Coordination Modes and Different Hapticities for Fullerene Organometallic Complexes
Abstract
:1. Introduction
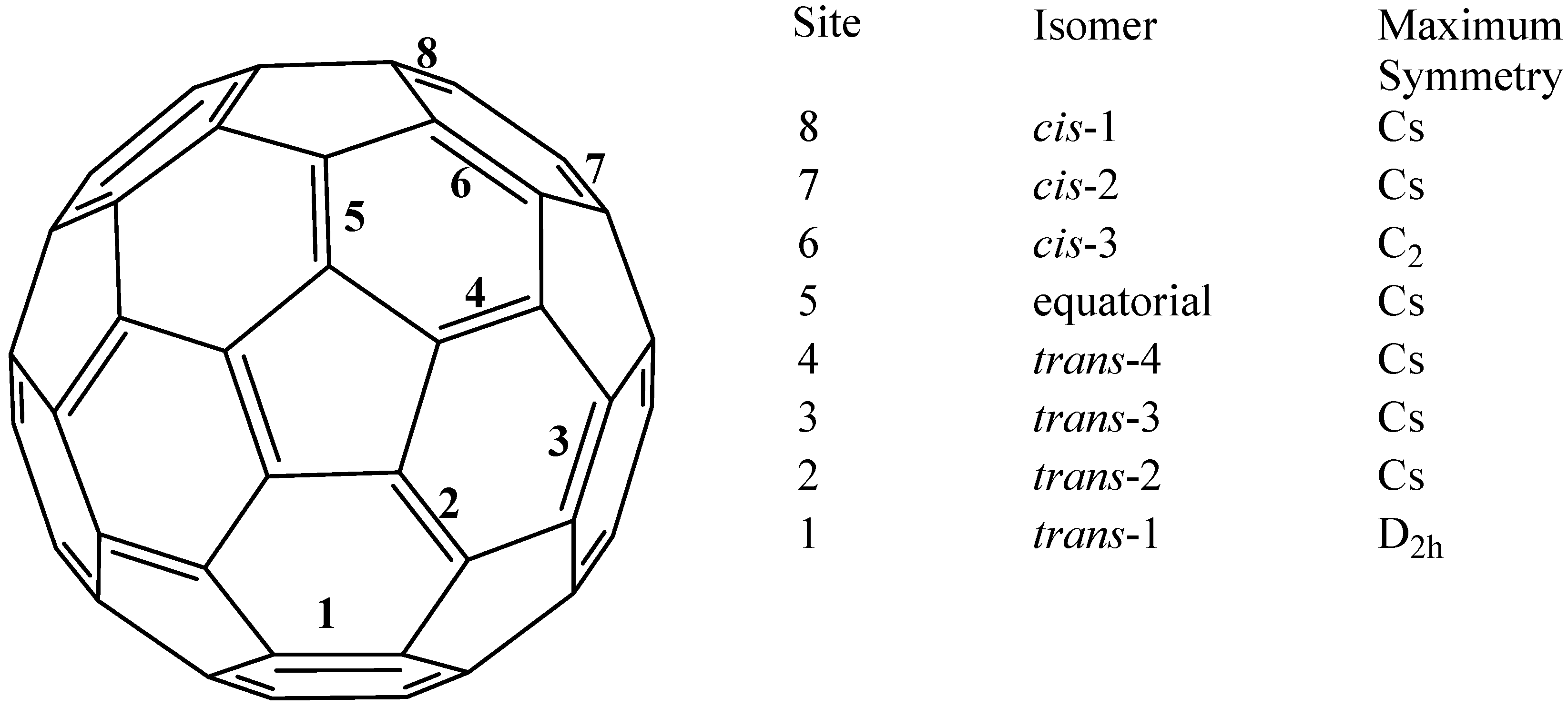
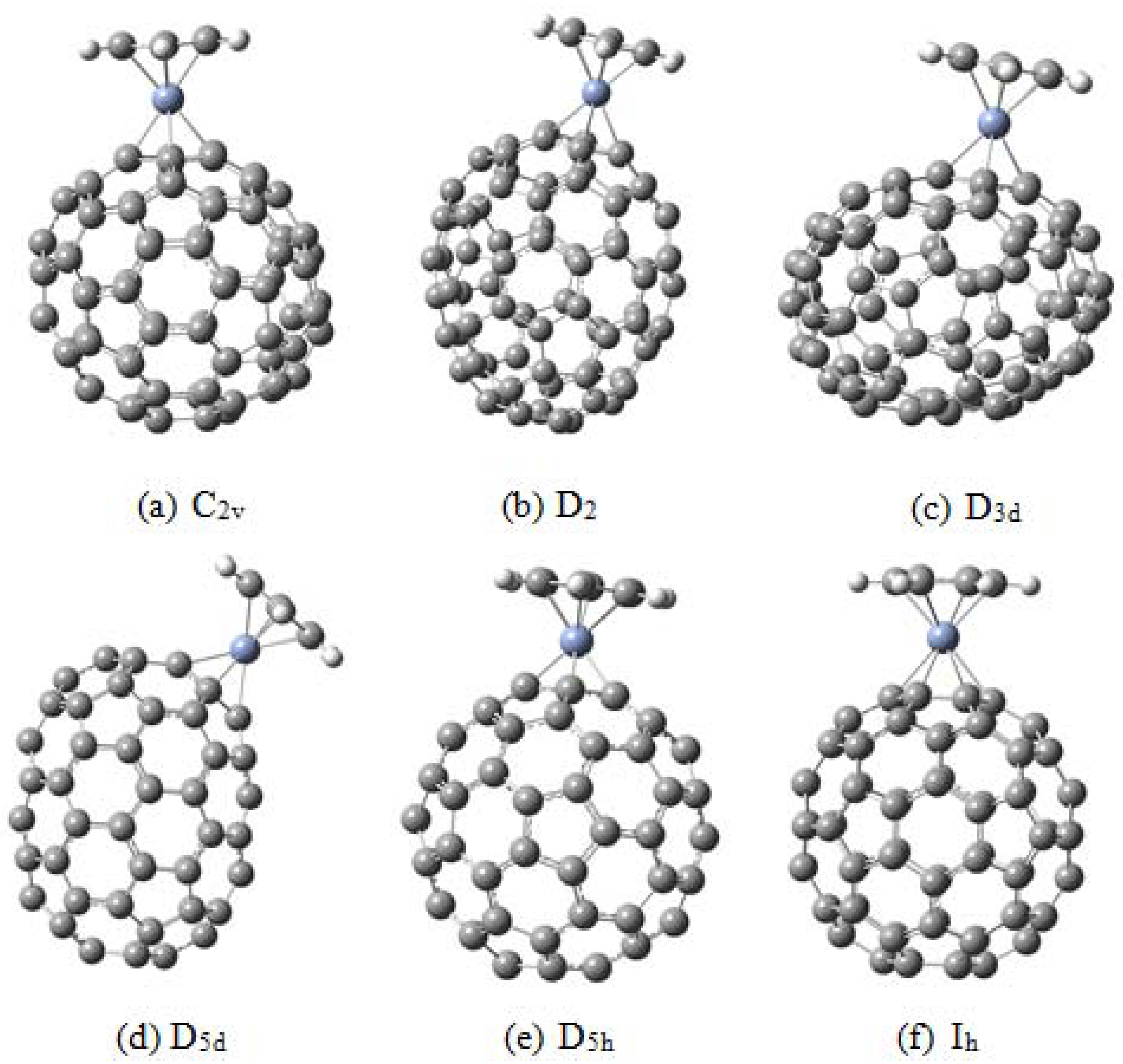
2. The Possibilities of Hapticity
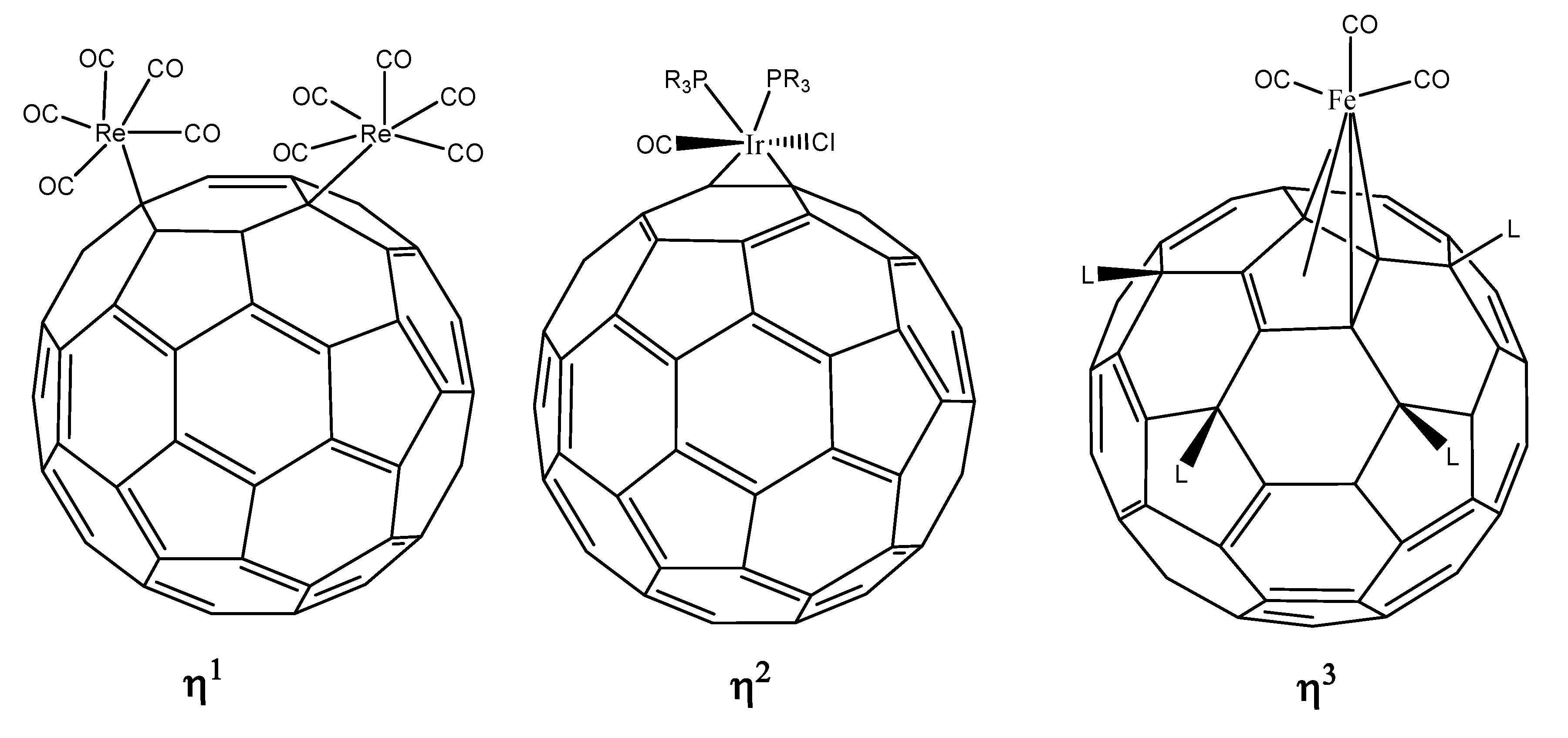
- ﹣ η1 hapticity with the metal atom directly above any carbon atom (an σ bond is expected);
- ﹣ η2 hapticity; this may be (6,6) or (6,5) type, although the last of these is not common;
