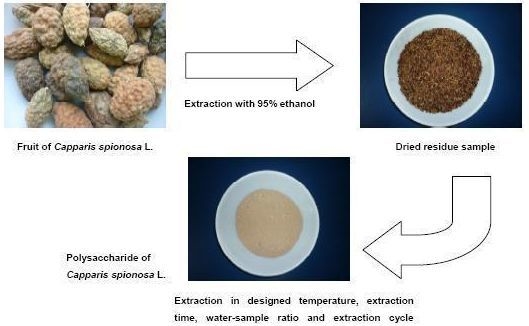
Optimizing the Extraction of Anti-tumor Polysaccharides from the Fruit of Capparis spionosa L. by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Extraction Temperature on the Yield of CSPS
2.2. Effect of Extraction Time on the Yield of CSPS


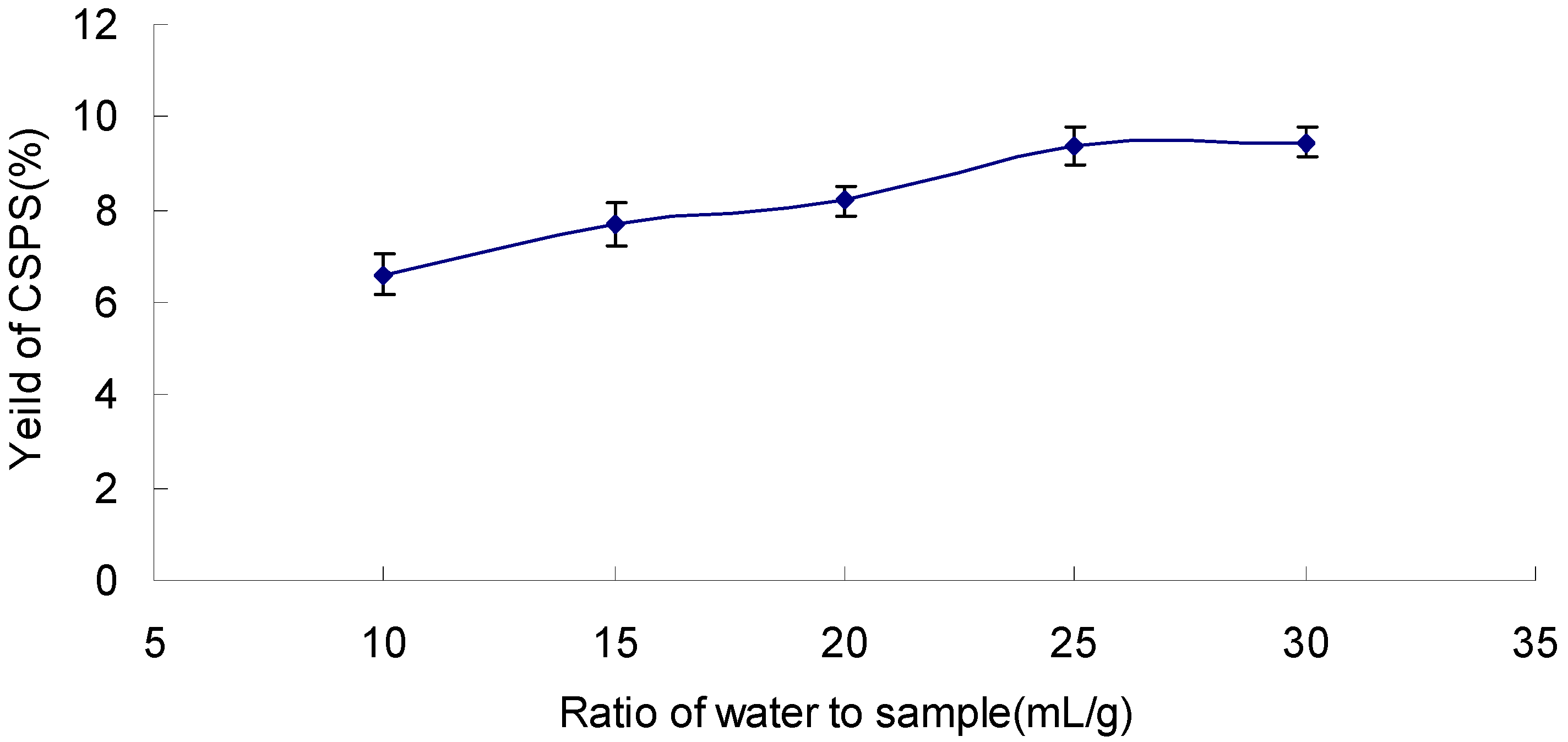
2.3. Effect of Ratio of Water to Sample on the Yield of CSPS
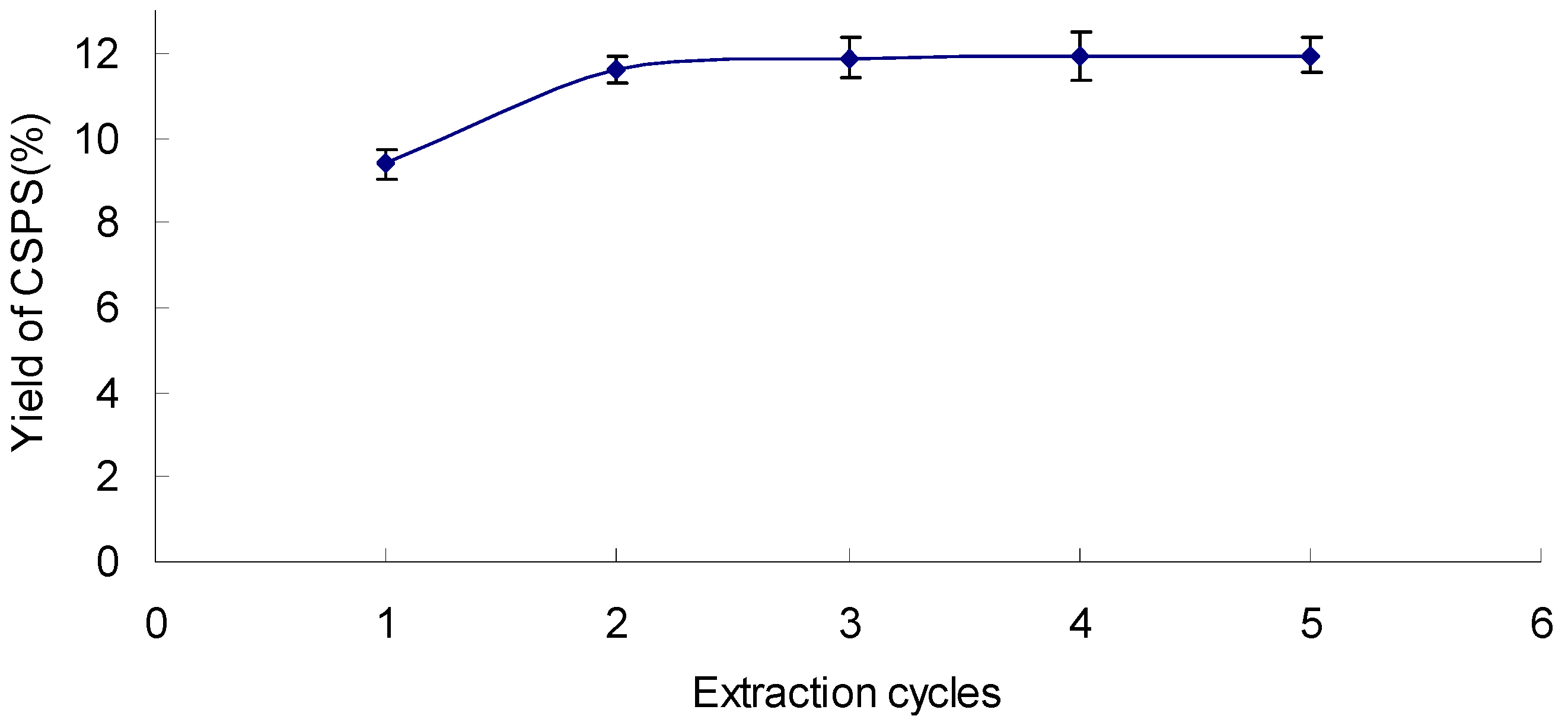
2.4. Effect of Extraction Cycles on the Yield of CSPS
2.5. Results of the Yield of CSPS of Optimization of the Procedure
| No. | X1/Extraction temperature (°C) | X2/Extraction time (min) | X3/Ratio of water to sample (mL/g) | X4/Extraction cycles | Yield of CSPS (%) |
|---|---|---|---|---|---|
| 1 | −1(80) | −1(90) | 0(25) | 0(2) | 9.028 |
| 2 | 1(100) | −1(90) | 0(25) | 0(2) | 11.584 |
| 3 | −1(80) | 1(150) | 0(25) | 0(2) | 11.370 |
| 4 | 1(100) | 1(150) | 0(25) | 0(2) | 11.458 |
| 5 | 0(90) | 0(120) | −1(20) | −1(1) | 8.206 |
| 6 | 0(90) | 0(120) | 1(30) | −1(1) | 9.466 |
| 7 | 0(90) | 0(120) | −1(20) | 1(3) | 10.962 |
| 8 | 0(90) | 0(120) | 1(30) | 1(3) | 12.190 |
| 9 | −1(80) | 0(120) | 0(25) | −1(1) | 7.052 |
| 10 | 1(100) | 0(120) | 0(25) | −1(1) | 9.150 |
| 11 | −1(80) | 0(120) | 0(25) | 1(3) | 10.214 |
| 12 | 1(100) | 0(120) | 0(25) | 1(3) | 11.792 |
| 13 | 0(90) | −1(90) | −1(20) | 0(2) | 8.566 |
| 14 | 0(90) | 1(150) | −1(20) | 0(2) | 10.826 |
| 15 | 0(90) | −1(90) | 1(30) | 0(2) | 10.250 |
| 16 | 0(90) | 1(150) | 1(30) | 0(2) | 11.090 |
| 17 | 0(80) | 0(120) | −1(20) | 0(2) | 9.722 |
