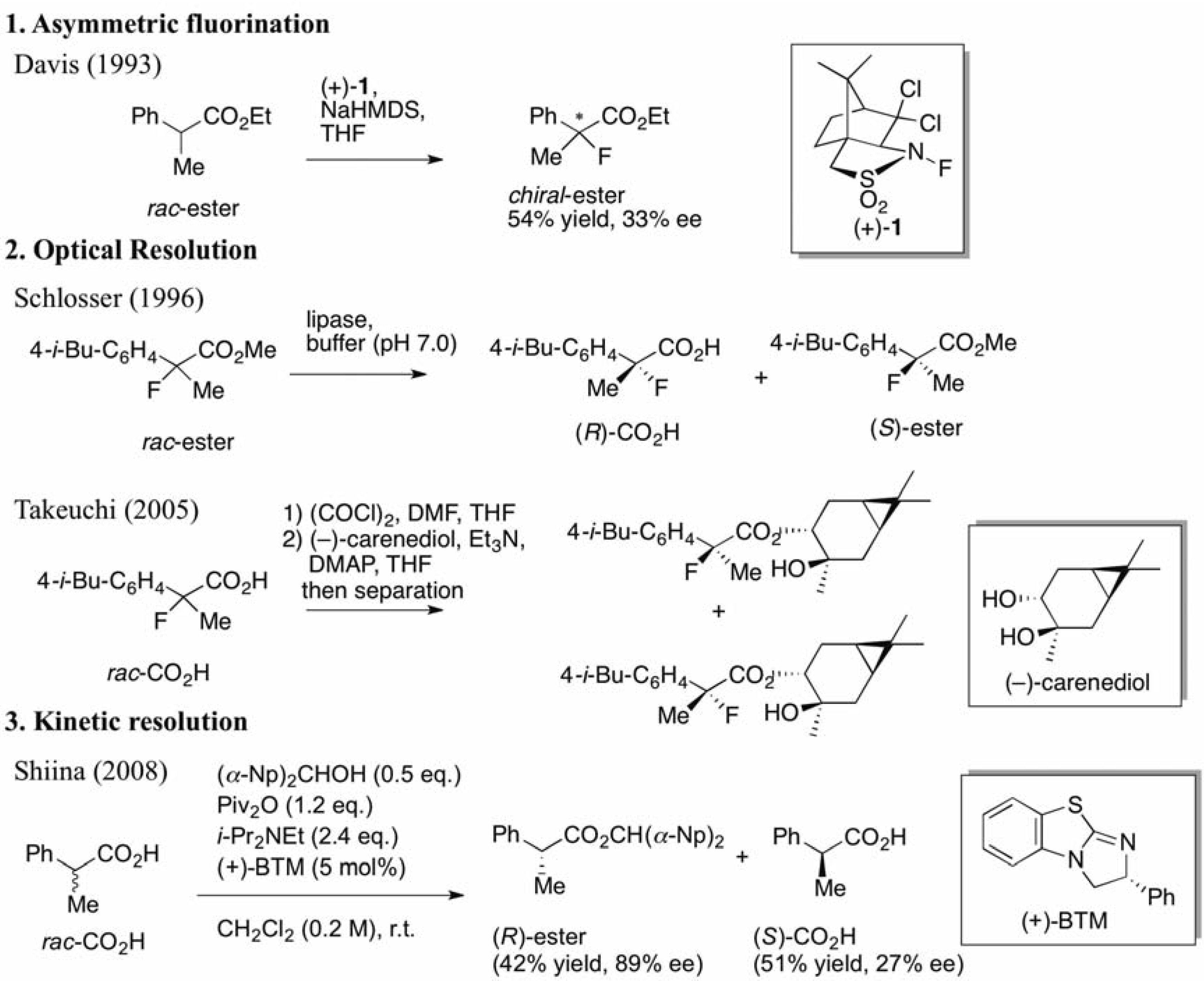
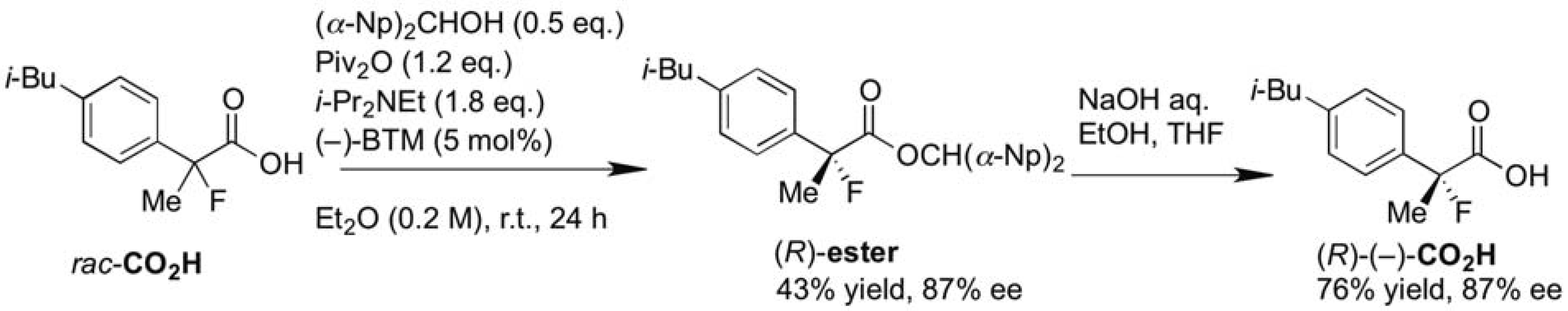
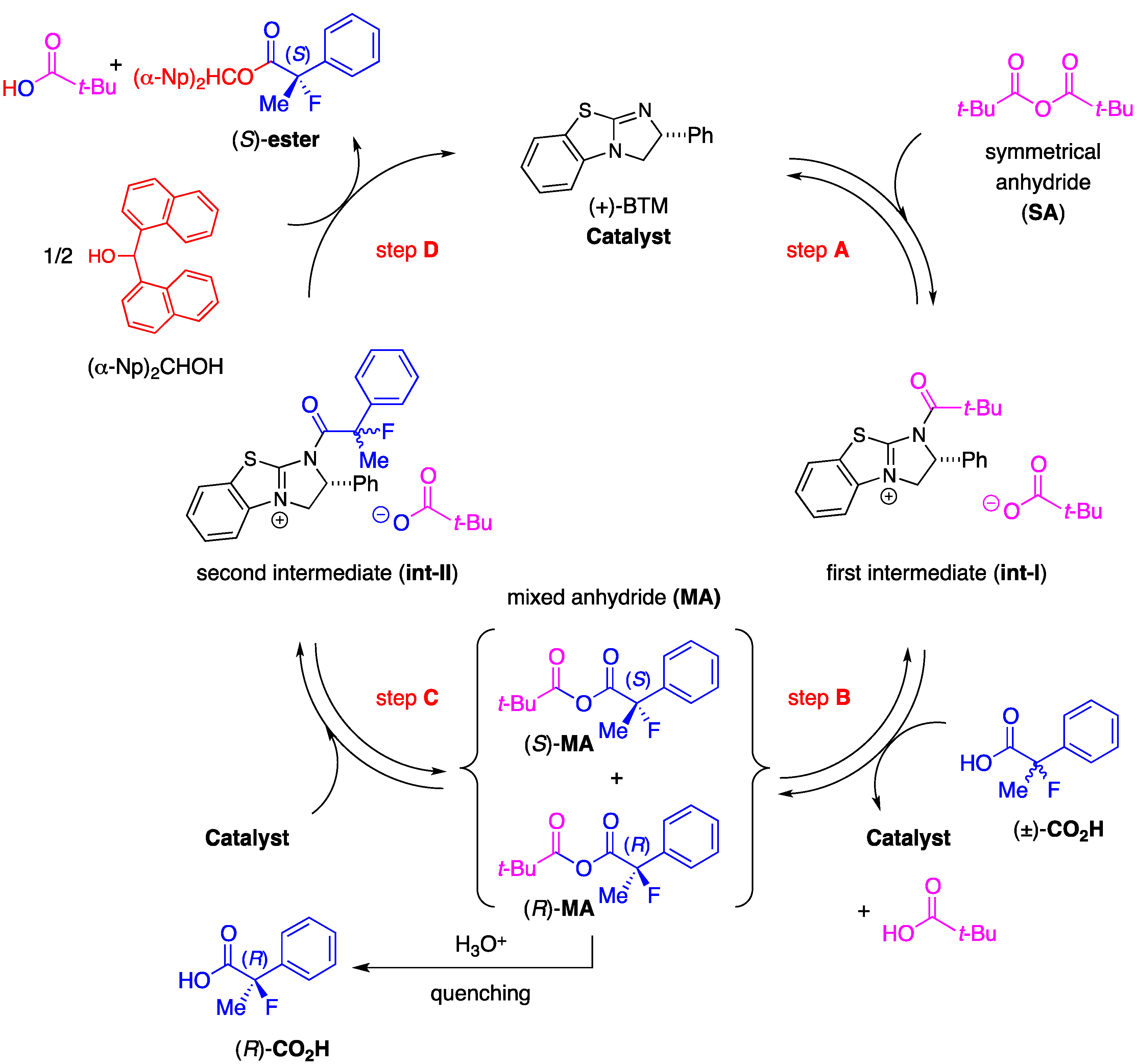
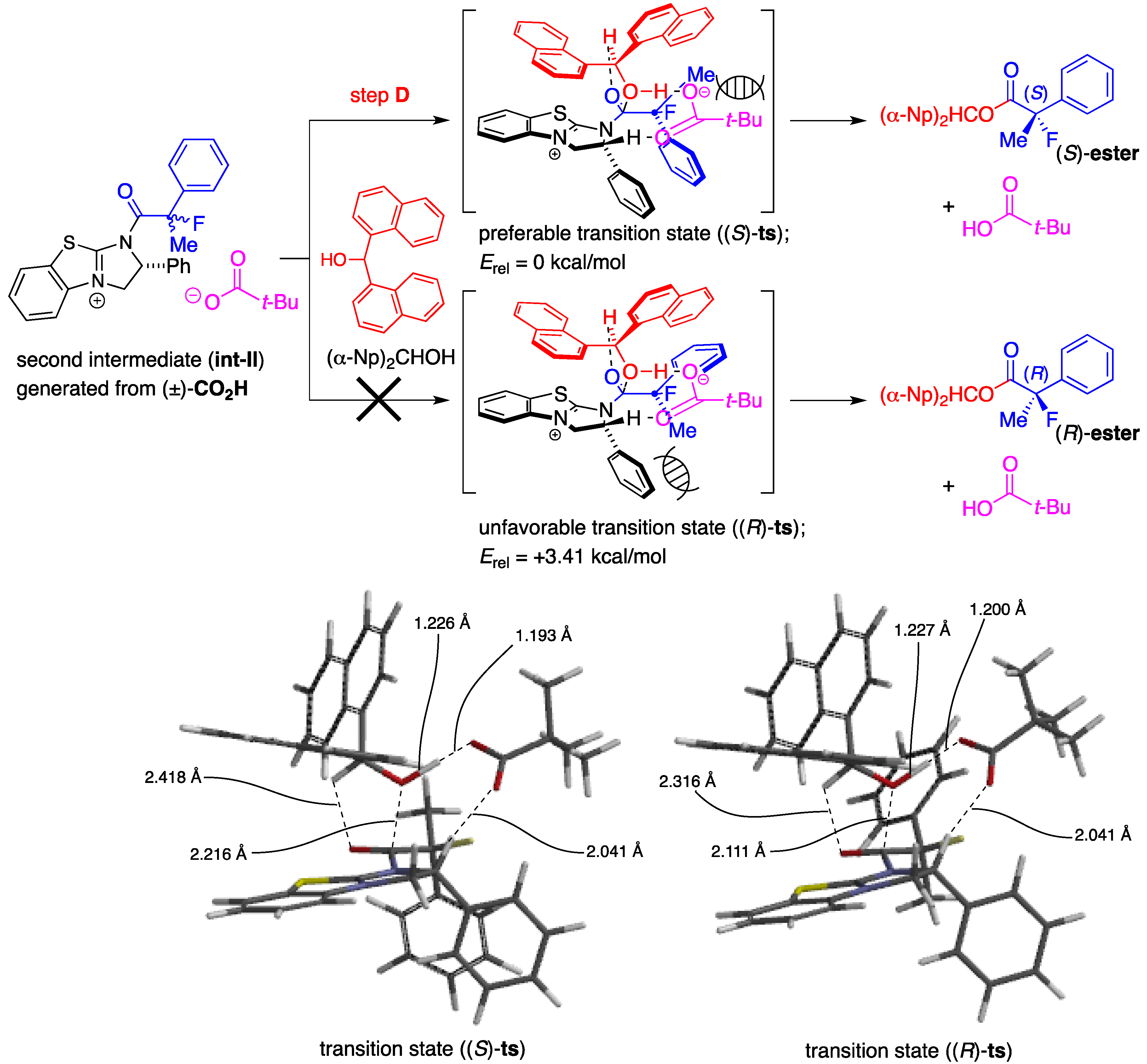
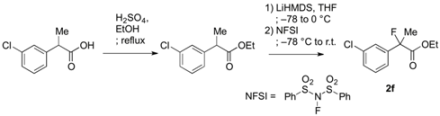
3.2. General Procedure for the Preparation of Racemic Ethyl 2-Fluoro-2-arylpropanoates 2a–k
To a solution of 2-(3-chlorophenyl)propanoic acid {prepared from 2-(3-chlorophenyl)acetic acid according to [
18] and [
22]} (920 mg, 4.98 mmol) in ethanol (10 mL) at 0 °C was added sulfuric acid (3 mL). The reaction mixture was stirred at 0 °C for 5 min, and then refluxed for 7 h. After cooling to room temperature, the reaction mixture was diluted with water (30 mL) and extracted with ethyl acetate. The organic layer was washed with saturated aqueous NaHCO
3 and brine, and then dried over sodium sulfate. After filtration of the mixture and evaporation of the solvent, ethyl 2-(3-chlorophenyl)propanoate (994 mg, 94% yield) was obtained as a pale yellow oil. This crude product was used in the next reaction without further purification.
To a stirred solution of ethyl 2-(3-chlorophenyl)propanoate (987 mg, 4.74 mmol) in THF (10 mL) at −78 °C was added LHMDS in THF (1.0 M, 5.69 mL, 5.69 mmol). The mixture was stirred at −78 °C for 20 min, and then at 0 °C for 20 min. After cooling to −78 °C, N-fluorobenzenesulfonimide (NFSI) (1.87 g, 5.93 mmol) in THF (10 mL) was added to the reaction mixture. After gradually raised to room temperature for 8 h, the reaction mixture was diluted with 1 M hydrochloric acid (10 mL) and water (20 mL). The mixture was extracted with hexane, and the organic layer was washed with water, and then dried over sodium sulfate. After filtration of the mixture and evaporation of the solvent, the crude product was purified by silica gel column chromatography (ethyl acetate/hexane = 1/30) to afford 2f (983 mg, 92% yield) as a colorless oil. If the fluorination was not sufficient and any precursor remained in the product, the fluorination process as described above was repeated.
