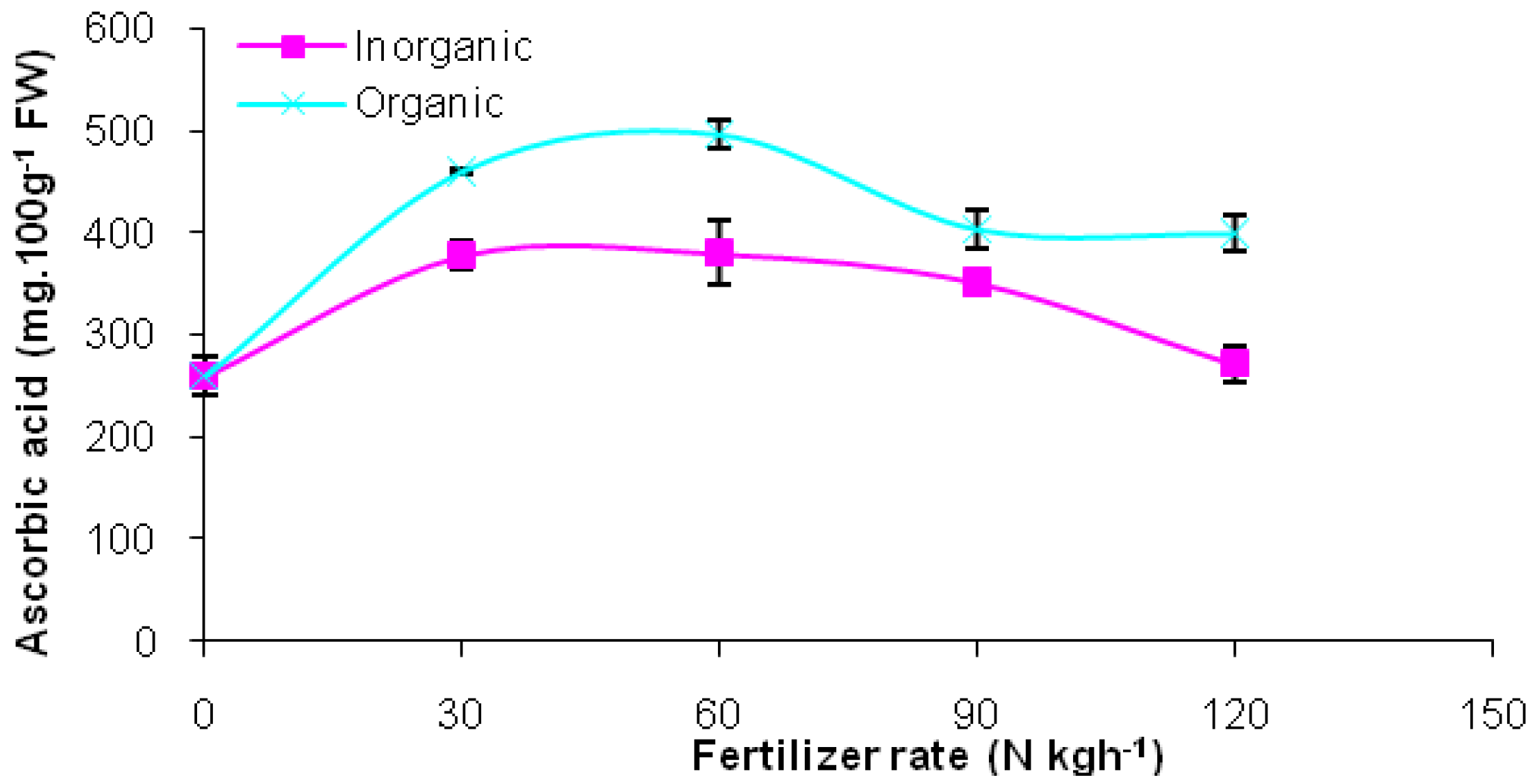
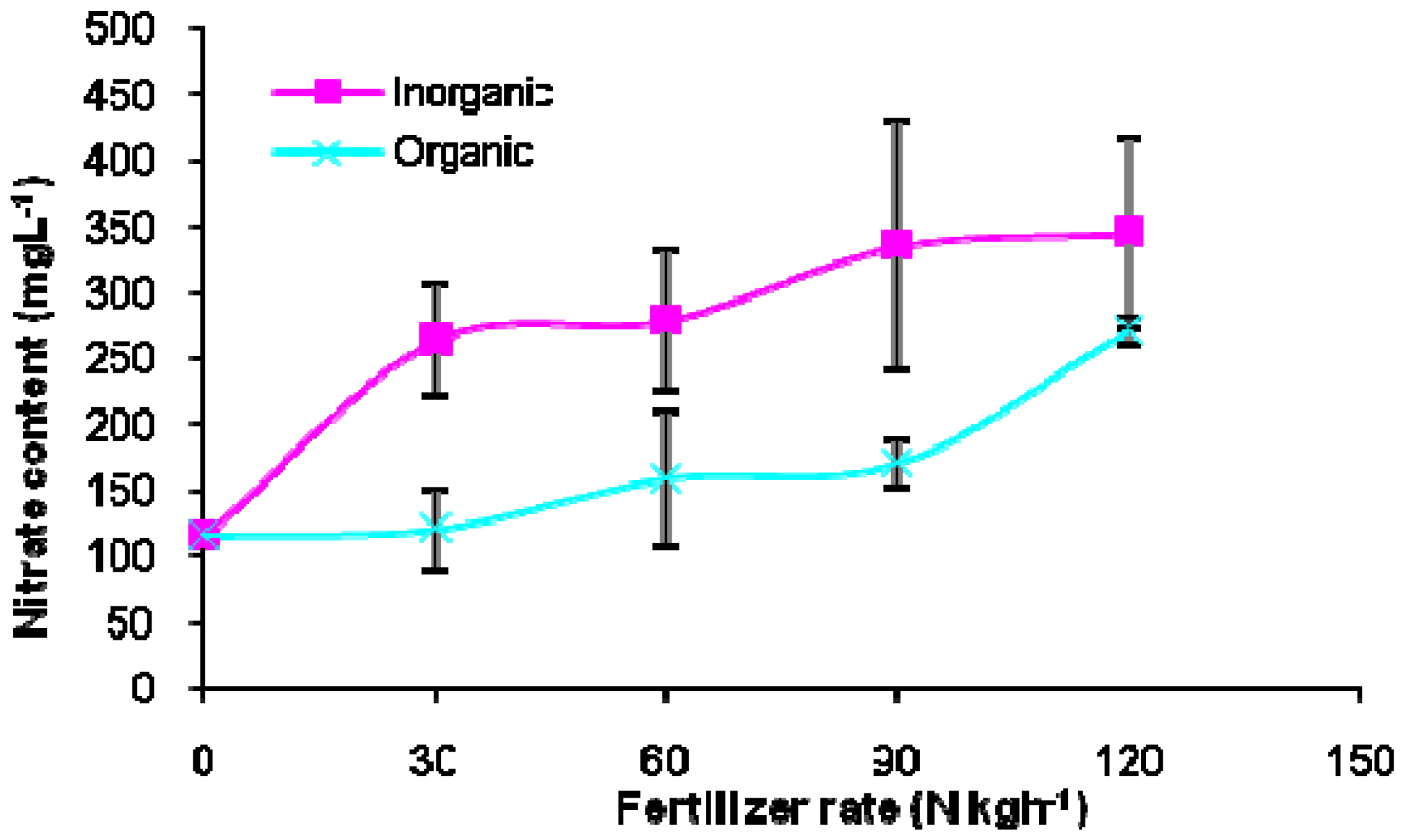
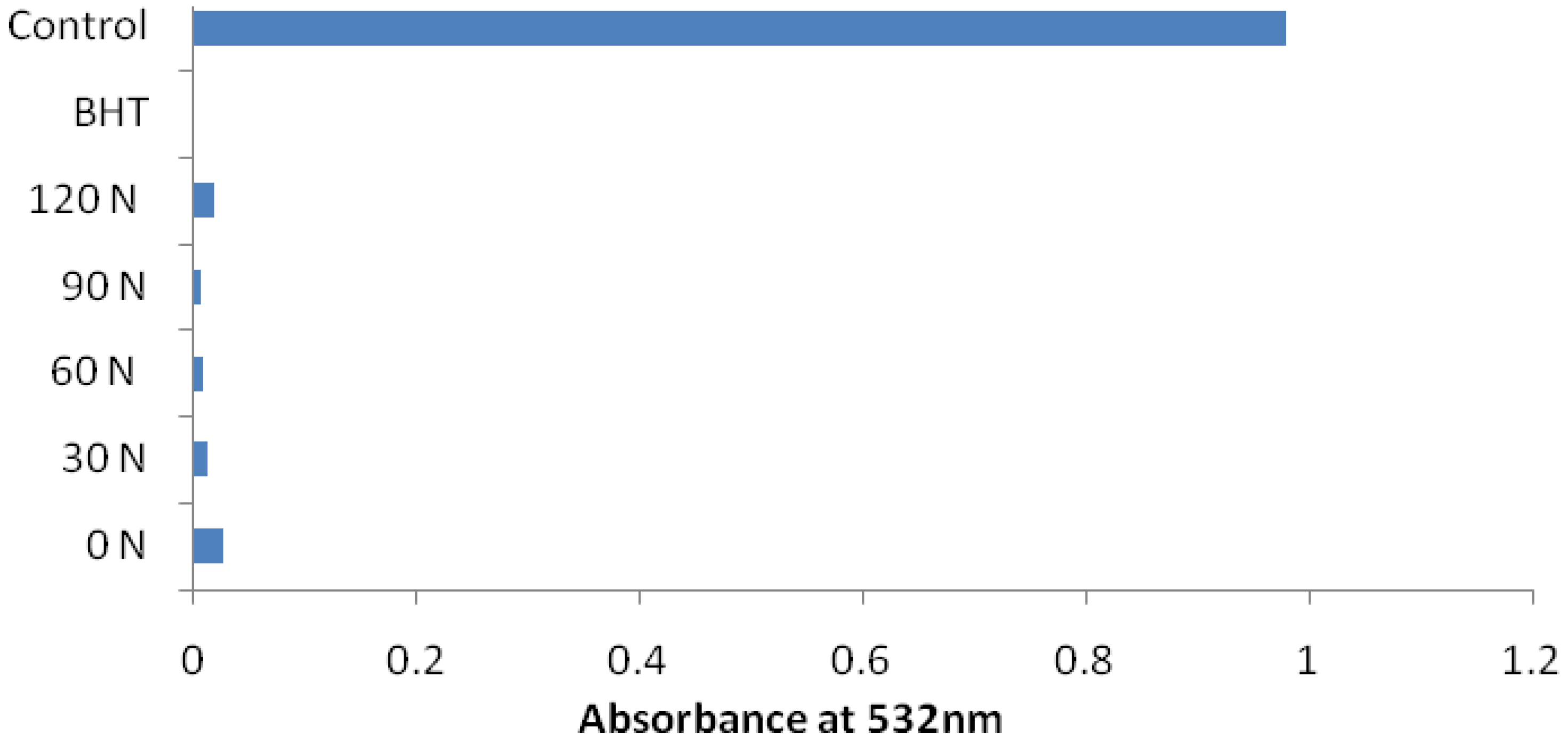
2. Results and Discussion
| Treatment | Ascorbic acid (mg·100 g−1 FW) | Nitrate (ppm) | Chlorophyll (mg/cm2) |
|---|---|---|---|
| Fertilizer sources Inorganic | 328.43a | 261.3a | 30.90a |
| Organic | 411.90b | 193.3b | 30.13a |
| Fertilizer rates (N kg h−1) | |||
| 0 | 259.7c | 115.0b | 24.45c |
| 30 | 419.8a | 241.7a | 29.25b |
| 60 | 438.8a | 243.3a | 31.10b |
| 90 | 378.3b | 244.2a | 35.83a |
| 120 | 354.3b | 292.5a | 31.95ab |
| Source × Rate | ns | ns | ns |
| Treatment | Nutrient content (%/g DW) | ||||
|---|---|---|---|---|---|