| 18 | 1(100) | 0(120) | −1(20) | 0(2) | 10.968 |
| 19 | −1(80) | 0(120) | 1(30) | 0(2) | 10.202 |
| 20 | 1(100) | 0(120) | 1(30) | 0(2) | 11.560 |
| 21 | 0(90) | −1(90) | 0(25) | −1(1) | 8.426 |
| 22 | 0(90) | 1(150) | 0(25) | −1(1) | 8.568 |
| 23 | 0(90) | −1(90) | 0(25) | 1(3) | 11.002 |
| 24 | 0(90) | 1(150) | 0(25) | 1(3) | 12.49 |
| 25 | 0(90) | 0(120) | 0(25) | 0(2) | 11.702 |
| 26 | 0(90) | 0(120) | 0(25) | 0(2) | 11.547 |
| 27 | 0(90) | 0(120) | 0(25) | 0(2) | 11.626 |
| 28 | 0(90) | 0(120) | 0(25) | 0(2) | 11.315 |
| 29 | 0(90) | 0(120) | 0(25) | 0(2) | 11.918 |
2.6. Model Fitting and Statistical Significance Analysis

| Parameter | Estimate | df | Standard error | 95%CI | F-value | p-value | |
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| intercept | 11.62 | 1 | 0.21 | 11.18 | 12.06 | ||
| X1 | 0.74 | 1 | 0.13 | 0.46 | 1.03 | 31.35 | <0.0001 |
| X2 | 0.58 | 1 | 0.13 | 0.29 | 0.86 | 19.00 | 0.0007 |
| X3 | 0.46 | 1 | 0.13 | 0.17 | 0.74 | 11.94 | 0.0039 |
| X4 | 1.84 | 1 | 0.13 | 1.20 | 1.77 | 124.09 | <0.0001 |
| X1X2 | −0.62 | 1 | 0.23 | −1.11 | −0.12 | 7.91 | 0.0179 |
| X1X3 | 0.028 | 1 | 0.23 | −0.47 | 0.52 | 0.015 | 0.9048 |
| X1X4 | −0.13 | 1 | 0.23 | −0.62 | 0.36 | 0.32 | 0.5809 |
| X2X3 | −0.35 | 1 | 0.23 | −0.85 | 0.14 | 2.83 | 0.1451 |
| X2X4 | 0.34 | 1 | 0.23 | −0.16 | 0.83 | 2.14 | 0.1656 |
| X3X4 | −0.008 | 1 | 0.23 | −0.50 | 0.49 | 0.005 | 0.9727 |
| X1X1 | −0.55 | 1 | 0.18 | −0.94 | −0.17 | 9.41 | 0.0083 |
| intercept | 11.62 | 1 | 0.21 | 11.18 | 12.06 | ||
