Functional and Preliminary Characterisation of Hydrocolloid from Tamarillo (Solanum betaceum Cav.) Puree
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrocolloid Yield of Various Tropical and Sub-Tropical Fruits Using Water Extraction Method
| Common name | Botanical name | Fraction | Moisture content (%) | Yield, Yf (% fresh weight) | Yield, Y (% dry weight) |
|---|---|---|---|---|---|
| Tamarillo (buah cinta) | Solanum betaceum Cav. | Pulp | 85.82 a ± 0.13 | 1.19 a ± 0.02 | 8.39 a ± 0.03 |
| Seed mucilage | 88.70 b ± 0.30 | 0.40 bf ± 0.08 | 3.54 b ± 0.16 | ||
| Puree | 85.78 a ± 0.09 | 1.18 ac ± 0.10 | 8.30 c ± 0.07 | ||
| Papaya | Carica papaya | Pulp | 84.64 c ± 0.04 | 1.11 acd ± 0.06 | 7.23 d ± 0.15 |
| Sapodilla (ciku) | Manilkara zapota | Pulp | 80.92 d ± 0.06 | 1.08 cd ± 0.11 | 5.66 e ± 0.23 |
| Mango | Mangifera indica | Pulp | 84.28 ce ± 0.03 | 1.04 d ± 0.13 | 6.62 f ± 0.07 |
| Kiwifruit | Actinidia deliciosa | Pulp | 87.01 f ± 0.04 | 0.90 e ± 0.04 | 6.93 g ± 0.15 |
| Mandarin orange | Citrus reticulata | Peel | 75.20 g ± 0.27 | 0.47 b ± 0.02 | 1.90 h ± 0.10 |
| Garden tomato | Lycopersicon esculentum | Whole without seeds | 91.24 h ± 0.15 | 0.42 bf ± 0.08 | 4.79 i ± 0.08 |
| Pineapple | Ananas comosus | Pulp | 89.95 i ± 0.03 | 0.40 bf ± 0.03 | 3.98 j ± 0.11 |
| Marian plum (kundang) | Bouea macrophylla | Pulp | 84.13 e ± 0.15 | 0.34 f ± 0.10 | 2.14 k ± 0.03 |
| Red dragon fruit | Hylocereus polyrhizus | Pulp | 87.69 j ± 0.08 | 0.33 f ± 0.11 | 2.68 l ± 0.07 |
| Guava | Psidium guajava | Whole without seeds | 91.29 h ± 0.08 | 0.20 g ± 0.02 | 2.30 m ± 0.06 |
| Water apple (jambu air) | Syzygium aqueum | Whole | 92.50 k ± 0.03 | 0.20 g ± 0.03 | 2.67 l ± 0.06 |