| N | P | K | Ca | Mg | |
| Fertilizer sources Inorganic | 3.22b | 0.44a | 2.22a | 0.31b | 0.13a |
| Organic | 3.49a | 0.41a | 2.18a | 0.34a | 0.14a |
| Fertilizer rates (N·kg h−1) | |||||
| 0 | 2.73d | 0.35b | 2.04b | 0.38b | 0.18b |
| 30 | 3.44c | 0.43a | 2.24ab | 0.52a | 0.19b |
| 60 | 3.63b | 0.45a | 2.23ab | 0.52a | 0.21a |
| 90 | 3.59bc | 0.45a | 2.20ab | 0.53a | 0.22a |
| 120 | 3.88a | 0.46a | 2.31a | 0.55a | 0.21a |
| Source × Rate | * | ns | ns | ns | ns |
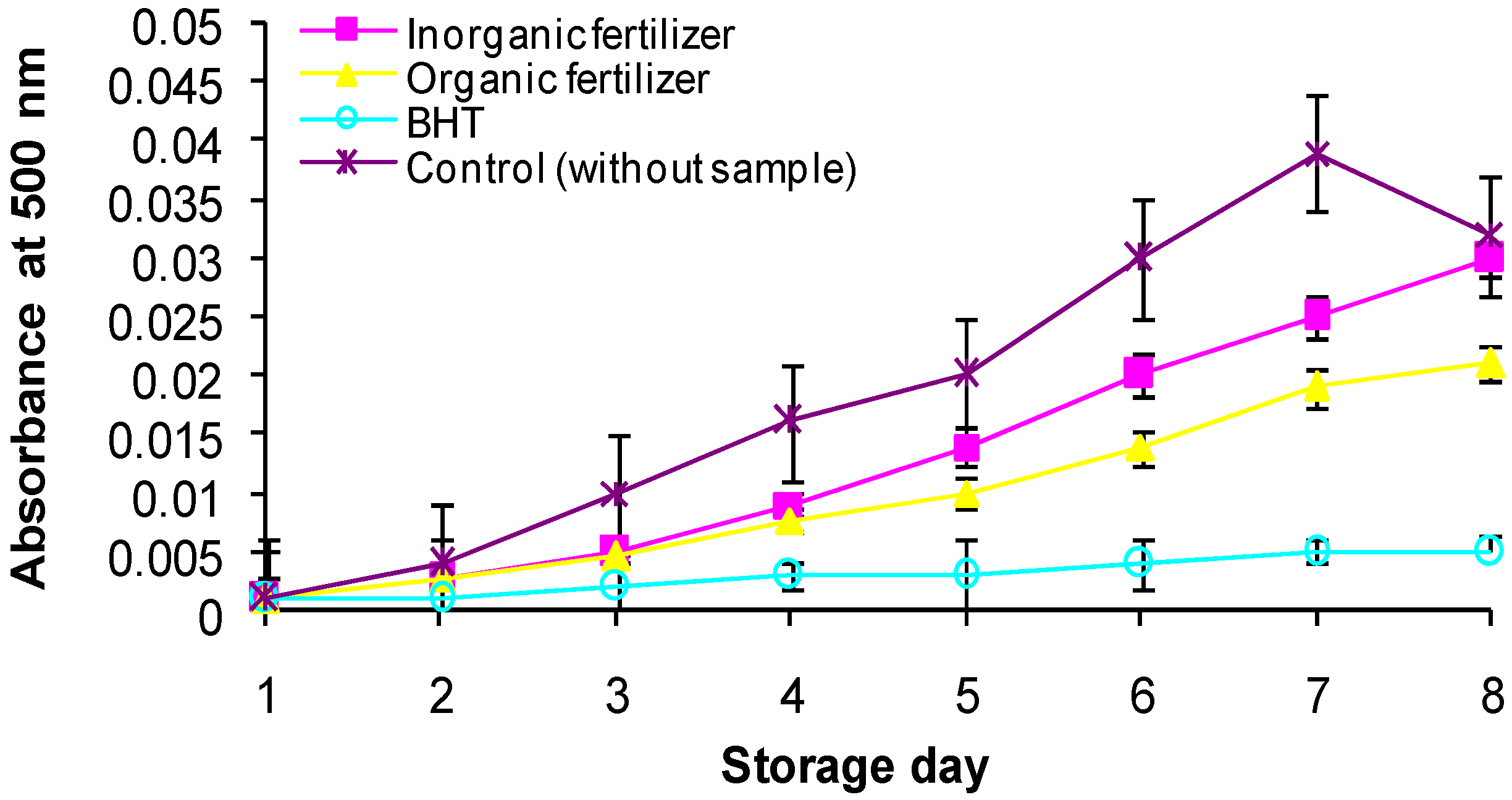
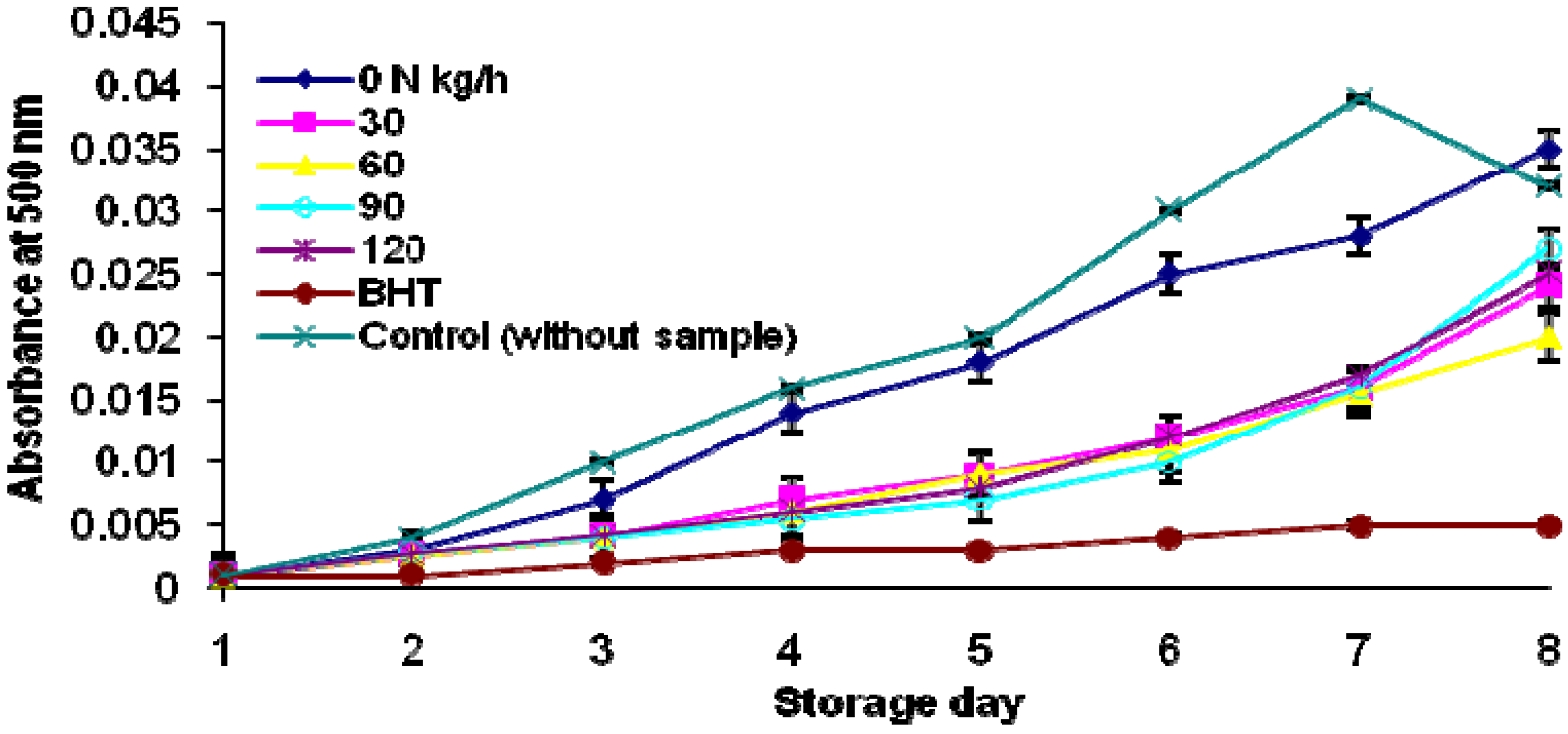
3. Experimental
4. Conclusions
Acknowledgments
References
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and Sorghum bicolour (L.). Afr. J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar]