| X2X2 | −0.48 | 1 | 0.18 | −0.87 | −0.097 | 7.19 | 0.0179 |
| X3X3 | −0.57 | 1 | 0.18 | −0.95 | −0.18 | 9.81 | 0.0074 |
| X4X4 | −1.13 | 1 | 0.18 | −1.51 | −0.74 | 38.92 | <0.0001 |
| Source | Sum of squares | df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 51.94 | 14 | 3.71 | 17.53 | <0.0001 | significant |
| Residual | 2.96 | 14 | 0.21 | |||
| Lack of fit | 2.77 | 10 | 0.28 | 5.71 | 0.0583 | |
| Pure error | 0.19 | 4 | 0.048 | |||
| Cor total | 54.90 | 28 |
2.7. Optimization of Extraction Conditions of CSPS
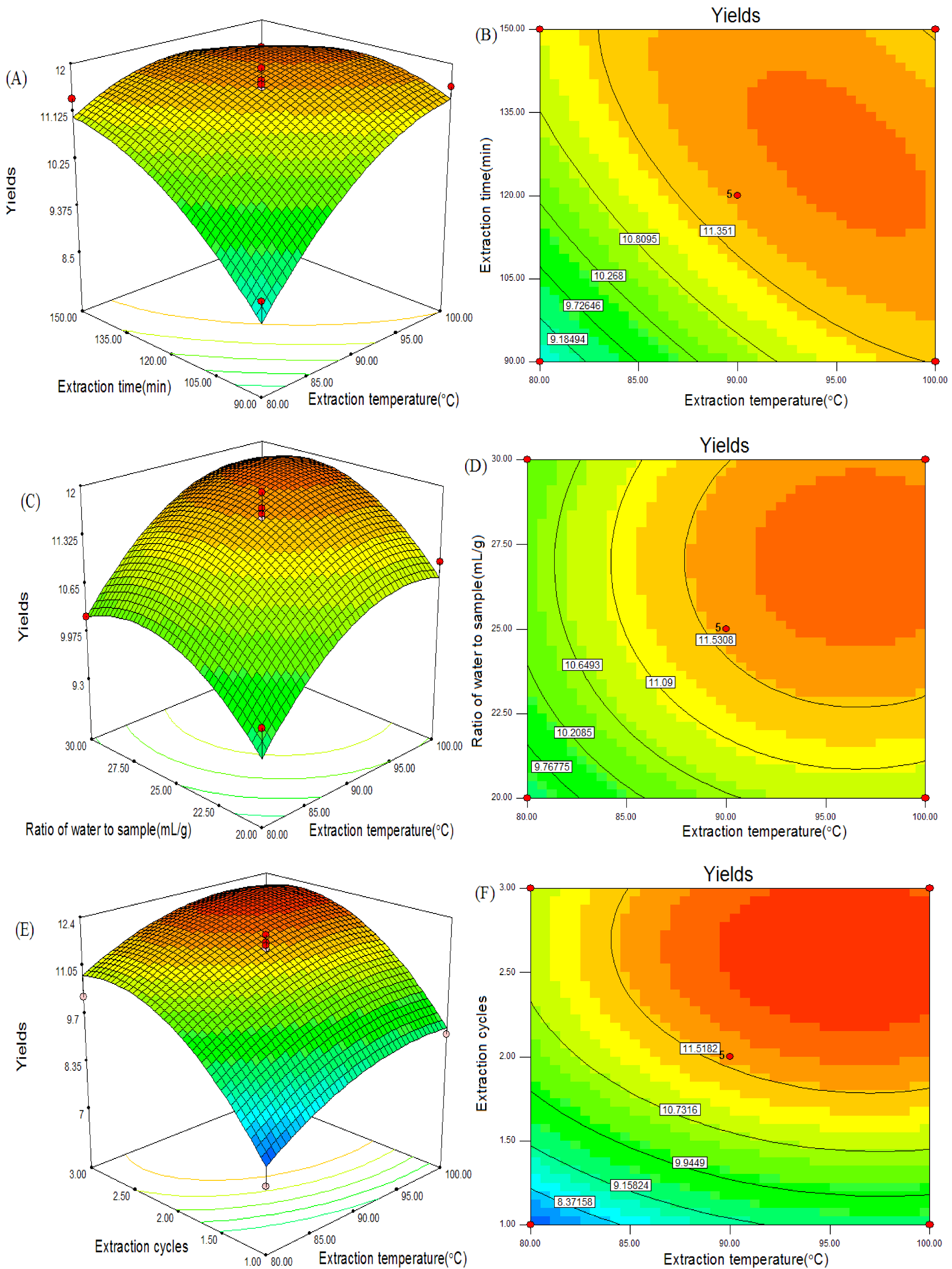
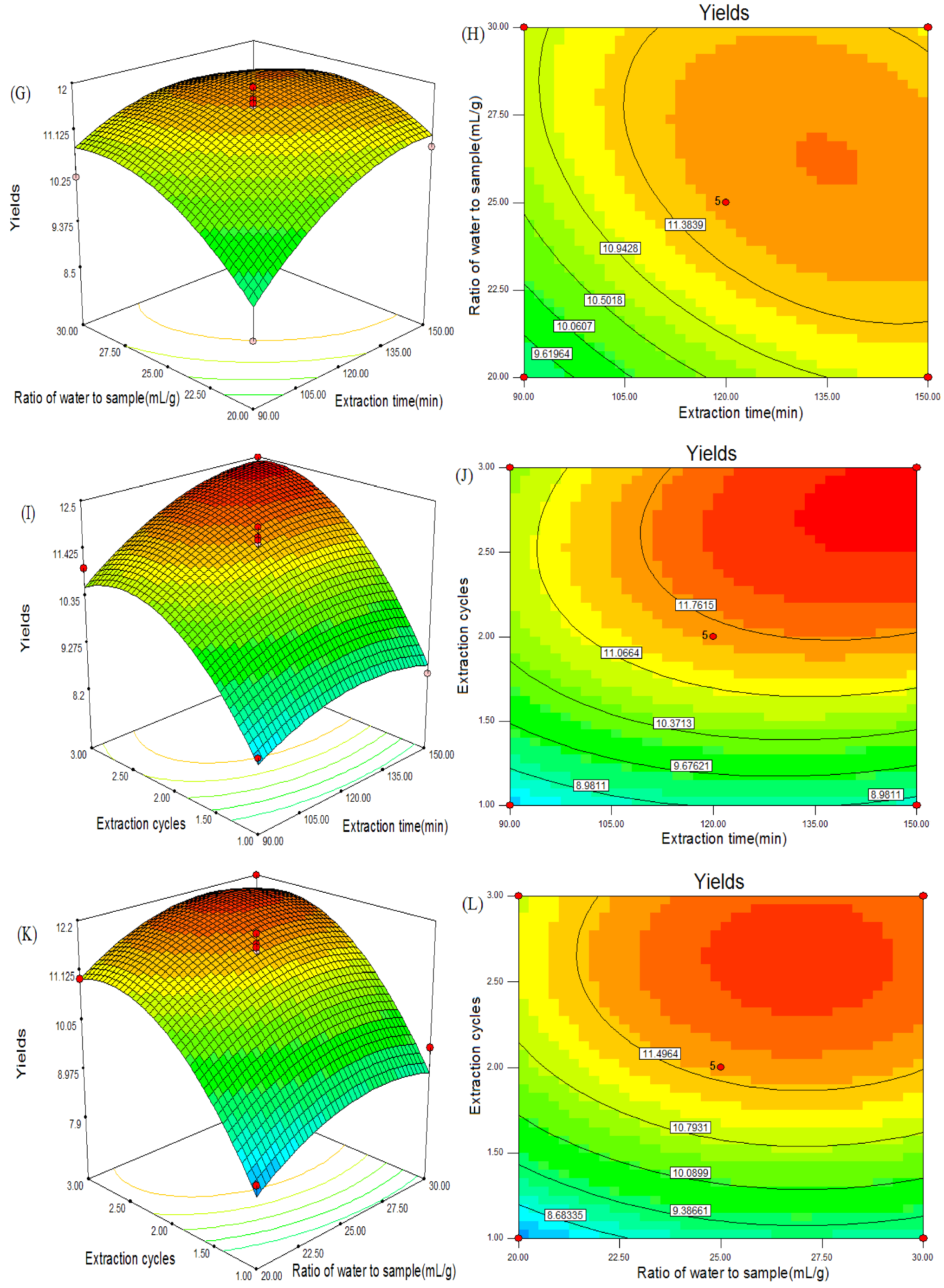
2.8. Verification of Predictive Model
| Extraction temperature (°C) | Extraction time (min) | Ratio of water to sample (mL/g) | Extraction cycles | Yield of CSPS (%) | |
|---|---|---|---|---|---|
| Predicted optimum condition | 92.46 | 138 | 26.06 | 2.74 | 12.94 |
| Experimental optimum condition | 92 | 140 | 26 | 3 | 13.01 ± 0.08 *a |
2.9. Anti-Tumor Activity of CSPS in Vivo
| Groups | Number | Dose (mg/kg) | Survival time (d) | Prolonging rate (%) |
|---|---|---|---|---|
| Control | 12 | Normal saline | 10.24 ± 2.97 | - |
| Low-CSPS | 12 | 50 | 12.66 ± 2.53 * | 23.63 |
| Mid-CSPS | 12 | 100 | 15.48 ± 3.15 ** | 51.17 |
| High-CSPS | 12 | 200 | 16.72 ± 2.31 ** | 63.28 |
| APS | 12 | 100 | 14.89 ± 2.35 ** | 45.41 |
3. Experimental
3.1. Materials
3.2. Extraction and Yield of CSPS
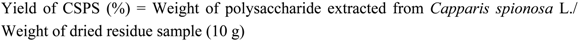
3.3. Optimization Design
| Independent variables | Factor level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 extraction temperature (°C) | 80 | 90 | 100 |