- ﹣ η3 hapticity; the metal atom linked to three carbon atoms, theoretically this could be on either a six or five-member ring;
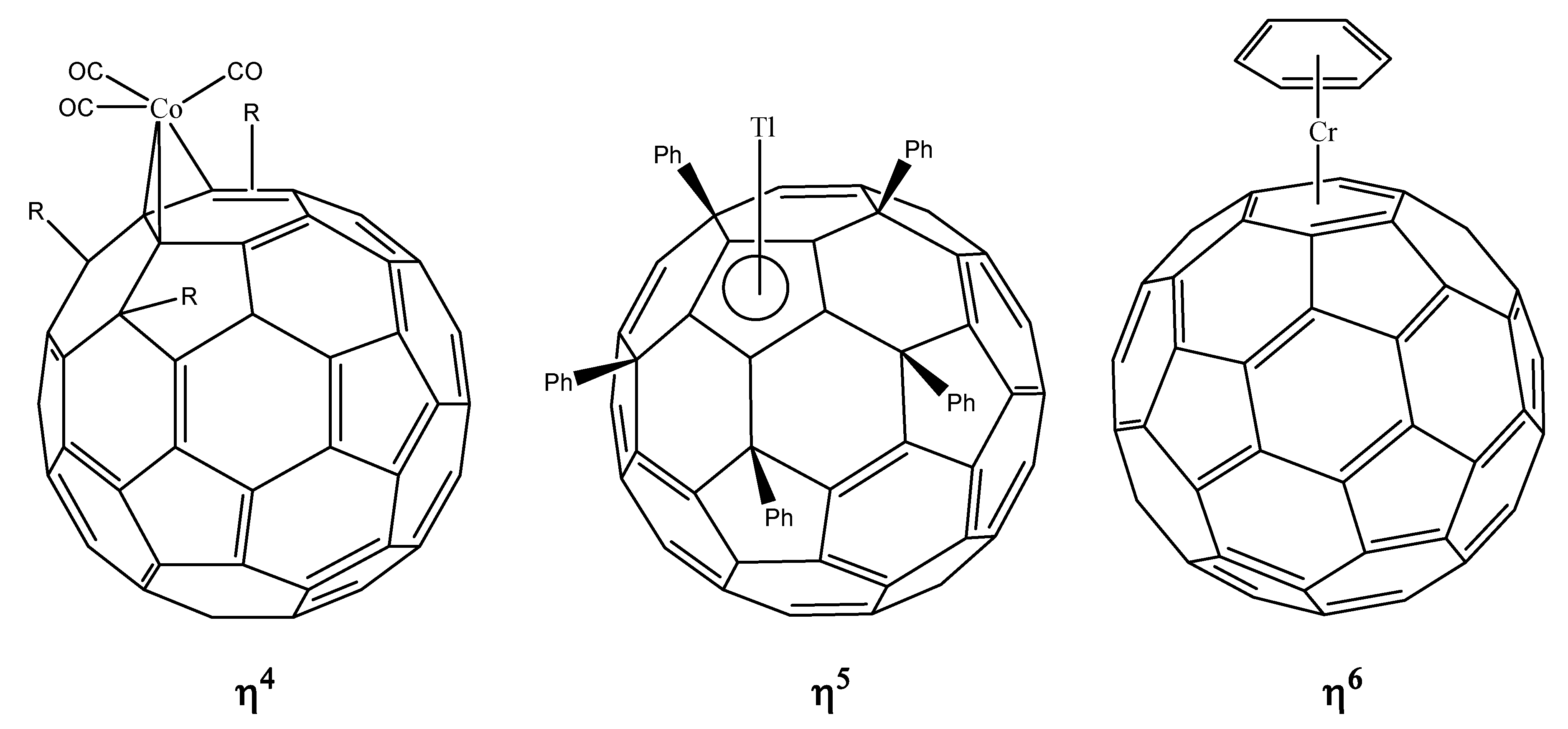
- ﹣ η4 hapticity, the metal atom linked to four carbon atoms;
- ﹣ η5 hapticity, metal atom linked directly above the center of a five-member ring;
- ﹣ η6 hapticity, metal atom linked directly above the center of a six-member ring.