- Riahi, A.; Hdider, C.; Sanaa, M.; Tarchoun, N.; ben Kheder, M.; Guezal, I. The influence of different organic fertilizers on yield and physico-chemical properties of organically grown tomato. J. Sustain. Agric. 2009, 33, 658–673. [Google Scholar]
- Seung, K.L.; Adel, A.K. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Hassan, A.; Predrag, L.; Irina, P.; Omar, S.; Uri, C.; Arieh, B. Fertilization-induced changes in growth parameters and antioxidant activity of medicinal plants used in traditional Arab medicine. Oxf. J. 2005, 2, 549–556. [Google Scholar]
- Alizadeh, A.; Khoshkhui, M.; Javidnia, K.; Firuzi, O.; Tafazoli, E.; Khalighi, A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensisi L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 2010, 4, 33–40. [Google Scholar]
- Ramesh, G.; Shivana, M.B.; Santa Ram, A. Interactive influence of organic manures and inorganic fertilizers on growth and yield of kalmegh (Andrographis paniculata Nees). Int. Res. J. Plant Sci. 2011, 2, 16–21. [Google Scholar]
- Toor, R.K.; Savage, G.P.; Heeb, A. Influence of different types of fertilizers on the major antioxidant components of tomatoes. J. Food Compost. Anal. 2006, 19, 20–27. [Google Scholar] [CrossRef]