| X2 extraction time (min) | 90 | 120 | 150 |
| X3 ratio of water to sample (mL/g) | 20 | 25 | 30 |
| X4 extraction cycles | 1 | 2 | 3 |
3.4. In Vivo Life Prolonging Experiments

3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
- Sample Availability: Samples of the compounds are available from the authors.
References
- Wang, C.; Chen, Y.; Hu, M.; Ding, J.; Xu, C.; Wang, R. In vitro antioxidant activities of the polysaccharides from Tricholoma lobayense. Int. J. Biol. Macromol. 2012, 50, 534–539. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, M.; Liao, S.; Yu, S.; Chi, J.; Wei, Z. Antioxidant activity of polysaccharide-enriched fractions extracted from pulp tissue of Litchi Chinensis Sonn. Molecules 2010, 15, 2152–2165. [Google Scholar] [CrossRef]
- Liu, Z.F.; Dong, F.; Ji, Y.B.; Miao, J.; Jin, L.N. A study on process of pharmacology of selenium polysaccharide. J. Beijing Union Univ. 2011, 25, 36–40. [Google Scholar]
- Tincer, G.; Yerlikaya, S.; Yagci, F.C.; Kahraman, T.; Atanur, O.M.; Erbatur, O.; Gursel, I. Immunostimulatory activity of polysaccharide-poly(I:C) nanoparticles. Biomaterials 2011, 32, 4275–4282. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef]
- Men, X.Y.; Wang, Y.F.; Zheng, W.J.; Zhu, Y.M.; Zhang, M.Y.; Jiang, X. The synthesis of selenium nanoparticles with polysaccharides from Undaria Pinnatifida (Charv.) Suringer and its antivirus effects on CVB3 in vitro. Chin. J. Health Lab. Technol. 2005, 15, 1153–1155. [Google Scholar]