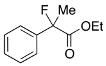
Ethyl 2-Fluoro-2-phenylpropanoate (2a) [4,7]
IR (neat): 1,754, 1,496, 1,123, 732, 696 cm−1; 1H-NMR (CDCl3): δ 7.53–7.48 (m, 2H, Ar), 7.42–7.29 (m, 3H, Ar), 4.24 (q, JH-H = 7.2 Hz, 2H, OEt), 1.93 (d, JH-F = 22.4 Hz, 3H, Me), 1.26 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 170.9 (d, JC-F = 26.4 Hz, 1), 139.3 (d, JC-F = 21.9 Hz), 128.5 (d, JC-F = 1.4Hz), 128.4, 124.6 (d, JC-F = 8.1 Hz), 94.6 (d, JC-F = 186.3 Hz, 2), 61.9 (Et), 24.8 (d, JC-F = 23.5 Hz, 3), 14.0 (Et); HR MS: calcd for C11H13FO2Na (M+Na+) 219.0788, found 210.0792.
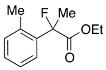
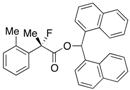
Ethyl 2-Fluoro-2-(o-tolyl)propanoate (2b)
IR (neat): 1,751, 1,491, 1,117, 741 cm−1; 1H-NMR (CDCl3): δ 7.44–7.37 (m, 1H, Ar), 7.29–7.12 (m, 3H, Ar), 4.24 (q, JH-H = 7.2 Hz, 2H, OEt), 2.37 (s, 3H, o-Me), 1.98 (d, JH-F = 22.4 Hz, 3H, 2-Me), 1.25 (t, JH-H = 7.2 Hz, 3H, OEt); >13C-NMR (CDCl3): δ 171.5 (d, JC-F = 26.5 Hz, 1), 136.7 (d, JC-F = 20.5 Hz), 136.7, 131.9, 128.9 (d, JC-F = 1.4 Hz), 125.9 (d, JC-F = 7.3 Hz), 125.8, 95.0 (d, JC-F = 183.3 Hz, 2), 61.9 (Et), 24.1 (d, JC-F = 25.0 Hz, 3), 20.2 (d, JC-F = 5.1 Hz, o-Me), 14.0 (Et); HR MS: calcd for C12H15FO2Na (M+Na+) 233.0948, found 233.0944.
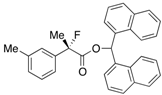
Ethyl 2-Fluoro-2-(m-tolyl)propanoate (2c)
IR (neat): 1,755, 1,608, 1,489, 1,122, 852, 731, 697 cm−1; 1H-NMR (CDCl3): δ 7.34–7.22 (m, 3H, Ar), 7.20–7.12 (m, 1H, Ar), 4.22 (q, JH-H = 7.2 Hz, 2H, OEt), 2.37 (s, 3H, m-Me), 1.91 (d, JH-F = 22.0 Hz, 3H, 2-Me), 1.26 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 71.0 (d, JC-F = 27.2 Hz, 1), 139.3 (d, JC-F = 22.7 Hz), 138.2, 129.3 (d, JC-F = 1.4 Hz), 128.3, 125.2 (d, JC-F = 8.9 Hz), 121.6 (d, JC-F = 8.2 Hz), 94.7 (d, JC-F = 186.4 Hz, 2), 61.9 (Et), 24.8 (d, JC-F = 24.8 Hz, 3), 21.5 (m-Me), 14.0 (Et); HR MS: calcd for C12H15FO2Na (M+Na+) 233.0948, found 233.0941.
Ethyl 2-Fluoro-2-(p-tolyl)propanoate (2d) [7]
IR (neat): 1,754, 1,616, 1,514, 1,114, 818, 739 cm−1; 1H-NMR (CDCl3): δ 7.41–7.33 (m, 2H, Ar), 7.21–7.09 (m, 2H, Ar), 4.21 (q, JH-H = 7.2 Hz, 2H, OEt), 2.35 (s, 3H, p-Me), 1.91 (d, JH-F = 22.4 Hz, 3H, 2-Me), 1.25 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 171.0 (d, JC-F = 27.2 Hz, 1), 138.4 (JC-F = 1.4 Hz), 136.4 (JC-F = 22.7 Hz), 129.1, 124.6 (JC-F = 8.2 Hz), 94.6 (JC-F = 185.7 Hz, 2), 61.9 (Et), 24.7 (JC-F = 24.2 Hz, 3), 21.1 (p-Me), 14.0 (Et); HR MS: calcd for C12H15FO2Na (M+Na+) 233.0948, found 233.0937.
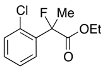
Ethyl 2-(2-Chlorophenyl)-2-fluoropropanoate (2e)
IR (neat): 1,747, 1,596, 1,474, 1,125, 750 cm−1; 1H-NMR (CDCl3): δ 7.63–7.58 (m, 1H, Ar), 7.41–7.27 (m, 3H, Ar), 4.33–4.20 (m, 2H, OEt), 1.96 (d, JH-F = 23.6 Hz, 3H, 2-Me), 1.26 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 69.5 (d, JC-F = 2 5.0 Hz, 1), 137.4 (d, JC-F = 22.0 Hz), 130.5, 131.5 (d, JC-F = 3.7 Hz), 130.5, 129.9 (d, JC-F = 1.5 Hz), 126.7 (d, JC-F = 12.5 Hz), 94.4 (d, JC-F = 180.4 Hz, 2), 62.2 (Et), 23.1 (d, JC-F = 25.0 Hz, 3), 13.9 (Et); HR MS: calcd for C11H12ClFO2Na (M+Na+) 253.0402, found 253.0405.
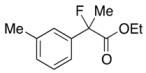
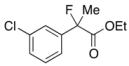
Ethyl 2-(3-Chlorophenyl)-2-fluoropropanoate (2f)
IR (neat): 1,756, 1,598, 1,477, 1,125, 792, 712 cm−1; 1H-NMR (CDCl3): δ 7.52–7.49 (m, 1H, Ar), 7.41–7.28 (m, 3H, Ar), 4.23 (q, JH-H = 7.2 Hz, 2H, OEt), 1.91 (d, JH-F = 22.4 Hz, 3H, 2-Me), 1.27 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 70.3 (d, JC-F = 26.4 Hz, 1), 141.3 (d, JC-F = 22.7 Hz), 134.5 (d, JC-F = 1.4 Hz), 129.8, 128.8, 125.0 (d, JC-F = 9.5 Hz), 122.8 (d, JC-F = 8.2 Hz), 94.1 (d, JC-F = 188.5 Hz, 2), 62.2 (Et), 24.9 (d, JC-F = 24.3 Hz, 3), 13.7 (Et); HR MS: calcd for C11H12ClFO2Na (M+Na+) 253.0402, found 253.0404.
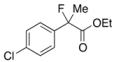
Ethyl 2-(4-Chlorophenyl)-2-fluoropropanoate (2g) [4,10]
IR (neat): 1,755, 1,600, 1,492, 1,125, 1,092, 833, 761 cm−1; 1H-NMR (CDCl3): δ 7.47–7.41 (m, 2H, Ar), 7.39–7.32 (m, 2H, Ar), 4.22 (q, JH-H = 7.2 Hz, 2H, OEt), 1.91 (d, JH-F = 22.0 Hz, 3H, 2-Me), 1.26 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 70.5 (d, JC-F = 26.5 Hz, 1), 137.9 (d, JC-F = 23.4 Hz), 134.7 (d, JC-F = 2.2Hz), 128.6, 126.1 (d, JC-F = 8.8 Hz), 94.2 (d, JC-F = 187.1 Hz, 2), 62.1 (Et), 24.8 (d, JC-F = 24.2 Hz, 3), 14.0 (Et); HR MS: calcd for C11H12ClFO2Na (M+Na+) 253.0402, found 253.0410.