3. η1 Hapticity
4. η2 Hapticity
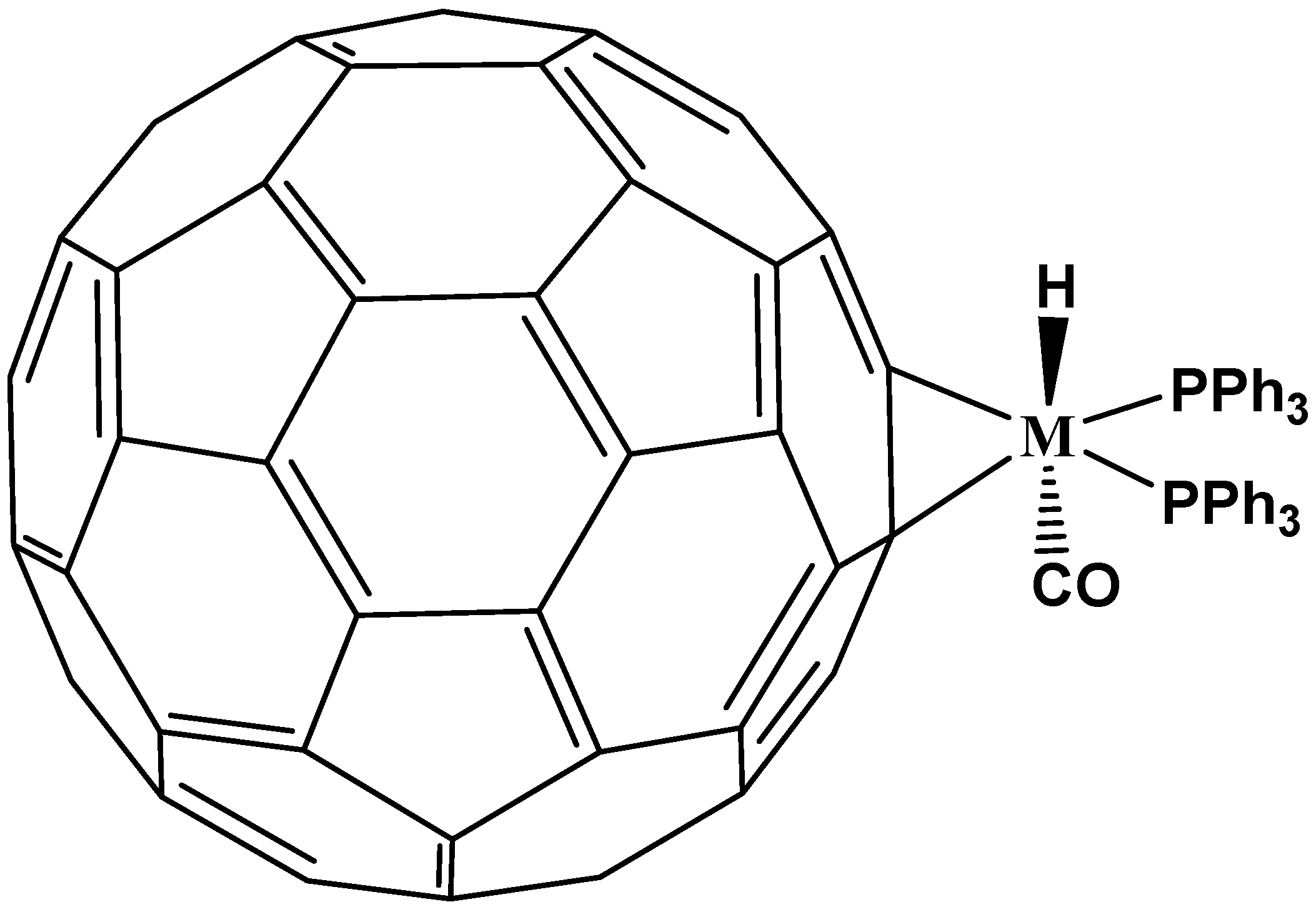
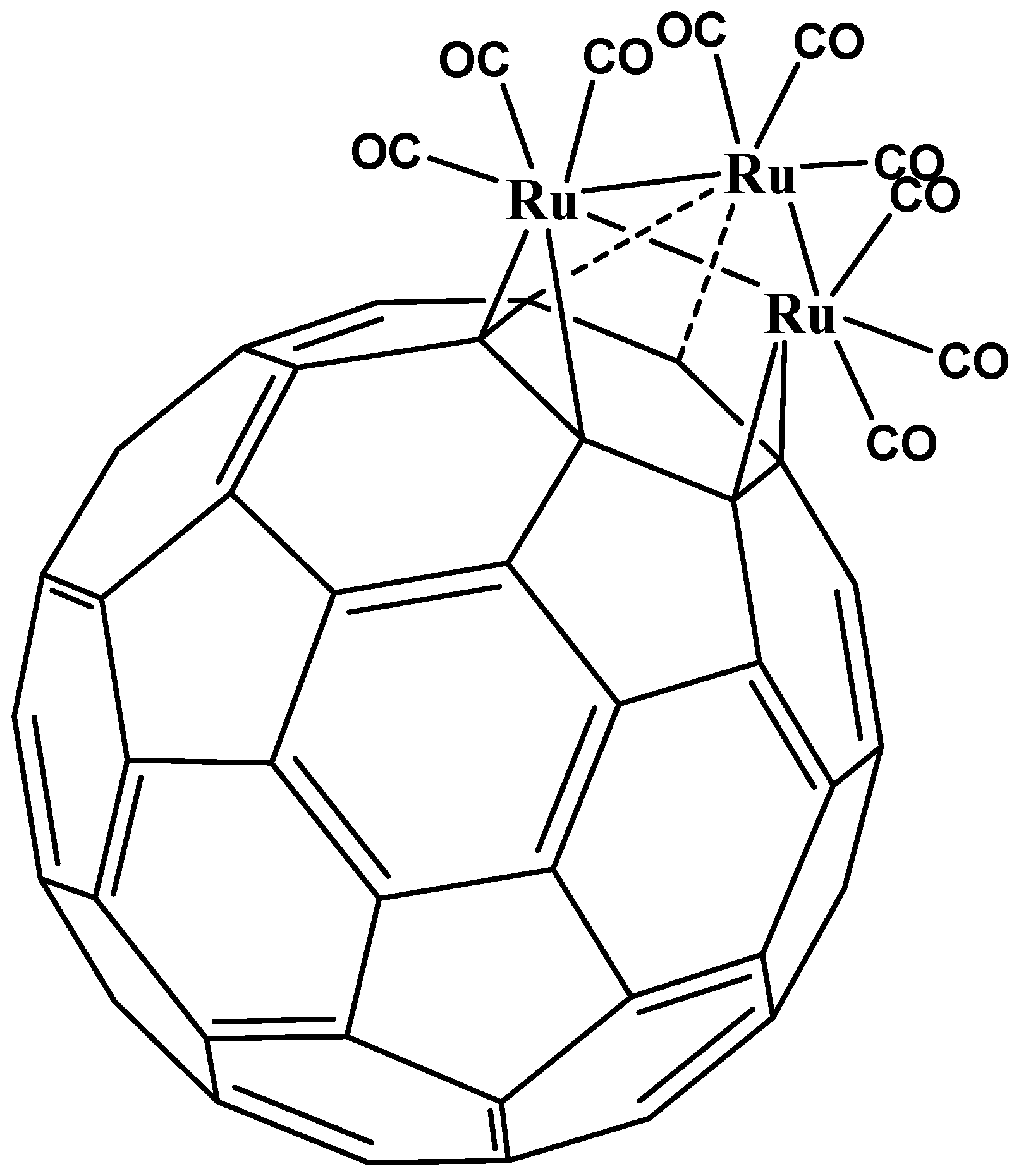
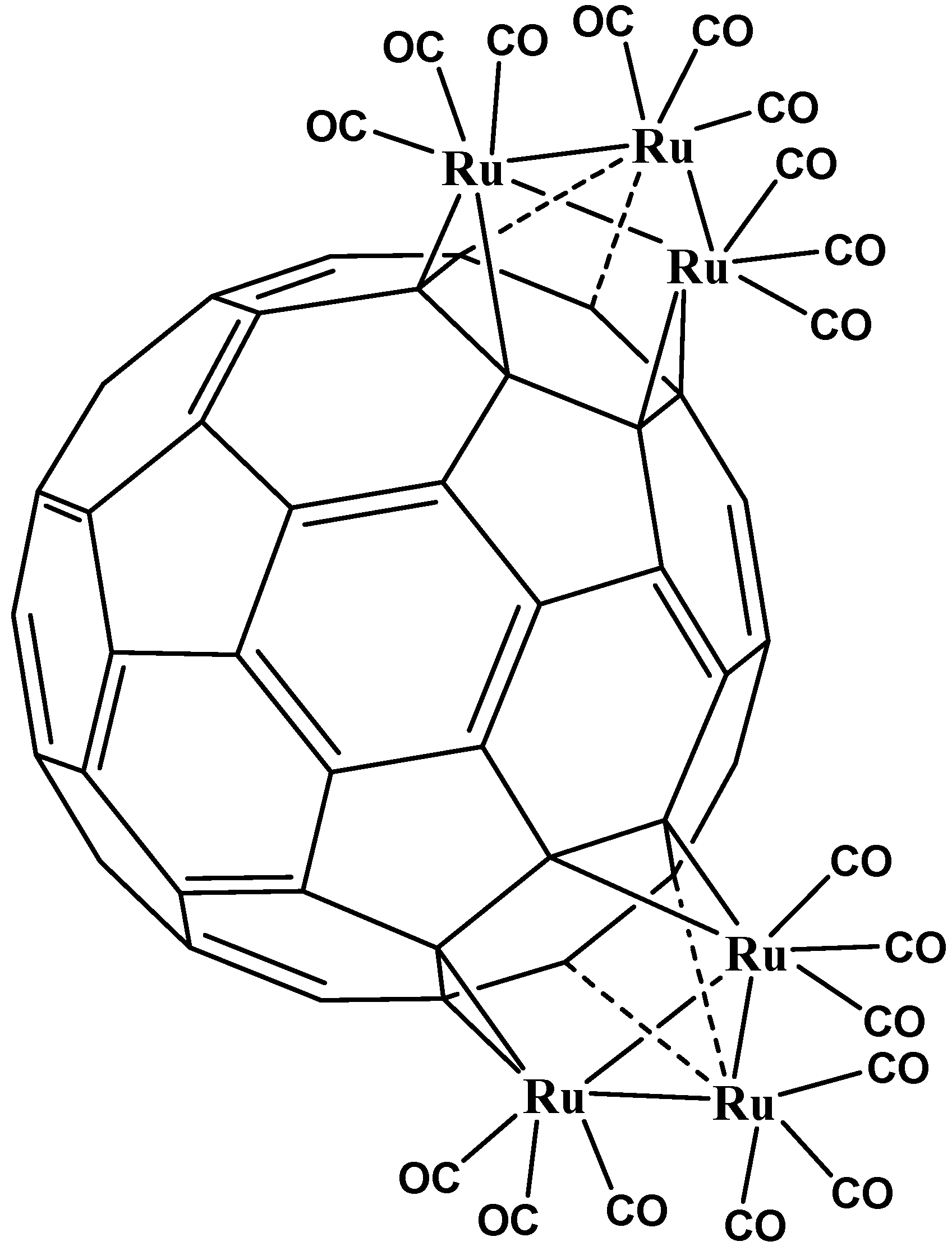
5. η3 and η4 Hapticities
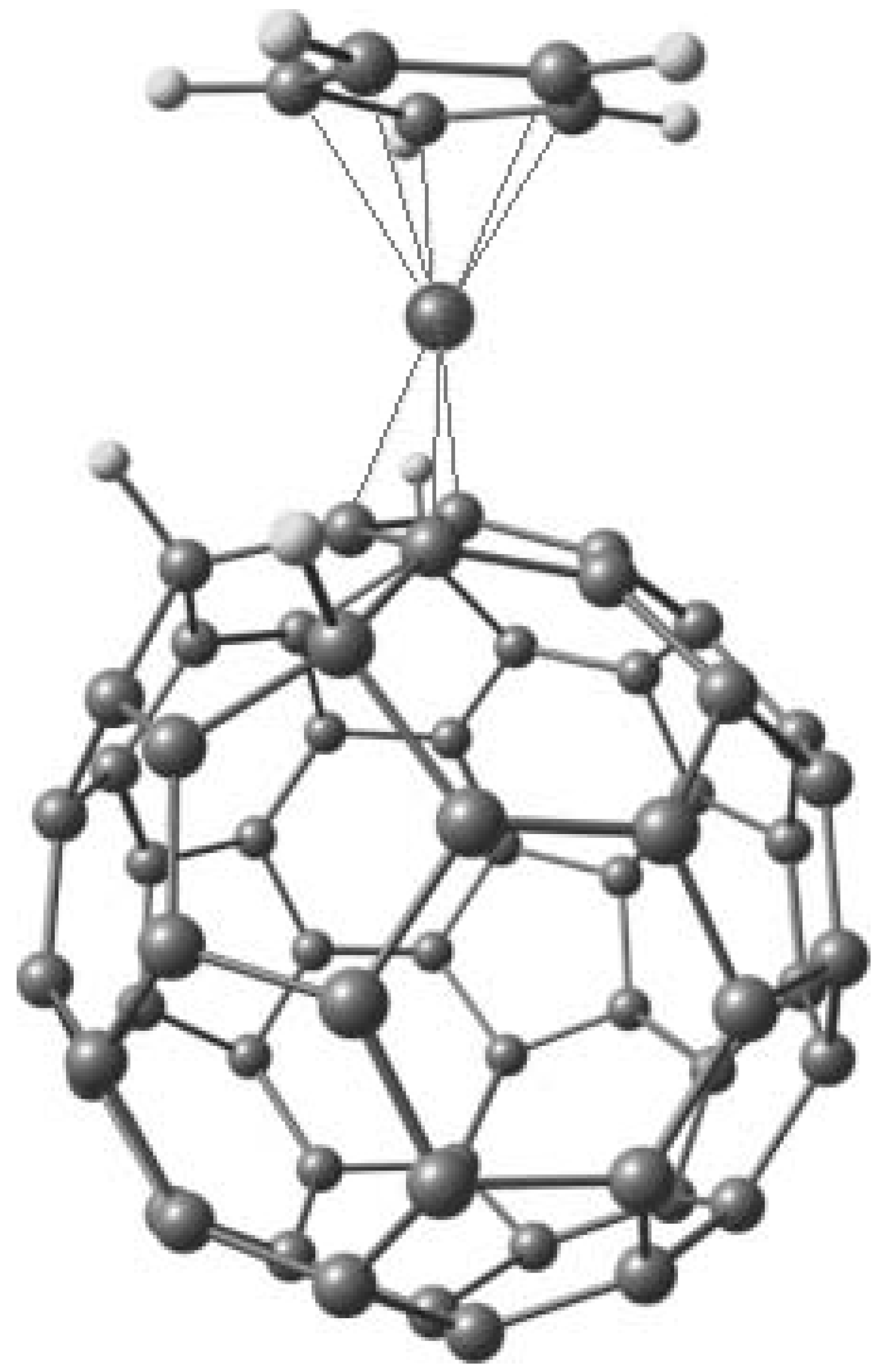
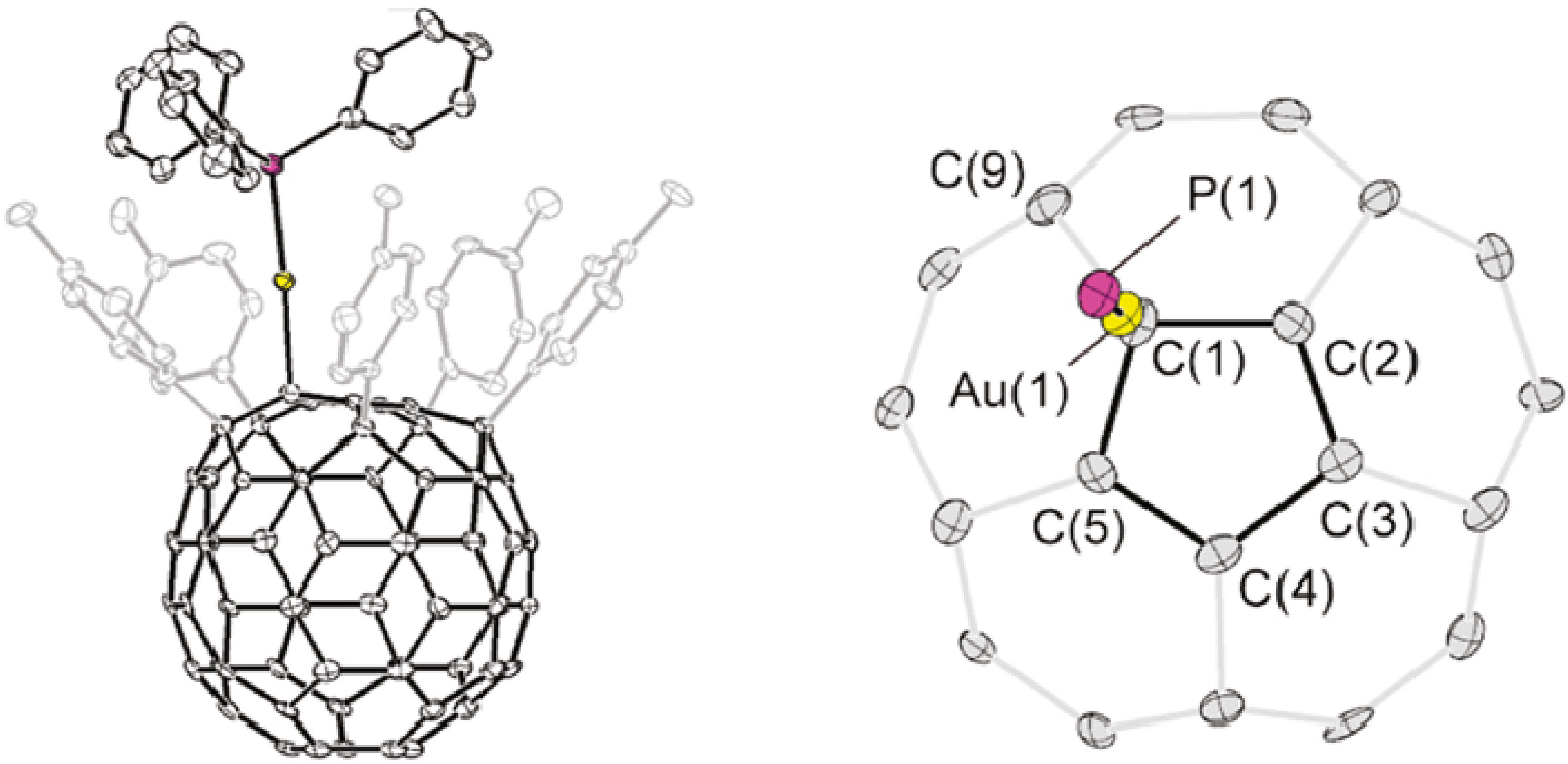

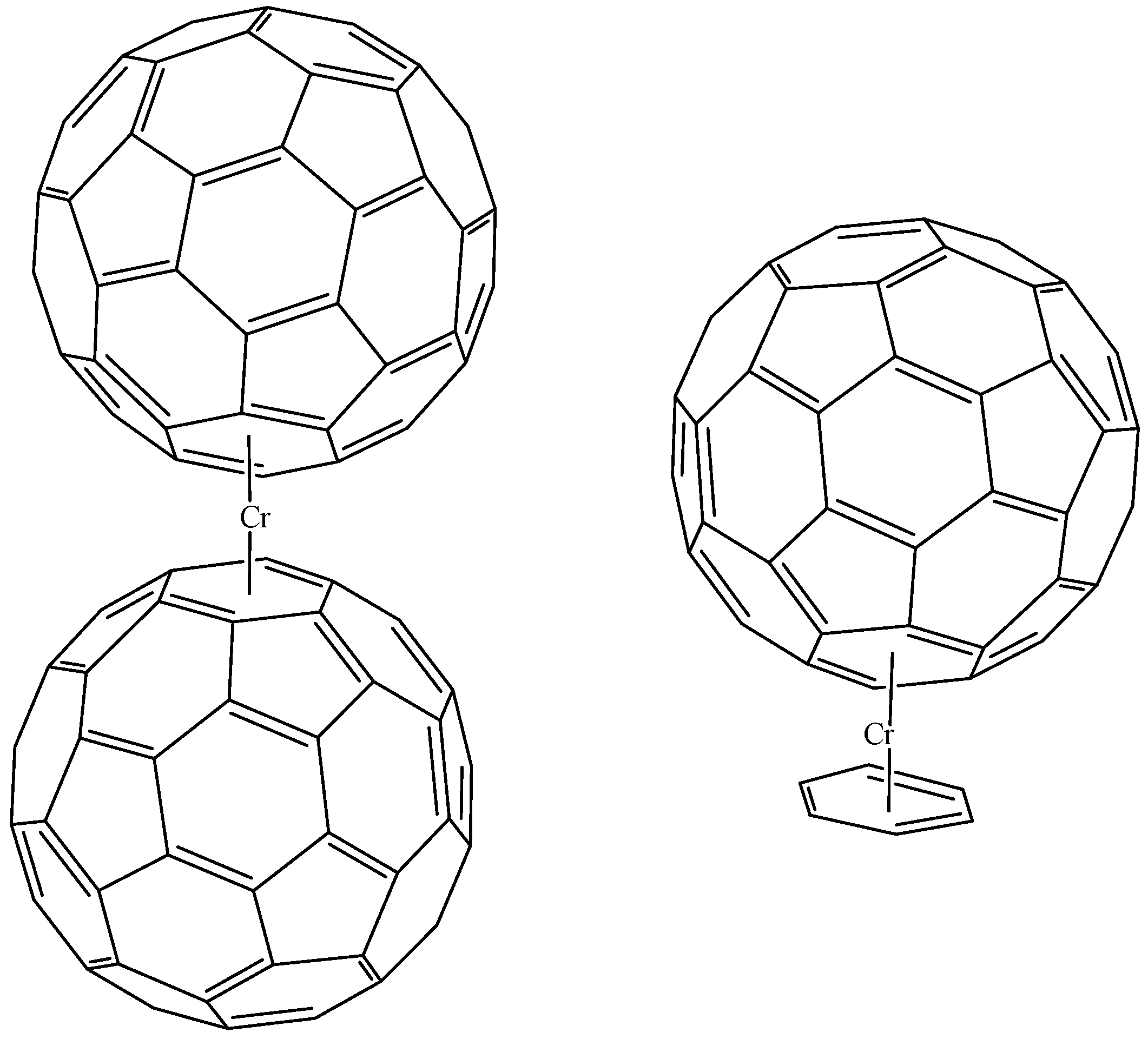
6. η5 Hapticity
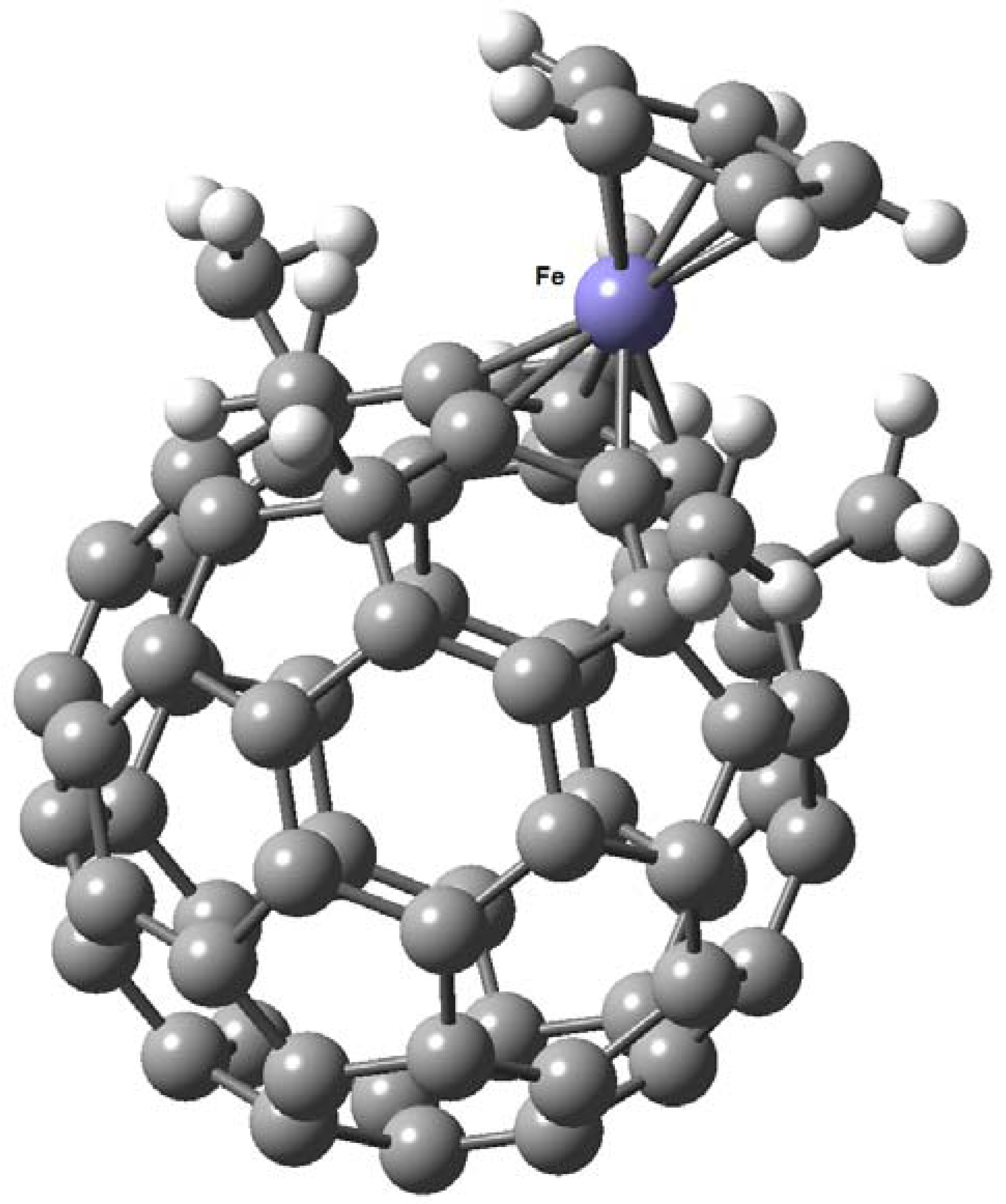
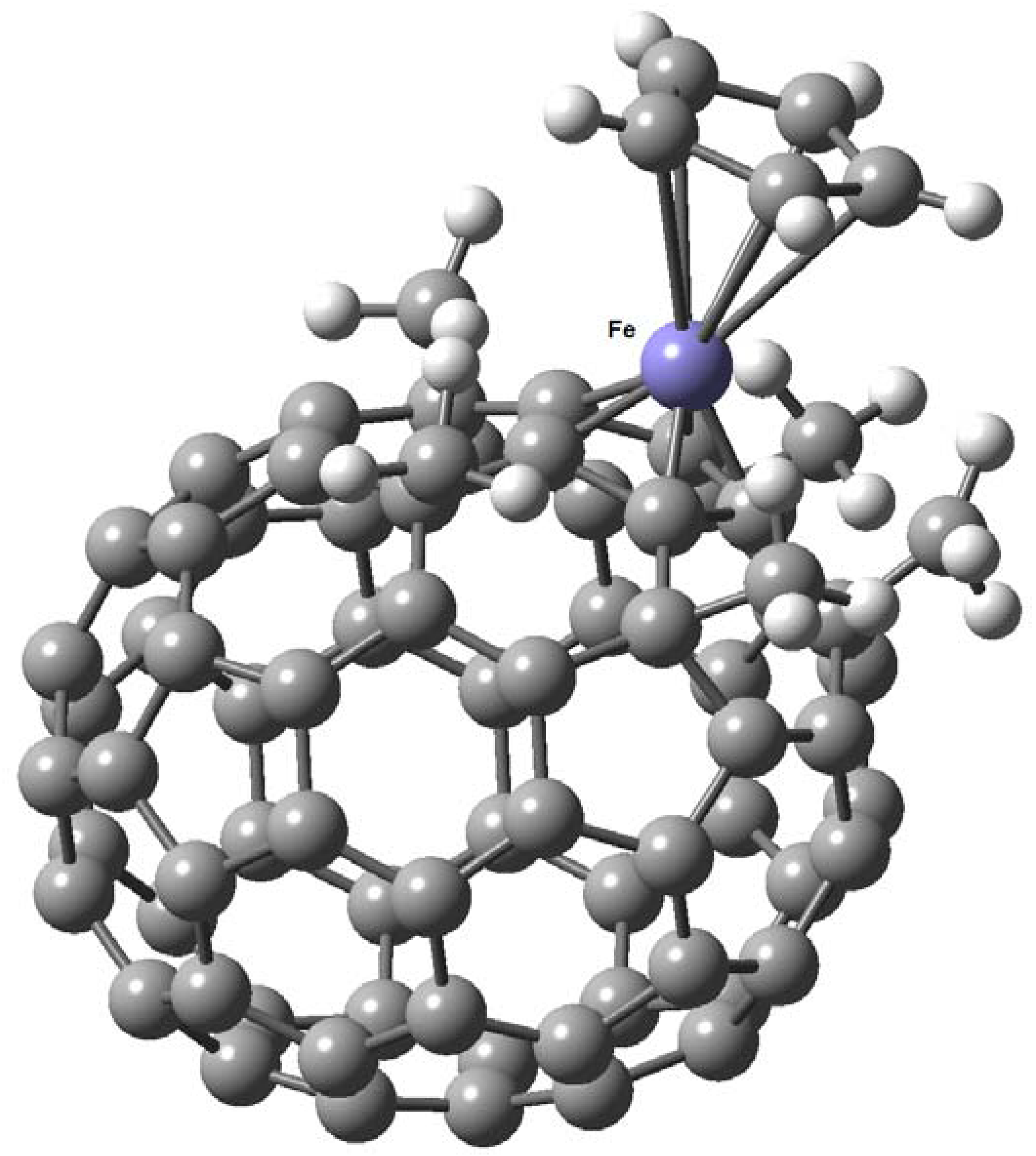

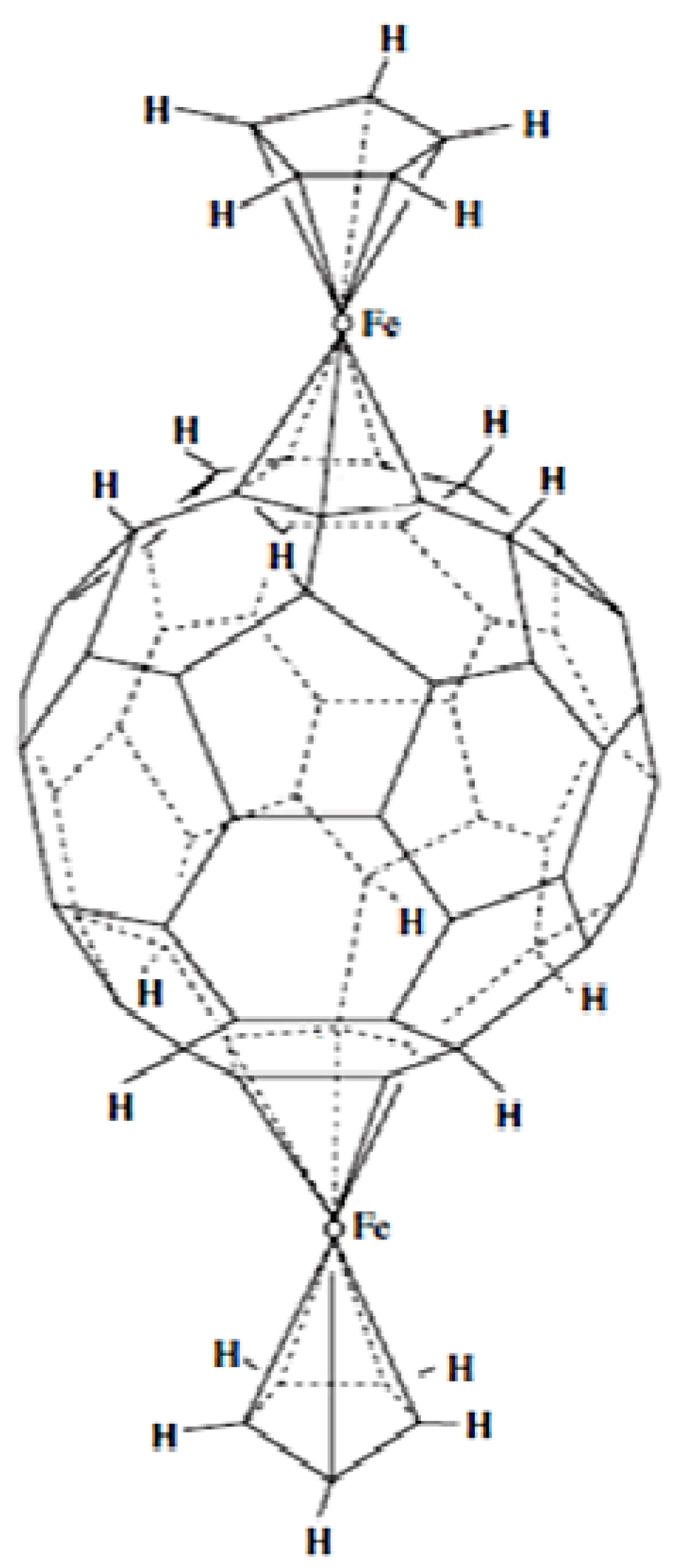
7. η6 Hapticity
9. Conclusions
Acknowledgements
References and Notes
- Fagan, P.J.; Calabrese, J.C.; Malone, B. The chemical nature of buckminsterfullerene and the characterization of a platinum derivative. Science 1991, 252, 1160–1161. [Google Scholar]
- Hawkins, J.M.; Meyer, A.; Lewis, T.A.; Loren, S.; Hollander, F.J. Crystal structure of osmilated C60. Confirmation of the soccer ball framework. Science 1991, 252, 312–313. [Google Scholar]
- Krätschmer, W.; Lamb, L.D.; Fastiropoulus, K.; Huffman, D.R. Solid C60 a new form of carbon. Nature 1990, 347, 354–356. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60-Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar]
- Balch, A.L.; Olmstead, M.M. Reactions of Transition Metal Complexes with Fullerenes (C60, C70, etc.) and Related Materials. Chem. Rev. 1998, 98, 2123–2165. [Google Scholar]
- Shinohara, H. Endohedral metallofulerenes. Rep. Prog. Phys. 2000, 63, 843–892. [Google Scholar] [CrossRef]
- Rodríguez-Fortea, A.; Balch, A.L.; Poblet, J.M. Endohedral metallofullerenes: A uniquehost-guest association. Chem. Soc. Rev. 2011, 40, 3551–3563. [Google Scholar] [CrossRef]
- Osuna, S.; Swart, M.; Solà, M. The reactivity of endohedral fullerenes. What can be learnt from computational studies? Phys. Chem. Chem. Phys. 2011, 13, 3585–3603. [Google Scholar]
- Yang, S.; Liu, F.; Chen, C.; Jiao, M.; Wei, T. Fullerenes encaging metal clusters—Clusterfullerenes. Chem. Commun. 2011, 47, 11822–11839. [Google Scholar] [CrossRef]
- Hirsch, A.; Lamparth, I.; Karfunkel, P.D.H.R. Fullerene Chemistry in three dimensions: Isolation of seven regioisomeric bisadducts and chiral trisadducts of C60 and di(ethoxicarbonyl)methylene, Angew. Chem. Int. Ed. Engl. 1994, 33, 437–438. [Google Scholar] [CrossRef]
- Lichtenberger, D.L.; Wright, L.L.; Gruhn, N.E.; Rempe, M.E. Electronic structure of exohedral interactions between C60 and transition metals. J. Organomet. Chem. 1994, 478, 213–221. [Google Scholar]