- Li, R.Y.; Gao, J.P. Primary study of pharmacological action in vivo with abdominal cavity S180 tumor bearing mice of the radix codonopsis coarse polysaccharides. J. Changzhi Med. Coll. 2011, 2, 94–96. [Google Scholar]
- Lan, M.B.; Guo, J.; Zhao, H.L.; Yuan, H.H. Antioxidant and anti-tumor activities of purified polysaccharides with low molecular weights from Magnolia officinalis. J. Med. Plan. Res. 2012, 6, 1025–1034. [Google Scholar]
- Tang, Y.L.; Luo, Q.; Ding, W.; Ding, X.; Yang, Z.R. Anti-tumor activity of polysaccharides extracted from two wild amanitas. J. Sichuan Univ. 2011, 42, 792–796. [Google Scholar]
- Ye, C.L.; Hu, W.L.; Dai, D.H. Extraction of polysaccharides and the antioxidant activity from the seeds of Plantago asiatica L. Int. J. Biol. Macromol. 2011, 49, 466–470. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Psaras, G.K. Development and Structure of Drought-tolerant Leaves of the Mediterranean Shrub Capparis spinosa L. Ann. Bot. 2003, 92, 377–383. [Google Scholar] [CrossRef]
- Adel, M. Screening of some indigenous qatari medicinal plants for antimicrobial activity. Phytother. Res. 2002, 16, 751–753. [Google Scholar] [CrossRef]
- Angelo, D.; Catania, S. Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J. Agric. Food Chem. 2002, 50, 1168–1171. [Google Scholar] [CrossRef]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef]
- Jacobson, R.L.; Schlein, Y. Phlebotomus papatasi and Leishmania major parasites express alpha-amylase and alpha-glucosidase. Acta Trop. 2001, 78, 41–49. [Google Scholar] [CrossRef]