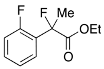
Ethyl 2-Fluoro-2-(2-fluorophenyl)propanoate (2h)
IR (neat): 1,747, 1,617, 1,586, 1,492, 1,121, 1,100, 754 cm−1; 1H-NMR (CDCl3): δ 7.56–7.49 (m, 1H, Ar), 7.39–7.32 (m, 1H, Ar), 7.22–7.15 (m, 1H, Ar), 7.11–7.01 (m, 1H, Ar), 4.19–4.32 (m, 2H, OEt), 1.96 (d, JH-F = 22.8 Hz, 3H, 2-Me), 1.26 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 169.9 (d, JC-F = 26.4 Hz, 3), 159.6 (dd, JC-F = 249.5, 4.4 Hz), 130.7 (dd, JC-F = 8.1, 1.5 Hz), 126.9 (d, JC-F = 12.5 Hz), 126.7 (dd, JC-F = 9.9, 3.3 Hz), 124.1 (d, JC-F = 3.0 Hz), 116.1 (d, JC-F = 22.0 Hz), 92.5 (d, JC-F = 182.6 Hz, 2), 62.1 (Et), 23.4 (dd, JC-F = 24.6, 2.6 Hz, 1), 13.9 (Et); HR MS: calcd for C11H12F2O2Na (M+Na+) 237.0698, found 237.0706.
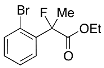
Ethyl 2-(2-Bromophenyl)-2-fluoropropanoate (2i)
IR (neat): 1,746, 1,592, 1,471, 1,124, 748, 638 cm−1; 1H-NMR (CDCl3): δ 7.64–7.55 (m, 2H, Ar), 7.42–7.34 (m, 1H, Ar), 7.25–7.18 (m, 1H, Ar), 4.27 (q, JH-H = 7.2 Hz, 2H, OEt), 1.98 (d, JH-F = 23.6 Hz, 3H, 2-Me), 1.27 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 169.3 (d, JC-F = 24.9 Hz, 1), 139.0 (d, JC-F = 21.2 Hz), 134.0, 130.0 (d, JC-F = 1.5 Hz), 127.4 (d, JC-F = 1.5 Hz), 127.0 (d, JC-F = 13.2 Hz), 120.6 (d, JC-F = 3.6 Hz), 95.2 (d, JC-F = 180.5 Hz, 2), 62.2 (Et), 23.3 (d, JC-F = 25.0 Hz, 3), 13.9 (Et); HR MS: calcd for C11H12BrFO2Na (M+Na+) 296.9897, found 296.9893.
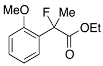
Ethyl 2-Fluoro-2-(2-methoxyphenyl)propanoate (2j)
IR (neat): 1,746, 1,604, 1,589, 1,492, 1,116, 752 cm−1; 1H-NMR (CDCl3): δ7.52–7.46 (m, 1H, Ar), 7.38–7.30 (m, 1H, Ar), 7.05–6.96 (m, 1H, Ar), 6.92–6.86 (m, 1H, Ar), 4.28–4.18 (m, 2H, OEt), 3.79 (s, 3H, OMe), 1.89 (d, JH-F = 22.8 Hz, 3H, 2-Me), 1.24 (t, JH-H = 7.2 Hz, 3H, OEt); 13C-NMR (CDCl3): δ 170.8 (d, JC-F = 25.0 Hz, 1), 156.1 (d, JC-F = 4.4 Hz), 130.1 (d, JC-F = 2.2 Hz), 128.1 (d, JC-F = 21.2 Hz), 125.9 (d, JC-F = 9.6 Hz), 120.6, 111.1, 93.1 (d, JC-F = 179.7 Hz, 2), 61.4 (Et), 55.4 (OMe), 23.0 (d, JC-F = 25.0 Hz, 3), 14.0 (Et); HR MS: calcd for C12H15FO3Na (M+Na+) 249.0897, found 249.0907.
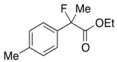
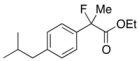
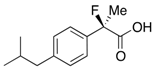
Ethyl 2-Fluoro-2-(4-isobutylphenyl)propanoate (2k) [4,6,7,10]
IR (neat): 1,756, 1,614, 1,512, 1,116, 849 cm−1; 1H-NMR (CDCl3): δ 7.45–7.35 (m, 2H, Ar), 7.17–7.10 (m, 2H, Ar), 4.22 (q, JH-H = 7.2 Hz, 2H, OEt), 2.47 (d, JH-H = 7.2 Hz, 2H, i-Bu), 2.00 (d, JH-F = 22.4 Hz, 3H, 2-Me), 1.92-1.78 (m, 1H, i-Bu), 1.26 (t, JH-H = 7.2 Hz, 3H, OEt), 0.90 (d, JH-H = 6.8 Hz, 6H, i-Bu); 13C-NMR (CDCl3): δ 171.1 (d, JC-F = 27.2 Hz, 1), 142.2 (d, JC-F = 1.4 Hz), 136.6 (d, JC-F = 22.7 Hz), 129.1, 124.4 (d, JC-F = 8.1 Hz), 94.6 (d, JC-F = 185.6 Hz, 2), 61.9 (Et), 45.0 (i-Bu), 30.1 (i-Bu), 24.7 (d, JC-F = 24.3 Hz, 3), 22.3 (i-Bu), 14.0 (Et); HR MS: calcd for C15H21FO2Na (M+Na+) 275.1418, found 275.1410.
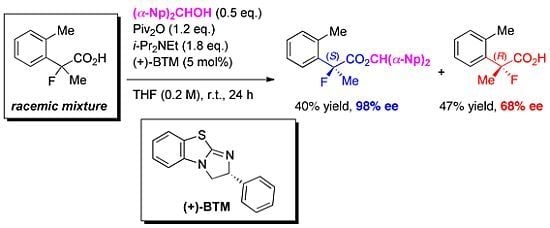
3.4. Procedure for Kinetic Resolution of Racemic 2-Fluoro-2-arylpropanoic Acid
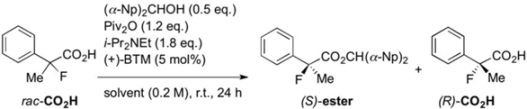
To a mixture of 2-(3-chlorophenyl)-2-fluoropropanoic acid (3f) (40.5 mg, 0.200 mmol), pivalic anhydride (48.7 μL, 0.240 mmol), and bis(α-naphthyl)methanol (28.4 mg, 0.100 mmol) in diethyl ether (1.0 mL) at room temperature were successively added diisopropylethylamine (62.7 μL, 0.360 μmol) and (+)-BTM (2.5 mg, 10 μmol). The reaction mixture was stirred for 24 h at room temperature, and then quenched with 1 M hydrochloric acid. The mixture was extracted with ethyl acetate, and the organic layer was dried over sodium sulfate. After filtration of the mixture and evaporation of the solvent, the crude product was purified by preparative thin layer chromatography on silica (toluene/hexane = 90/10) to afford the corresponding ester (S)-4f (38.6 mg, 40% yield, 76% ee) as a colorless oil. The polar fraction including 3f was further purified by preparative thin layer chromatography on silica (ethyl acetate/hexane/formic acid = 10/40/1) to afford the recovered optically active (R)-3f (20.4 mg, 50% yield, 54% ee) as a white solid.
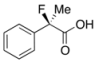
2-Fluoro-2-phenylpropanoic Acid ((R)-3a) [4,8,13] [Table 1, Entry 4, and Table 2, Entry 1, 70% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/10/0.01, flow rate = 1.0 mL/min): tR = 11.7 min (15.0%), tR = 13.9 min (85.0%); 1H-NMR (CDCl3): δ 10.47 (br s, 1H, CO2H), 7.62–7.28 (m, 5H, Ar), 1.96 (d, JH-F = 22.4 Hz, 3H, Me); 13C-NMR (CDCl3): δ 176.6 (d, JC-F = 28.7 Hz, 1), 138.3 (d, JC-F = 21.9 Hz), 128.9 (d, JC-F = 1.4 Hz), 128.6, 124.7 (d, JC-F = 8.8 Hz), 94.1 (d, JC-F = 186.4 Hz, 2), 24.4 (d, JC-F = 23.4 Hz, 3); HR MS: calcd for C9H9FO2Na (M+Na+) 191.0479, found 191.0479. Analytical data on racemic compound: Mp: 54-56 °C (hexane); IR (KBr): 2,939, 1,716, 1,601, 1,496, 1,146, 727, 697 cm−1.