- Lichtenberger, D.L.; Wright, L.L.; Gruhn, N.E.; Rempe, M.E. Electronic structure and bonding of C60 to metals. Synth. Metals 1993, 59, 353–367. [Google Scholar] [CrossRef]
- Sokolov, V.I. Fullerenes as a new type of ligands for transition metals. Russ. J. Coord. Chem. 2007, 33, 723–737. [Google Scholar]
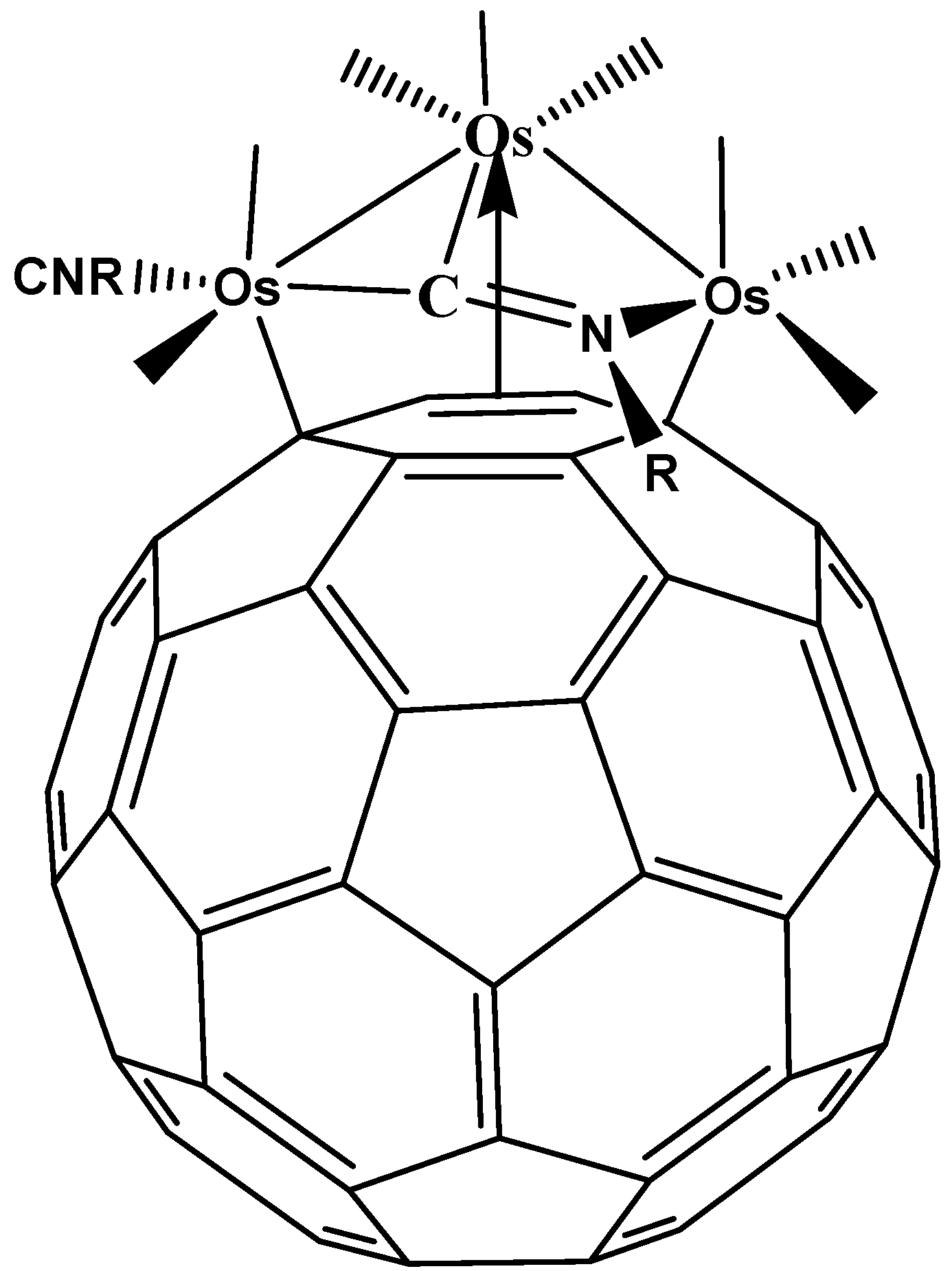
- Song, H.; Lee, K.; Park, J.T.; Chang, H.Y.; Choi, M.-G. Fluxional process and structural characterization of μ3-η2η2η2-C60 triosmium cluster complexes, Os3(CO)9-n(PMe3)nμ3-η2η2η2-C60 (n = 1,2,3). J. Organomet. Chem. 2000, 599, 49–56. [Google Scholar]
- Usatov, A.V.; Martynova, E.V.; Dolgushin, F.M.; Peregudov, A.S.; Antipin, M.Y.; Novikov, Y.N. Selective insertion of dioxygen into an (η2-C60)-Ir bond: Synthesis and Structure of the first metallacyclic complex with a σ-coordinated fullerene molecule. Eur. J. Inorg.Chem. 2003, 29–33. [Google Scholar]
- Zhang, S.; Brown, T.L.; Du, Y.; Shapley, J.R. Metalation of C60 with pentacarbonylrhenium radicals. Reversible formation of {C60-Re(CO5)}2. J. Am. Chem. Soc. 2003, 115, 6705–6709. [Google Scholar]
- Arce, M.J.; Viado, A.L.; An, Y.-Z.; Khan, S.I.; Rubin, Y. Triple scission of a six membered ring on the surface of C60 via consecutive pericyclic reactions and oxidative cobalt insertion. J. Am. Chem. Soc. 1996, 118, 3775–3776. [Google Scholar]
- Han, Y.-K.; Kim, K.H.; Kim, J.C.; Park, B.K.; Park, J.T. 60-Fullerene metal cluster complexes: Understanding η1 and η2[6,5] bonding modes of metallofullerenes. Eur. J. Inorg. Chem. 2010, 1530–1535. [Google Scholar]
- Olmstead, M.M.; Maitra, K.; Balch, A.L. Formation of a curved silver nitrate network that conforms to the shape of C60 and encapsulated the fullerene-Structural characterization of C60{Ag(NO3)}5. Angew. Chem. Int. Ed. Engl. 1999, 38, 231–233. [Google Scholar] [CrossRef]
- Song, H.; Lee, K.; Choi, M.-G.; Park, J.T. [60]Fullerene as a versatile four electron donor ligand. Organometallics 2002, 21, 1756–1758. [Google Scholar] [CrossRef]
- Konarev, D.V.; Khasanov, S.S.; Otsuka, A.; Lyubovskaya, R.N.; Yoshida, Y.; Otsuka, A. The interaction of C60, C70 and C60(CN)2 radical anions with cobalt(II) tetraphenylporphyrin in solid multicomponent complexes. Chem. Eur. J. 2003, 9, 3837–3848. [Google Scholar] [CrossRef]