- Ji, Y.B.; Guo, S.D.; Ji, C.F. Progress of study on Capparis spinosa L. J. Harbin Univ. Commerce 2006, 22, 5–10. [Google Scholar]
- Liu, J.C.; Miao, S.; Wen, X.C.; Sun, Y.X. Optimization of polysaccharides (ABP) extraction from the fruiting bodies of Agaricus blazei Murill. using response surface methodology (RSM). Carbohydr. Polym. 2009, 78, 704–709. [Google Scholar] [CrossRef]
- Varnalis, A.I.; Brennan, J.G.; MacDougall, D.B.; Gilmour, S.G. Optimisation of high temperature puffing potato cubes using response surface methodology. J. Food Eng. 2004, 61, 153–163. [Google Scholar] [CrossRef]
- Muralidhar, R.V.; Chirumamil, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, J.C.; Kennedyb, J.F. Extraction optimization of antioxidant polysaccharides from the fruiting bodies of Chroogomphis rutilus (Schaeff.: Fr.) O.K. Miller by Box-Behnken statistical design. Carbohydr. Polym. 2010, 82, 209–214. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lv, F.X.; Lu, Z.X. Optimization of cultivation conditions for exopolysaccharide and mycelial biomass by Clitocybe sp. using Box-Behnken Design. Sci. Agric. Sin. 2005, 1, 145–150. [Google Scholar]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R. Response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Lu, D.X.; Cui, J.; Liu, Y.X.; Jin, Y.L.; Fan, X.Y. Effects of Schisandrin B combined with cisplatin on the survival time of H22 mice with tumor. Heilongjiang Med. Pharm. 2008, 31, 9–10. [Google Scholar]