2-Fluoro-2-(o-tolyl)propanoic Acid ((R)-3b/) [Table 2, Entry 2, 68% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/10/0.01, flow rate = 0.75 mL/min): tR = 14.4 min (83.9%), tR = 18.2 min (16.1%); 1H-NMR (CDCl3): δ 9.56 (br s, 1H, CO2H), 7.46–7.16 (m, 4H, Ar), 2.42 (d, JH-F = 3.2 Hz, 3H, o-Me), 2.04 (d, JH-F = 22.4 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 176.6 (d, JC-F = 27.7 Hz, 1), 137.0, 135.6 (d, JC-F = 20.6 Hz), 132.1 (d, JC-F = 1.4 Hz), 129.4 (d, JC-F = 1.4 Hz), 126.2 (d, JC-F = 6.5 Hz), 125.9, 94.6 (d, JC-F = 184.2 Hz, 2), 24.0 (d, JC-F = 25.0 Hz, 3), 20.4 (d, JC-F = 5.8 Hz, o-Me); HR MS: calcd for C10H10FO2 (M−H+) 181.0665, found 181.0663. Analytical data on racemic compound: Mp: 105–106 °C (CH2Cl2/hexane); IR (KBr): 2,926, 1,733, 1,605, 1,492, 1,126, 729 cm−1.
2-Fluoro-2-(m-tolyl)propanoic Acid ((R)-3c) [Table 2, Entry 3, 61% ee]
HPLC (CHIRALPAK AS-H, i-PrOH/hexane/TFA = 1/50/0.05, flow rate = 0.75 mL/min): tR = 19.3 min (80.5%), tR = 23.2 min (19.5%); 1H-NMR (CDCl3): δ 9.46 (br s, 1H, CO2H), 7.38–7.14 (m, 4H, Ar), 2.37 (s, 3H, m-Me) 1.95 (d, JH-F = 22.4 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 76.3 (d, JC-F = 27.9 Hz, 1), 138.4, 138.2 (d, JC-F = 22.7 Hz), 129.7 (d, JC-F = 1.5 Hz), 128.5, 125.3 (d, JC-F = 8.1 Hz), 121.8 (d, JC-F = 8.1 Hz), 94.2 (d, JC-F = 186.3 Hz, 2), 24.4 (d, JC-F = 24.3 Hz, 3), 21.5 (m-Me); HR MS: calcd for C10H10FO2Na (M+Na+) 205.0635, found 205.0629. Analytical data on racemic compound: Mp: 36–39 °C (hexane); IR (KBr): 2,919, 1,713, 1,609, 1,488, 1,129, 827, 721, 697 cm−1.
2-Fluoro-2-(p-tolyl)propanoic Acid ((R)-3d) [Table 2, Entry 5, 78% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/10/0.01, flow rate = 0.75 mL/min): tR = 16.3 min (10.9%), tR = 21.2 min (89.1%); 1H-NMR (CDCl3): δ 8.42 (br s, 1H, CO2H), 7.47–7.32 (m, 2H, Ar), 7.23–7.13 (m, 2H, Ar), 2.35 (s, 3H, p-Me), 1.90 (d, JH-F = 22.0 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 176.2 (d, JC-F = 28.0 Hz, 1), 138.9 (JC-F = 1.4 Hz), 135.4 (JC-F = 22.7 Hz), 129.3, 124.7 (JC-F = 8.1 Hz), 94.2 (JC-F = 186.4 Hz, 2), 24.3 (JC-F = 23.4 Hz, 3), 21.1 (p-Me); HR MS: calcd for C10H10FO2Na (M+Na+) 205.0635, found 205.0628. Analytical data on racemic compound: Mp: 68–71 °C (hexane); IR (KBr): 2,997, 1,711, 1,613, 1,513, 1,114, 818, 731 cm−1.
2-(2-Chlorophenyl)-2-fluoropropanoic Acid ((R)-3e) [Table 2, Entry 6, 43% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/10/0.01, flow rate = 0.75 mL/min): tR = 15.8 min (71.4%), tR = 17.5 min (28.6%); 1H-NMR (CDCl3): δ 9.65 (br s, 1H, CO2H), 7.64–7.56 (m, 1H, Ar), 7.45–7.29 (m, 3H, Ar), 2.02 (d, JH-F = 23.2 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 75.2 (d, JC-F = 23.2 Hz, 1), 136.2 (d, JC-F = 21.2 Hz), 132.1 (d, JC-F = 3.7 Hz), 130.7, 130.4 (d, JC-F = 1.4 Hz), 127.0 (d, JC-F = 10.3 Hz), 127.0, 93.9 (d, JC-F = 181.9 Hz, 2), 23.0 (d, JC-F = 23.0 Hz, 3); HR MS: calcd for C9H7ClFO2 (M–H+) 201.0119, found 201.0114. Analytical data on racemic compound: Mp: 114–116 °C (CH2Cl2/hexane); IR (KBr): 2,998, 1,712, 1,594, 1,475, 1,137, 748 cm−1.
2-(3-Chlorophenyl)-2-fluoropropanoic Acid ((R)-3f) [Table 2, Entry 8, 54% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/10/0.01, flow rate = 0.75 mL/min): tR = 8.7 min (22.9%), tR = 10.1 min (77.1%); 1H-NMR (CDCl3): δ 8.84 (br s, 1H, CO2H), 7.58–7.49 (m, 1H, Ar), 7.48–7.30 (m, 3H, Ar), 1.95 (d, JH-F = 22.0 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 75.2 (d, JC-F = 27.9 Hz, 1), 140.2 (d, JC-F = 22.7 Hz), 134.7 (d, JC-F = 1.4 Hz), 129.9, 129.2, 125.1 (d, JC-F = 9.6 Hz), 122.9 (d, JC-F = 8.8 Hz), 93.7 (d, JC-F = 187.8 Hz, 2), 24.6 (d, JC-F = 23.4 Hz, 3); HR MS: calcd for C9H7ClFO2 (M–H+) 201.0119, found 201.0116. Analytical data on racemic compound: Mp: 64–66 °C (CH2Cl2/hexane); IR (KBr): 2,986, 1,714, 1,597, 1,479, 1,146, 797, 702 cm−1.
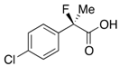
2-(4-Chlorophenyl)-2-fluoropropanoic Acid ((R)-3g) [4] [Table 2, Entry 9, 74% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/50/0.05, flow rate = 0.75 mL/min): tR = 33.6 min (13.0%), tR = 37.1 min (87.0%); 1H-NMR (CDCl3): δ 9.16 (br s, 1H, CO2H), 7.50–7.43 (m, 2H, Ar), 7.42–7.34 (m, 2H, Ar), 1.95 (d, JH-F = 22.4 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 75.4 (d, JC-F = 27.9 Hz, 1), 136.8 (d, JC-F = 22.7 Hz), 135.2 (d, JC-F = 1.5 Hz), 128.8, 126.2 (d, JC-F = 8.9 Hz), 93.9 (d, JC-F = 187.1 Hz, 2), 24.5 (d, JC-F = 23.4 Hz, 3); HR MS: calcd for C9H7ClFO2 (M–H+) 201.0119, found 201.0117. Analytical data on racemic compound: Mp: 90–92 °C (CH2Cl2/hexane); IR (KBr): 2,995, 1,722, 1,599, 1,493, 1,127, 1,098, 830, 761 cm−1.