- Al-Kharusi, L.; Elmardi, M.O.; Ali, A.; Al-Said, F.A.; Abdelbasit, K.M.; Al-Rawahy, S. Effect of mineral and organic fertilizers on the chemical characteristics and quality of date fruits. Int. J. Agric. Biol. 2009, 11, 290–296. [Google Scholar]
- Bimova, P.; Pokluda, R. Impact of organic fertilizers on total antioxidant capacity of head cabbage. Hortic. Sci. (Prague) 2009, 36, 21–25. [Google Scholar]
- Khalil, M.Y.; Moustafa, A.A.; Naguib, N.Y. Growth, phenolic compounds and antioxidant activity of some medicinal plants grown under organic farming condition. J. Agric. Sci. 2007, 3, 451–457. [Google Scholar]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Zhao, X.; Carey, E.E.; Iwamoto, T. Fertilizer source and high tunnel production environment affect antioxidant levels of Pac choi. HortScience 2006, 41, 1000–1001. [Google Scholar]
- Lila, M.A. The nature-versus-nurture debate on bioactive phytochemicals: The genome versus terroir. J. Sci. Food Agric. 2006, 86, 2510–2515. [Google Scholar] [CrossRef]
- Carsky, R.S.; Lwuafor, E.N.O. Contribution of Soil Fertility Research and Maintenance to Improve Maize Production and Productivity in Sub-Sahara Africa. In Proceedings of the Regional Maize Workshop on Strategy for Sustainable Maize Production in West and Central Africa; Badu-Aparku, B., Fakorede, M.A.B., Quedraogo, M., Quin, F.M., Eds.; IIAA-Cotonou, Benin Republic: 21–15 April 1997; pp. 3–20.