- Han, X.; Liu, A.J.; Zhao, X.H.; Li, Y.D.; Zheng, G.Q.; Zha, G.R. Immunizing effects of cocultures of H22 hepatocarcinoma cells and cartilage polysaccharide on murine H22 hepatocarcinoma. J. Food Sci. 2010, 75, 265–273. [Google Scholar] [CrossRef]
- Ji, Y.B.; Dong, F.; Gao, S.Y.; Zou, X. Apoptosis induced by Capparis spionosa polysaccharide in human HepG2. Chin. Tradit. Herb. Drugs 2008, 9, 1364–1367. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, Y.-B.; Dong, F.; Ma, D.-B.; Miao, J.; Jin, L.-N.; Liu, Z.-F.; Zhang, L.-W. Optimizing the Extraction of Anti-tumor Polysaccharides from the Fruit of Capparis spionosa L. by Response Surface Methodology. Molecules 2012, 17, 7323-7335. https://doi.org/10.3390/molecules17067323
Ji Y-B, Dong F, Ma D-B, Miao J, Jin L-N, Liu Z-F, Zhang L-W. Optimizing the Extraction of Anti-tumor Polysaccharides from the Fruit of Capparis spionosa L. by Response Surface Methodology. Molecules. 2012; 17(6):7323-7335. https://doi.org/10.3390/molecules17067323
Chicago/Turabian StyleJi, Yu-Bin, Fang Dong, Dong-Bin Ma, Jing Miao, Li-Na Jin, Zhen-Feng Liu, and Ling-Wen Zhang. 2012. "Optimizing the Extraction of Anti-tumor Polysaccharides from the Fruit of Capparis spionosa L. by Response Surface Methodology" Molecules 17, no. 6: 7323-7335. https://doi.org/10.3390/molecules17067323
APA StyleJi, Y.-B., Dong, F., Ma, D.-B., Miao, J., Jin, L.-N., Liu, Z.-F., & Zhang, L.-W. (2012). Optimizing the Extraction of Anti-tumor Polysaccharides from the Fruit of Capparis spionosa L. by Response Surface Methodology. Molecules, 17(6), 7323-7335. https://doi.org/10.3390/molecules17067323