2-Fluoro-2-(2-fluorophenyl)propanoic Acid ((R)-3h) [Table 2, Entry 10, 37% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/50/0.05, flow rate = 0.75 mL/min): tR = 64.9 min (68.5%), tR = 81.5 min (31.5%); 1H-NMR (CDCl3): δ 9.48 (br s, 1H, CO2H), 7.59–7.49 (m, 1H, Ar), 7.43–7.34 (m, 1H, Ar), 7.23–7.16 (m, 1H, Ar), 7.15–7.05 (m, 1H, Ar), 2.02 (d, JH-F = 22.8 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 175.3 (d, JC-F = 27.2 Hz, 1), 159.8 (dd, JC-F = 249.5, 4.4 Hz), 131.2 (dd, JC-F = 8.8, 1.5 Hz), 126.9 (dd, JC-F = 9.2, 3.3 Hz), 125.6 (dd, JC-F = 22.7, 12.5 Hz), 124.2 (d, JC-F = 3.6 Hz), 116.3 (d, JC-F = 21.9 Hz), 92.1 (d, JC-F = 184.2 Hz, 2), 23.0 (dd, JC-F = 24.2, 2.9 Hz, 3); HR MS: calcd for C9H7F2O2 (M−H+) 185.0414, found 185.0412. Analytical data on racemic compound: Mp: 116–117 °C (CH2Cl2/hexane); IR (KBr): 2,984, 1,734, 1,618, 1,587, 1,510, 1,493, 1,124, 1,101, 815, 762, 749 cm−1.
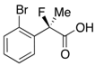
2-(2-Bromophenyl)-2-fluoropropanoic Acid ((R)-3i) [Table 2, Entry 11, 51% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/10/0.01, flow rate = 0.75 mL/min): tR = 18.6 min (75.6%), tR = 22.3 min (24.4%); 1H-NMR (CDCl3): δ 9.73 (br s, 1H, CO2H), 7.67–7.55 (m, 2H, Ar), 7.43–7.34 (m, 1H, Ar), 7.30–7.19 (m, 1H, Ar), 2.04 (d, JH-F = 23.6 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 174.7 (d, JC-F = 25.0 Hz, 1), 137.7 (d, JC-F = 20.5 Hz), 134.3, 130.5 (d, JC-F = 1.4 Hz), 127.5, 127.4 (d, JC-F = 11.0 Hz), 121.2 (d, JC-F = 2.9 Hz), 94.8 (d, JC-F = 181.9 Hz, 2), 23.2 (d, JC-F = 25.0 Hz, 3); HR MS: calcd for C9H7BrFO2 (M−H+) 244.9613, found 244.9612. Analytical data on racemic compound: Mp: 95–96 °C (CH2Cl2/hexane); IR (KBr): 3,000, 1,723, 1,591, 1,572, 1,474, 1,132, 760, 745, 641 cm−1.
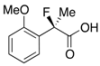
2-Fluoro-2-(2-methoxyphenyl)propanoic Acid ((R)-3j) [Table 2, Entry 12, 20% ee]
HPLC (CHIRALPAK OJ-H, i-PrOH/hexane/TFA = 1/50/0.05, flow rate = 0.75 mL/min): tR = 48.9 min (59.8%), tR = 54.5 min (40.2%); 1H-NMR (CDCl3): δ 9.23 (br s, 1H, CO2H), 7.53–7.47 (m, 1H, Ar), 7.45–7.31 (m, 1H, Ar), 7.11–6.98 (m, 1H, Ar), 7.98–6.84 (m, 1H, Ar), 3.82 (s, 3H, OMe), 1.96 (d, JH-F = 22.4 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 175.8 (d, JC-F = 26.4 Hz, 1), 156.6 (d, JC-F = 3.0 Hz), 130.8 (d, JC-F = 2.2 Hz), 126.9 (d, JC-F = 20.5 Hz), 120.8, 111.7, 92.8 (d, JC-F = 181.2 Hz, 2), 55.6 (OMe), 22.7 (d, JC-F = 25.0 Hz, 3); HR MS: calcd for C10H10FO3 (M−H+) 197.0614, found 197.0605. Analytical data on racemic compound: Mp: 83–84 °C (CH2Cl2/hexane); IR (KBr): 2,986, 1,721, 1,604, 1,587, 1,495, 1,125, 754 cm−1.
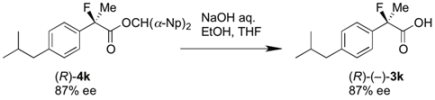
2-Fluoro-2-(4-isobutylphenyl)propanoic Acid ((R)-3k) [4,5,8,9,11,12,13] [Table 2, Entry 13, 63% ee]
HPLC (CHIRALPAK AS-H, i-PrOH/hexane/TFA = 1/50/0.05, flow rate = 0.75 mL/min): tR = 15.1 min (81.5%), tR = 17.9 min (18.5%); 1H-NMR (CDCl3): δ 9.83 (br s, 1H, CO2H), 9.44–9.39 (m, 2H, Ar), 9.19–9.13 (m, 2H, Ar), 2.47 (d, JH-H = 7.2 Hz, 2H, i-Bu), 1.95 (d, JH-F = 22.0 Hz, 3H, 2-Me), 1.91–1.80 (m, 1H, i-Bu), 0.90 (d, JH-H = 6.8 Hz, 6H, i-Bu); 13C-NMR (CDCl3): δ 176.0 (d, JC-F = 27.9 Hz, 1), 142.7, 135.6 (d, JC-F = 22.7 Hz), 129.3, 124.5 (d, JC-F = 8.1 Hz), 94.4 (d, JC-F = 185.7 Hz, 2), 45.0 (i-Bu), 30.1 (i-Bu), 24.4 (d, JC-F = 23.4 Hz, 3), 22.3 (i-Bu); HR MS: calcd for C13H17FO2Na (M+Na+) 247.1105, found 247.1105. Analytical data on racemic compound: Mp: 71–72 °C (CH2Cl2/hexane); IR (KBr): 2,970, 1,717, 1,612, 1,509, 1,112, 849 cm−1.
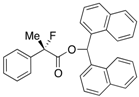
Di(naphthalen-1-yl)methyl 2-Fluoro-2-phenylpropanoate ((Di(naphthalen-1-yl)methyl 2-Fluoro-2-phenylpropanoate)-4a) [Table 1, Entry 4, and Table 2, Entry 1, 87% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 1.0 mL/min): tR = 12.7 min (6.3%), tR = 21.0 min (93.7%); 1H-NMR (CDCl3): δ 8.37 (s, 1H, 1'-H), 7.93–7.67 (m, 6H, Ar), 7.52–7.01 (m, 13H, Ar), 1.94 (d, JH-F = 22.8 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 169.9 (d, JC-F = 27.9 Hz, 1), 138.6 (d, JC-F = 22.7 Hz), 134.0, 133.9, 133.8, 133.7, 133.1, 130.9, 129.3, 129.1, 128.8, 128.75, 128.67, 128.4, 126.7, 126.6, 126.1, 125.9, 125.8, 125.6, 125.1, 125.1, 124.9 (d, JC-F = 8.2 Hz), 123.3, 123.2, 94.7 (d, JC-F = 186.4 Hz, 2), 72.6 (1'), 23.9 (d, JC-F = 23.4 Hz, 3); HR MS: calcd for C30H23FO2Na (M+Na+) 457.1574, found 457.1584. Analytical data on racemic compound: Mp: 150–151 °C (CH2Cl2/hexane); IR (KBr): 3,065, 1,749, 1,600, 1,510, 1,123, 777, 698 cm−1.