- Christin, H.C.; Dean, A.K.; David, E.K. Nitrogen concentration affects nutrient and carotenoid accumulation in parsley. J. Plant Nutr. 2005, 28, 285–297. [Google Scholar] [CrossRef]
- Biesiada, A.; Sokol-Letowska, A.; Kucharska, A. The effect of nitrogen fertilization on yielding and antioxidant activity of lavender (Lavandula angustifolia). Acta Sci. 2008, 7, 33–40. [Google Scholar]
- Liu, D.; Liu, W.; Zhu, D.; Geng, M.; Zhou, W.; Yang, T. Nitrogen effects on total flavonoids, chlorogenic acid and antioxidant activity of the medicinal plant Chrysanthemum morifolium. J. Plant Nutr. Soil Sci. 2010, 173, 268–274. [Google Scholar] [CrossRef]
- Zameer Khan, M.; Ehsan Akhtar, M.; Naeem Safdar, M.; Masd Mahmood, M.; Ahmad, S.; Ahmed, N. Effect of source and level of potash on yield and quality of potato tubers. Pak. J. Bot. 2010, 42, 3137–3145. [Google Scholar]
- Jeppsson, N. The effects of fertilizer rate on vegetative growth, yield and fruit quality, with special respect to pigments, in black chokeberry (Aronia melanocarpa) cv. ‘Viking’. Sci. Hortic. 2000, 83, 127–137. [Google Scholar]
- Woese, K.; Lange, D.; Boess, C.; Bögl, K.W. A comparison of organically and conventionally grown foods—Results of a review of the relevant literature. J. Sci. Food Agric. 1997, 74, 281–293. [Google Scholar]
- Shui, G.; Leong, L.P.; Wong, S.P. Rapid screening and characterization of antioxidants of Cosmos caudatus using liquid chromatography. J. Chromatogr. B 2005, 827, 127–138. [Google Scholar] [CrossRef]
- Ismail, S. Sayuran Tradisional Ulam dan Penyedap Rasa; Universiti Kebangsaan Malaysia: Bangi, Malaysia, 2000. [Google Scholar]
- Aires, A.; Rosa, E.; Carvalho, R. Effect of nitrogen and sulfur fertilization on glucosinates in the leaves and roots of broccoli sprouts (Brassica oleracea var italic). J. Sci. Food Agric. 2006, 86, 1512–1516. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Gerendas, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Mohebbi, M.; Maleki, A. Effect of water stress on some seed characteristics of isabgol (Plantago Ovata Forsk) in Zanjan (Iran). Adv. Environ. Biol. 2010, 4, 10–13. [Google Scholar]
- Tarozzi, A.; Hrelia, S.; Angeloni, C.; Morroni, F.; Biagi, P.; Guardigli, M.; Cantelli-Forti, G.; Hrelia, P. Antioxidant effectiveness of organically and non-organically grown red oranges in cell culture systems. Eur. J. Nutr. 2006, 45, 152–158. [Google Scholar] [CrossRef]
- Heaton, S. Organic Farming, Food Quality and Human Health: A Review of the Evidence; Soil Association: Bristol, UK, 2001; pp. 1–87. [Google Scholar]
- Barker, A.V. Plant Nutrients: Deficiency Symptoms in Laboratory, Problem set and Examination Manual; University of Massachusetts: Amherst, MA, USA, 1999. [Google Scholar]
- Eghball, B.; Power, J.F. Phousporus and nitrogen based manure and compost application corn production and soil phosphorus. Soil Sci. Soc. Am. J. 1999, 63, 895–901. [Google Scholar]
- Winchester, P.D.; Huskins, J.; Ying, J. Agrichemicals in surface water and birth defects in the United States. Acta Paediat. 2009, 98, 664–669. [Google Scholar] [CrossRef]
- Brown, J.R. Nitrate in Soils and Plants. 1993. Available online: http://extension.missouri.edu/p/G9804/ (accessed on 20 October 2011).