- Konarev, D.V.; Khasanov, S.S.; Otsuka, A.; Otsuka, A.; Lyubovskaya, R.N. Peculiarities of C60− coordination cobalt(II) octaethylporphyrin in ionic multicomponent complexes: Observation of the reversible formation of Co-C(C60−) coordination bonds. Chem. Eur. J. 2006, 12, 5225–5230. [Google Scholar] [CrossRef]
- Howard, J.A.; Tomietto, M.; Wilkinson, D.A. Electron Paramagnetic Resonance study of the reaction of Ag atoms with C60 in cyclohexane on a rotating cryostat. J. Am. Chem. Soc. 1991, 113, 7870–7872. [Google Scholar]
- Kim, H.H.; Jung, J.; Han, Y.-K. Geometric and electronic structures of Os3(CO)9(μ3-η2η2η2-C60), Os3(CO)8(P(CH3)3(μ3-η2η2η2-C60) and their anions (Q = −1 to −4): Reduction induced conversion of π to σ C60 -Metal complexes. Organometallics 2004, 23, 3865–3869. [Google Scholar] [CrossRef]
- Bashilov, V.V.; Petrovski, P.V.; Sokolov, V.I. Synthesis of the first optically active organometallic fullerene derivative by η2 functionalization of buckminsterfullerene by Pd(0) complexes. Russ. Chem. Bull. 1993, 42, 392–393. [Google Scholar] [CrossRef]
- Hawkins, J.M.; Meyer, A.; Lewis, T.A. Crystal structure of osmilated C60. Confirmation of the soccer ball framework. Science 1991, 252, 312–313. [Google Scholar]
- Wu, Y.-Y.; Yeh, W.-Y. Synthesis of diphosphino-fullerene 1,2,4,15-(PPh2)2(H)2C60 and its complexation with triosmium carbonyl complexes. Organometallics 2011, 30, 4792–4795. [Google Scholar] [CrossRef]
- Chen, C.-S.; Lin, C.-S.; Yeh, W.-Y. Synthesis of Mo(CO)2(η3-PPh2(o-C6H4)C(=O)H)2 and its reaction with C60. J. Organomet. Chem. 2011, 696, 1474–1478. [Google Scholar] [CrossRef]
- Ivanova, V.N. Fullerene compounds with transition metals MnC60: Preparation, structure and properties. J. Struct. Chem. 2000, 41, 135–148. [Google Scholar] [CrossRef]
- Nunzi, F.; Sgamellotti, A.; Re, N.; Floriani, C. A density functional study of C60 transition metal complexes. Organometallics 2000, 19, 1628–1634. [Google Scholar] [CrossRef]
- Chatt, J.; Duncanson, L.A. Olefin co-ordination compounds. Part III. Infra-red spectra and structure: attempted preparation of acetylene complexes. J. Chem. Soc. 1953, 2939–2947. [Google Scholar]
- Dewar, M.J.S.; Ford, G.P. Relationship between olefinic .pi. complexes and three membered rings. J. Am. Chem. Soc. 1979, 101, 783–791. [Google Scholar] [CrossRef]