Di(naphthalen-1-yl)methyl 2-Fluoro-2-(o-tolyl)propanoate ((S)-4b) [Table 2, Entry 2, 98% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 19.8 min (0.8%), tR = 26.9 min (99.2%); 1H-NMR (CDCl3): δ 8.45 (s, 1H, 1’-H), 8.12–8.07 (m, 1H, Ar), 7.92–7.69 (m, 5H, Ar), 7.55–7.38 (m, 4H, Ar), 7.36–7.21 (m, 4H, Ar), 7.19–7.03 (m, 3H, Ar), 6.98-6.92 (m, 1H, Ar), 2.14 (s, 3H, o-Me), 1.95 (d, JH-F = 22.8 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 170.3 (d, JC-F = 27.9 Hz, 1), 137.4, 136.0 (d, JC-F = 20.5 Hz), 134.0, 134.0, 133.8, 133.7, 131.9 (d, JC-F = 1.5 Hz), 131.3, 130.7, 129.4, 129.02, 128.97, 128.92, 128.7, 126.9, 126.8, 126.5, 126.4, 126.3, 126.0, 125.8, 125.7, 125.1 (d, JC-F = 6.5 Hz), 125.0, 123.5, 123.2, 95.0 (d, JC-F = 184.9 Hz, 2), 72.4 (1'), 23.8 (d, JC-F = 25.0 Hz, 3), 20.3 (d, JC-F = 5.9 Hz, o-Me); HR MS: calcd for C31H25FO2Na (M+Na+) 471.1736, found 471.1731. Analytical data on racemic compound: Mp: 142–144 °C (CH2Cl2/hexane); IR (KBr): 1,751, 1,600, 1,510, 1,124, 778, 733 cm−1.
Di(naphthalen-1-yl)methyl 2-Fluoro-2-(m-tolyl)propanoate ((S)-4c) [Table 2, Entry 3, 84% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 16.3 min (8.0%), tR = 25.5 min (92.0%); 1H-NMR (CDCl3): δ 8.38 (s, 1H, 1'-H), 7.92–7.69 (m, 6H, Ar), 7.52–7.03 (m, 12H, Ar), 2.19 (s, 3H, m-Me), 1.93 (d, JH-F = 22.4 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 70.0 (d, JC-F = 27.9 Hz, 1), 138.5 (d, JC-F = 22.0 Hz), 138.2, 134.1, 134.0, 133.8, 133.7, 131.1, 130.9, 129.4 (d, JC-F = 1.5 Hz), 129.2, 129.1, 128.8, 128.7, 128.3, 126.63, 126.57, 126.1, 125.85, 125.79, 125.7, 125.6 (d, JC-F = 7.3 Hz), 125.1, 125.1, 123.3, 123.2, 121.9 (d, JC-F = 8.1 Hz), 94.7 (d, JC-F = 186.4 Hz, 2), 72.5 (1'), 23.9 (d, JC-F = 23.5 Hz, 3), 21.3 (m-Me); HR MS: calcd for C31H25FO2Na (M+Na+) 471.1731, found 471.1713. Analytical data on racemic compound: Mp: 140–141 °C (CH2Cl2/hexane); IR (KBr): 1,744, 1,600, 1,510, 1,125, 800, 724, 700 cm−1.
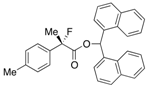
Di(naphthalen-1-yl)methyl 2-Fluoro-2-(p-tolyl)propanoate ((S)-4d) [Table 2, Entry 5, 84% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 20.5 min (8.1%), tR = 38.0 min (91.9%); 1H-NMR (CDCl3): δ 8.36 (s, 1H, 1'-H), 7.90–7.69 (m, 6H, Ar), 7.50–7.19 (m, 8H, Ar), 7.16–7.01 (m, 4H, Ar), 2.33 (s, 3H, p-Me), 1.92 (d, JH-F = 22.4 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 170.1 (d, JC-F = 27.9 Hz, 1), 138.5 (d, JC-F = 1.5 Hz), 135.7 (d, JC-F = 22.7 Hz), 134.1, 134.0, 133.8, 133.7, 131.1, 130.9, 129.2, 129.1, 129.0, 129.0, 128.8, 128.7, 126.6, 126.5, 126.1, 125.8, 125.7, 125.6, 125.1, 125.1, 124.9 (d, JC-F = 8.1 Hz), 123.3 (d, JC-F = 7.3 Hz), 94.6 (d, JC-F = 185.7 Hz, 2), 72.5 (1'), 23.8 (d, JC-F = 23.5 Hz, 3), 21.1 (p-Me); HR MS: calcd for C31H25FO2Na (M+Na+) 471.1731, found 471.1720. Analytical data on racemic compound: Mp: 133–134 °C (CH2Cl2/hexane); IR (KBr): 1,749, 1,600, 1,511, 1,118, 804, 777, 734 cm−1.
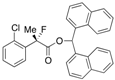
Di(naphthalen-1-yl)methyl 2-(2-Chlorophenyl)-2-fluoropropanoate ((S)-4e) [Table 2, Entry 6, 91% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 23.8 min (4.6%), tR = 34.1 min (95.4%); 1H-NMR (CDCl3): δ 8.49 (s, 1H, 1'-H), 8.09–7.75 (m, 6H, Ar), 7.57–7.19 (m, 12H, Ar), 1.97 (d, JH-F = 23.2 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 68.6 (d, JC-F = 25.0 Hz, 1), 136.7 (d, JC-F = 22.0 Hz), 134.1, 133.9, 133.8, 133.8, 131.9 (d, JC-F = 3.6 Hz), 131.1, 131.0, 130.6, 130.0, 129.24, 129.17, 128.78, 128.76, 127.0 (d, JC-F = 11.8 Hz), 126.8, 126.59, 126.56, 126.54, 126.1, 125.8, 125.8, 125.08, 125.05, 123.6, 123.6, 94.5 (d, JC-F = 183.4 Hz, 2), 72.9 (1'), 23.1 (d, JC-F = 24.2 Hz, 3); HR MS: calcd for C30H22ClFO2Na (M+Na+) 491.1185.0402, found 491.1166. Analytical data on racemic compound: Mp: 171–175 °C (CH2Cl2/hexane); IR (KBr): 1,745, 1,599, 1,511, 1,257, 1,122, 804, 775, 756 cm−1.
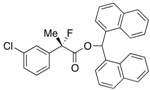
Di(naphthalen-1-yl)methyl 2-(3-Chlorophenyl)-2-fluoropropanoate ((S)-4f) [Table 2, Entry 8, 76% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 15.2 min (12.0%), tR = 23.4 min (88.0%); 1H-NMR (CDCl3): δ 8.37 (s, 1H, 1'-H), 7.95-7.71 (m, 6H, Ar), 7.54–7.08 (m, 12H, Ar), 1.92 (d, JH-F = 22.0 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 69.3 (d, JC-F = 27.2 Hz, 1), 140.6 (d, JC-F = 23.4 Hz), 134.5 (d, JC-F = 1.4 Hz), 133.81, 133.79, 133.74, 131.0, 130.9, 129.7, 129.4, 129.3, 128.86, 128.85, 128.84, 128.82, 126.7, 126.6, 126.04, 125.91, 125.87, 125.7, 125.2 (d, JC-F = 8.8 Hz), 125.12, 125.10, 123.12, 123.10, 123.08 (d, JC-F = 8.1 Hz), 94.2 (d, JC-F = 188.6 Hz, 2), 73.0 (1'), 24.1 (d, JC-F = 23.4 Hz, 3); HR MS: calcd for C30H22ClFO2Na (M+Na+) 491.1185, found 491.1194. Analytical data on racemic compound: Mp: 113–115 °C (CH2Cl2/hexane); IR (KBr): 1,748, 1,598, 1,510, 1,127, 798, 777, 712 cm−1.