- Sowinska, K.A.; Uklanska, C.M. Effect of nitrogen fertilization on yield and quality of endive. Veg. Crops Res. Bull. 2009, 70, 193–201. [Google Scholar] [CrossRef]
- Benbrook, C.; Zhao, X.; Yáñez, J.; Davies, N.; Andrews, P. New Evidence Confirms the Nutritional Superiority of Plant-Based Organic Foods. State of Science Review. The Organic Center: Boulder, CO, USA, 2008. [Google Scholar]
- Rembialkowska, E. Quality of plant products from organic agriculture. J. Sci. Food Agric. 2007, 87, 2757–2762. [Google Scholar] [CrossRef]
- Leroy, B.M.M.; Bommele, L.; Reheul, D.; Moen, M.; de Neve, S. The application of vegetable, fruit and garden waste (VFG) compost in addition to cattle slurry in a silage maize monoculture: Effects on soil fauna and yield. Eur. J. Soil Biol. 2007, 43, 91–100. [Google Scholar] [CrossRef]
- Hunter, D.; Foster, M.; McArthur, J.O.; Ojha, R.; Petocz, P.; Samman, S. Evaluation of the micronutrient composition of plant foods produced by organic and conventional agricultural methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 571–582. [Google Scholar] [CrossRef]
- Howard, L.R.; Pandjaitan, N.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J. Agric. Food Chem. 2002, 50, 5891–5896. [Google Scholar] [CrossRef]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equation for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochem. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Zhang, T. Quick Nitrate Test for Hybrid Sudangrass and Pearl Millet Hays; Forage Management College: Stillwater, OK, USA, 1998; Volume 31, pp. 2127–2132. [Google Scholar]
- Ranggana, S. Manual of Analysis of Frutis and Vegetable Products; Tata McGraw-Hill Publishing Co. Ltd.: New Delhi, India, 1977. [Google Scholar]
- Kikuzaki, H.; Nakatani, N. Antioxidant effect of some ginger constituents. J. Food Sci. 1993, 587, 1407–1410. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hassan, S.A.; Mijin, S.; Yusoff, U.K.; Ding, P.; Wahab, P.E.M. Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of Cosmos caudatus in Response to Organic and Mineral-Based Fertilizer Rates. Molecules 2012, 17, 7843-7853. https://doi.org/10.3390/molecules17077843
Hassan SA, Mijin S, Yusoff UK, Ding P, Wahab PEM. Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of Cosmos caudatus in Response to Organic and Mineral-Based Fertilizer Rates. Molecules. 2012; 17(7):7843-7853. https://doi.org/10.3390/molecules17077843
Chicago/Turabian StyleHassan, Siti Aishah, Salumiah Mijin, Umi Kalsom Yusoff, Phebe Ding, and Puteri Edaroyati Megat Wahab. 2012. "Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of Cosmos caudatus in Response to Organic and Mineral-Based Fertilizer Rates" Molecules 17, no. 7: 7843-7853. https://doi.org/10.3390/molecules17077843
APA StyleHassan, S. A., Mijin, S., Yusoff, U. K., Ding, P., & Wahab, P. E. M. (2012). Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of Cosmos caudatus in Response to Organic and Mineral-Based Fertilizer Rates. Molecules, 17(7), 7843-7853. https://doi.org/10.3390/molecules17077843