- Loboda, O. A DFT study on exohedral metallofullerenes: Structural and electronic properties. Fuller. Nanotub. Carbon Nanostruct. 2009, 17, 457–475. [Google Scholar] [CrossRef]
- Mavunkal, I.J.; Chi, Y.; Peng, S.M.; Lee, G.-H. Preparation and Structure of Cp*2Ru2(μ-Cl)(μ-X)C60, X=H,Cl. Novel Dinuclear Fullerene Complexes with and without Direct Ruthenium-Ruthenium Bonding. Organometallics 1995, 14, 4454–4456. [Google Scholar]
- Hsu, H.-F.; Wilson, S.R.; Shapley, J.R. Triruthenium cluster complexes of C70. Synthesis and structural characterization of {Ru3(CO)9}x(μ3-η2,η2,η2-C70)] (x = 1, 2). Chem. Commun. 1997, 12, 1125–1126. [Google Scholar]
- Chen, Z.; King, R.B. Spherical aromaticity: Recent works in fullerenes, polyhedral boranes and related structures. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef]
- Stankevich, I.V.; Sokolov, V.I. Advances in fullerene chemistry. Russ. Chem. Bull. Int. Ed. 2004, 53, 1824–1845. [Google Scholar] [CrossRef]
- Stankevich, I.V.; Chistyakov, A.L. Metal complexes of allyl derivatives of C60 fullerene: Molecular and electronic prediction from density functional calculations. Russ. Chem. Bull. 2003, 52, 1272–1279. [Google Scholar] [CrossRef]
- Halim, M.; Kennedy, R.D.; Suzuki, M.; Khan, S.I.; Diaconescu, P.L.; Rubin, Y. Complexes of gold(I), silver and cooper with pentaaryl [60] fulleride. J. Am. Chem. Soc. 2011, 133, 6841–6851. [Google Scholar]
- Chistyakov, A.L.; Stankevich, I.V. On the possibility of existence of η4-π complexes of corannulene (C20H10) and C60 fullerene derivatives. Russ. Chem. Bull. 2002, 51, 230–239. [Google Scholar] [CrossRef]
- Rogers, J.R.; Marynick, D.S. Theoretical estimates of the η6 bonding capability of buckminsterfullerene in transition metal complex. Chem. Phys. Lett. 1993, 205, 197–199. [Google Scholar] [CrossRef]
- Kang, H.S. A theoretical study of fullerene-ferrocene hybrids. J. Comp. Chem. 2006, 28, 594–600. [Google Scholar] [CrossRef]
- Chistyakov, A.L.; Stankevich, I.V. Cyclopentadienyl type η5-π-complexes of C60 fullerene derivatives with indium and thalium: Simulation of molecular and electronic structure by the MNDO/PM3 method. Russ. Chem. Bull. 1997, 46, 1832–1837. [Google Scholar] [CrossRef]
- Nakamura, E. Bucky ferrocene: Hybrid of ferrocene and fullerene. Pure Appl. Chem. 2003, 75, 427–434. [Google Scholar] [CrossRef]