Di(naphthalen-1-yl)methyl 2-(3-Chlorophenyl)-2-fluoropropanoate ((S)-4g) [Table 2, Entry 9, 75% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 19.3 min (12.3%), tR = 40.0 min (87.7%); 1H-NMR (CDCl3): δ 8.34 (s, 1H, 1'-H), 7.93–7.74 (m, 5H, Ar), 7.75–7.64 (m, 1H, Ar), 7.52–7.06 (m, 12H, Ar), 1.95 (d, JH-F = 22.0 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 69.5 (d, JC-F = 27.9 Hz, 1), 137.1 (d, JC-F = 22.7 Hz), 134.8 (d, JC-F = 2.2 Hz), 133.81, 133.76, 131.0, 130.9, 129.4, 129.3, 129.0, 128.9, 128.8, 128.5, 128.5, 126.7, 126.6, 126.4 (d, JC-F = 8.1 Hz), 126.0, 125.91, 125.86, 125.6, 125.1, 125.1, 123.13, 123.12, 94.3 (d, JC-F = 187.8 Hz, 2), 73.0 (1'), 23.0 (d, JC-F = 23.4 Hz, 3); HR MS: calcd for C30H22ClFO2K (M+K+) 507.0929, found 507.0940. Analytical data on racemic compound: Mp: 129–131 °C (CH2Cl2/hexane); IR (KBr): 1,749, 1,510, 1,490, 1,121, 1,093, 838, 778 cm−1.
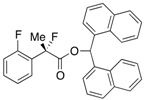
Di(naphthalen-1-yl)methyl 2-Fluoro-2-(2-fluorophenyl)propanoate ((S)-4h) [Table 2, Entry 10, 60% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 20.2 min (20.2%), tR = 24.9 min (79.8%); 1H-NMR (CDCl3): δ 8.45 (s, 1H, 1'-H), 8.10–7.78 (m, 6H, Ar), 7.53–7.19 (m, 10H, Ar), 7.12–7.04 (m, 1H, Ar), 6.99–6.89 (m, 1H, Ar), 1.96 (d, JH-F = 22.8 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 169.0 (d, JC-F = 26.5 Hz, 1), 158.4 (dd, JC-F = 254.5, 4.3 Hz), 134.0 (dd, JC-F = 8.9, 1.5 Hz), 133.80, 133.78, 131.1, 131.0, 130.8, 130.7, 129.3, 129.3, 129.2, 129.2, 128.8, 128.8, 126.9 (dd, JC-F = 10.0, 3.3 Hz), 126.64, 126.59, 126.42 (dd, JC-F = 19.1, 14.0 Hz), 126.2, 125.84, 125.80, 125.1, 125.1, 124.1 (d, JC-F = 3.7 Hz), 123.3, 116.1 (d, JC-F = 22.0 Hz), 92.8 (d, JC-F = 185.6 Hz, 2), 72.8 (1'), 23.1 (dd, JC-F = 24.3, 2.9 Hz, 3); HR MS: calcd for C30H22F2O2Na (M+Na+) 475.1480, found 475.1485. Analytical data on racemic compound: Mp: 144–146 °C (CH2Cl2/hexane); IR (KBr): 1,738, 1,618, 1,599, 1,585, 1,120, 1,096, 774, 759 cm−1.
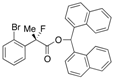
Di(naphthalen-1-yl)methyl 2-(2-Bromophenyl)-2-fluoropropanoate ((S)-4i) [Table 2, Entry 11, 94% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 27.4 min (2.8%), tR = 38.4 min (97.2%); 1H-NMR (CDCl3): δ 8.50 (s, 1H, 1'-H), 8.10–8.03 (m, 1H, Ar), 8.01–7.77 (m, 5H, Ar), 7.55–7.22 (m, 11H, Ar), 7.18–7.11 (m, 1H, Ar), 1.98 (d, JH-F = 23.2 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 168.5 (d, JC-F = 25.7 Hz, 1), 138.2 (d, JC-F = 21.2 Hz), 134.14, 134.08, 133.9, 133.8, 133.8, 131.1, 131.0, 130.1, 129.24, 129.16, 128.8, 128.8, 127.4, 127.31, 127.30, 126.6, 126.6 (d, JC-F = 10.2 Hz), 126.2, 125.81, 125.79, 125.09, 125.07, 123.6, 123.6, 120.9 (d, JC-F = 3.6 Hz), 95.4 (d, JC-F = 183.3 Hz, 2), 72.9 (1'), 23.3 (d, JC-F = 24.9 Hz, 3); HR MS: calcd for C30H22BrFO2Na (M+Na+) 535.0679, found 535.0684. Analytical data on racemic compound: Mp: 181–183 °C (CH2Cl2/hexane); IR (KBr): 1,744, 1,598, 1,510, 1,121, 796, 774, 756, 639 cm−1.
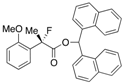
Di(naphthalen-1-yl)methyl 2-Fluoro-2-(2-methoxyphenyl)propanoate ((S)-4j) [Table 2, Entry 12, 89% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/9, flow rate = 0.75 mL/min): tR = 14.3 min (5.6%), tR = 16.9 min (94.4%); 1H-NMR (CDCl3): δ 8.52 (s, 1H, 1'-H), 8.13–8.06 (m, 1H, Ar), 8.00–7.92 (m, 1H, Ar), 7.88–7.72 (m, 4H, Ar), 7.50–7.19 (m, 10H, Ar), 6.96–6.86 (m, 1H, Ar), 6.65-6.57 (m, 1H, Ar), 3.04 (s, 3H, OMe), 1.95 (d, JH-F = 22.8 Hz, 3H, 2-Me); 13C-NMR (CDCl3): δ 169.8 (d, JC-F = 26.4 Hz, 1), 156.1 (d, JC-F = 3.6 Hz), 134.6, 134.5, 133.8, 133.8, 131.1, 131.0, 130.1 (d, JC-F = 1.4 Hz), 129.0, 128.8, 128.7, 126.6, 126.5, 126.5, 126.1, 126.0 (d, JC-F = 9.6 Hz), 125.74, 125.69, 125.1, 125.1, 125.0, 125.0, 123.7, 123.6, 120.2, 110.6, 93.3 (d, JC-F = 180.4 Hz, 2), 72.0 (1'), 54.5 (OMe), 22.8 (d, JC-F = 25.0 Hz, 3); HR MS: calcd for C31H25FO3Na (M+Na+) 487.1680, found 487.1685. Analytical data on racemic compound: Mp: 152–154 °C (CH2Cl2/hexane); IR (KBr): 1,734, 1,601, 1,589, 1,510, 1,495, 1,119, 801, 788, 776, 749 cm−1.
Di(naphthalen-1-yl)methyl 2-Fluoro-2-(4-isobutylphenyl)propanoate ((S)-4k) [Table 2, Entry 13, 88% ee]
HPLC (CHIRALPAK AD-H, i-PrOH/hexane = 1/50, flow rate = 0.75 mL/min): tR = 20.9 min (5.8%), tR = 29.4 min (94.2%); 1H-NMR (CDCl3): δ 8.38 (s, 1H, 1'-H), 7.95–7.68 (m, 6H, Ar), 7.51–7.19 (m, 8H, Ar), 7.14–7.00 (m, 4H, Ar), 2.47 (d, JH-H = 7.2 Hz, 2H, i-Bu), 1.92 (d, JH-F = 22.4 Hz, 3H, 2-Me), 1.92–1.79 (m, 1H, i-Bu), 0.91 (d, JH-H = 6.4 Hz, 6H, i-Bu); 13C-NMR (CDCl3): δ 170.1 (d, JC-F = 28.6 Hz, 1), 142.3 (d, JC-F = 1.5 Hz), 135.9 (d, JC-F = 22.7 Hz), 134.1, 134.0, 133.8, 133.7, 131.1, 130.9, 129.3, 129.10, 129.06, 128.8, 128.7, 126.7, 126.5, 126.2, 125.9, 125.8, 125.5, 125.09, 125.07, 124.8 (d, JC-F = 7.3 Hz), 123.3, 123.2, 94.6 (d, JC-F = 185.7 Hz, 2), 72.4 (1'), 45.0 (i-Bu), 30.1 (i-Bu), 23.9 (d, JC-F = 23.5 Hz, 3), 22.4 (i-Bu); HR MS: calcd for C34H31FO2K (M+K+) 529.1945, found 529.1949. Analytical data on racemic compound: IR (KBr): 1,755, 1,599, 1,511, 1,118, 849, 793, 777 cm−1.