- Sawamura, M.; Kuninobu, Y.; Toganoh, M.; Matsuo, Y.; Yamanaka, M.; Nakamura, E. Hybrid of ferrocene and fullerene. J. Am. Chem. Soc. 2002, 124, 9354–9355. [Google Scholar]
- Nakamura, E.; Sawamura, M. Chemistry of η5-fullerene metal complexes. Pure Appl. Chem. 2001, 73, 355–359. [Google Scholar] [CrossRef]
- Kaji, T.; Shimada, T.; Inoue, H.; Kuninobu, Y.; Matsuo, Y.; Nakamura, E.; Saiki, K. Molecular orientation and electronic structure of epitaxial bucky ferrocene (Fe(C60(Ch3)5)C5H5) thin films. J. Phys. Chem. B 2004, 108, 9914–9918. [Google Scholar] [CrossRef]
- Toganoh, M.; Matsuo, Y.; Nakamura, E. Rhenium templated regioselective polyhydrogenation reaction of [60] Fullerene. Angew. Chem. 2003, 115, 3654–3656. [Google Scholar] [CrossRef]
- Toganoh, M.; Matsuo, Y.; Nakamura, E. Synthesis of ferrocene/hydrofullerene hybrid and functionalized bucky ferrocenes. J. Am. Chem. Soc. 2003, 125, 13974–13975. [Google Scholar] [CrossRef]
- Matsuo, Y.; Nakamura, E. Ruthenium (II) complexes of pentamethylated [60] fullerene. Alkyl, alkynyl, chloro, isocyanide and phosphine complexes. Organometallics 2003, 22, 2554–2563. [Google Scholar]
- Matsuo, Y.; Iwashita, A.; Nakamura, E. Synthesis and derivatization of iridium (I) and Iridium (III) pentamethyl [60] fullerene complexes. Organometallics 2005, 24, 89–95. [Google Scholar]
- Gal’pern, E.G.; Stankevich, I.V.; Chistyakov, A.L. Modeling of the molecular and electronic structures of multiple decker sandwich macromolecules with η5-π bonds on the basis of biscyclopentadienyl derivatives C60H10 of a C60 fullerene. Phys. Sol. State 2001, 43, 989–992. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tahara, K.; Nakamura, E. Synthesis and electrochemistry of double-decker buckyferrocenes. J. Am. Chem. Soc. 2006, 128, 7154–7155. [Google Scholar] [CrossRef]
- Marczak, R.; Wielopolski, M.; Gayathri, S.S.; Guldi, D.M.; Matsuo, Y.; Matsuo, K.; Tahara, K.; Nakamura, E. Uniquely shaped double decker buckyferrocenes-distinct donor-acceptor interactions. J. Am. Chem. Soc. 2008, 130, 16207–16215. [Google Scholar]
- Nakamura, E.; Isobe, H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology and nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar]
- Stewart, M.P.; Butterick, R.III.; Sneddon, R.G.; Matsuo, Y.; Geiger, W.E. Voltammetry of half sandwich manganese group complexes of η6-PhC3B7H9 and η5C60Bn2PhH2, two ligands that are cyclopentadienyl mimics. Inorg. Chim. Acta 2010, 364, 251–254. [Google Scholar] [CrossRef]
- Gal’pern, E.G.; Gambaryan, N.P.; Stankevich, I.V.; Chistyakov, A.L. Fullerene C60 as a η5 and η6 ligand in sandwich type π-complexes with transition metals. Russ. Chem. Bull. 1994, 43, 547–550. [Google Scholar] [CrossRef]
- Francis, M.D.; Boltalina, O.V.; Nixon, J.F.; Taylor, R. Evidence for η6 coordination to a fullerene. Fuller. Nanotub. Carbon Nanostruct. 2004, 11, 115–121. [Google Scholar]
- Salcedo, R. Fullerenocene. Polyhedron 2009, 28, 431–436. [Google Scholar] [CrossRef]
- Sokolov, V.I.; Gasanov, R.G.; Goh, L.Y.; Weng, Z.; Chistyakov, A.L.; Stankevich, I.V. Cyclopentadienyl) Chromiumtricarbonyl dimers as a source of metal-centered free radicals to form stable-η5 bonded spin-adducts with fullerene. J. Organomet. Chem. 2005, 690, 2370–2375. [Google Scholar]
- Molina, B.; Pérez-Manríquez, L.; Salcedo, R. On the π coordination of organometallic fullerene complexes. Molecules 2011, 16, 4652–4659. [Google Scholar] [CrossRef]
- Poater, J.; Fradera, X.; Durán, M.; Solà, M. An insight into the local aromaticities of polycyclic aromatic hydrocarbons and fullerenes. Chem. Eur. J. 2003, 9, 1113–1122. [Google Scholar] [CrossRef]
- Chen, S.-C.; Lin, C.-S.; Yeh, W.-Y. Synthesis of Mo(CO)2(η3-PPh2(o-C6H4)C(=O)H)2 and its reaction with C60. J. Organomet. Chem. 2011, 696, 1474–1478. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Soto, D.; Salcedo, R. Coordination Modes and Different Hapticities for Fullerene Organometallic Complexes. Molecules 2012, 17, 7151-7168. https://doi.org/10.3390/molecules17067151
Soto D, Salcedo R. Coordination Modes and Different Hapticities for Fullerene Organometallic Complexes. Molecules. 2012; 17(6):7151-7168. https://doi.org/10.3390/molecules17067151
Chicago/Turabian StyleSoto, Delia, and Roberto Salcedo. 2012. "Coordination Modes and Different Hapticities for Fullerene Organometallic Complexes" Molecules 17, no. 6: 7151-7168. https://doi.org/10.3390/molecules17067151
APA StyleSoto, D., & Salcedo, R. (2012). Coordination Modes and Different Hapticities for Fullerene Organometallic Complexes. Molecules, 17(6), 7151-7168. https://doi.org/10.3390/molecules17067151