| Jackfruit | Artocarpus heterophyllus | Pulp | 77.92 l ± 0.33 | 0.2 0g ± 0.05 | 0.91 n ± 0.09 |
| Honeydew | Cucumis melo | Whole without seeds | 94.36 m ± 0.03 | 0.20 g ± 0.02 | 3.55 b ± 0.23 |
| Red apple | Malus pumila | Whole without seeds | 83.56 n ± 0.23 | 0.16 g ± 0.06 | 0.97 o ± 0.04 |
| Cupuassu | Theobroma grandiflorum | Pulp | NA | NA | 7.00 g ± NA [16] * |
| Longan | Dimocarpus longan | Pulp | NA | NA | 4.46 i ± 0.09 [17] * |
| Gold kiwifruit | Actinidia chinensis | Whole | 80.99 d ± 0.14 | NA | 6.69 f ± NA [18] * |
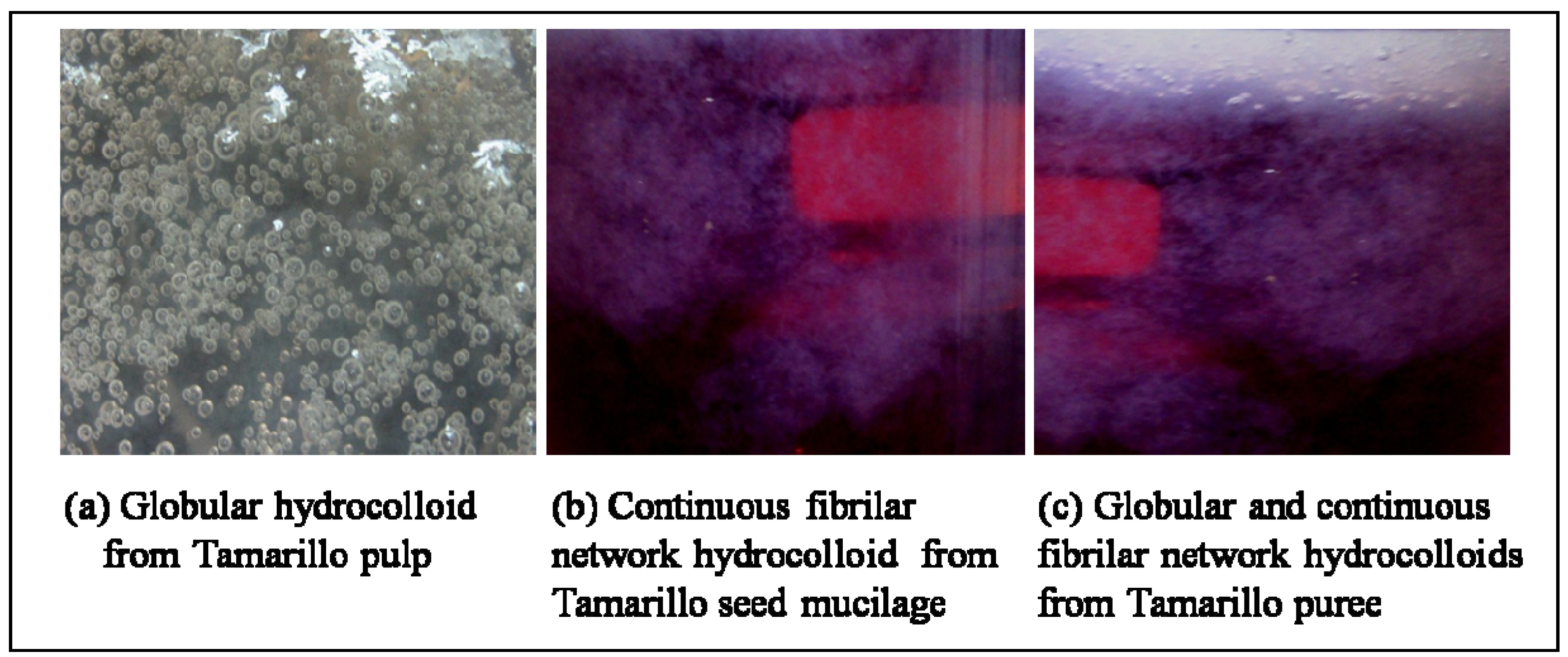
2.2. Proximate Compositions of Tamarillo Hydrocolloid
| Component | Composition |
|---|---|
| Moisture (%) | 10.65 ± 0.32 |
| Dry matter (%) | 89.35 ± 0.32 |
| Ash (% dry weight) | 0.80 ± 0.09 |
| Protein (% dry weight) | 21.18 ± 0.06 |
| Starch (% dry weight) | 0.83 ± 0.06 |
| Dietary fibre by difference a (% dry weight) | 66.48 ± 0.52 |
2.3. Functional Properties of Tamarillo Hydrocolloid in Comparison to that of Commercial Hydrocolloids
2.3.1. Water-Holding Capacity (WHC) and Oil-holding Capacity (OHC)
| Type of hydrocolloid | WHC (g water/g dry sample) | OHC (g oil/g dry sample) |
|---|---|---|
| THwater | 5.82 a ± 0.75 | 2.00 ab ± 0.07 |
| Agar-agar | 7.99 b ± 0.80 | 2.25 b ± 0.07 |
| Apple pectin | 6.71 ab ± 0.52 | 2.11 ab ± 0.17 |
| Bovine gelatine | 0.00 c ± 0.00 | 1.06 cfg ± 0.03 |
| Carrageenan | 28.21 d ± 0.92 | 1.31 dh ± 0.05 |
| Citrus pectin | 1.38 c ± 0.06 | 1.55 de ± 0.09 |
| CMC | 0.00 c ± 0.00 | 1.58 e ± 0.03 |
| Gum arabic | 0.28 c ± 0.15 | 1.00 cf ± 0.10 |
| Karaya gum | 24.39 e ± 0.17 | 1.12 cfgh ± 0.02 |
| Sodium alginate | 0.00 c ± 0.00 | 1.22 fgh ± 0.02 |
| Wheat starch | 0.74 c ± 0.02 | 0.92 c ± 0.03 |
| Xanthan gum | 62.63 f ± 0.91 | 1.28 gh ± 0.03 |
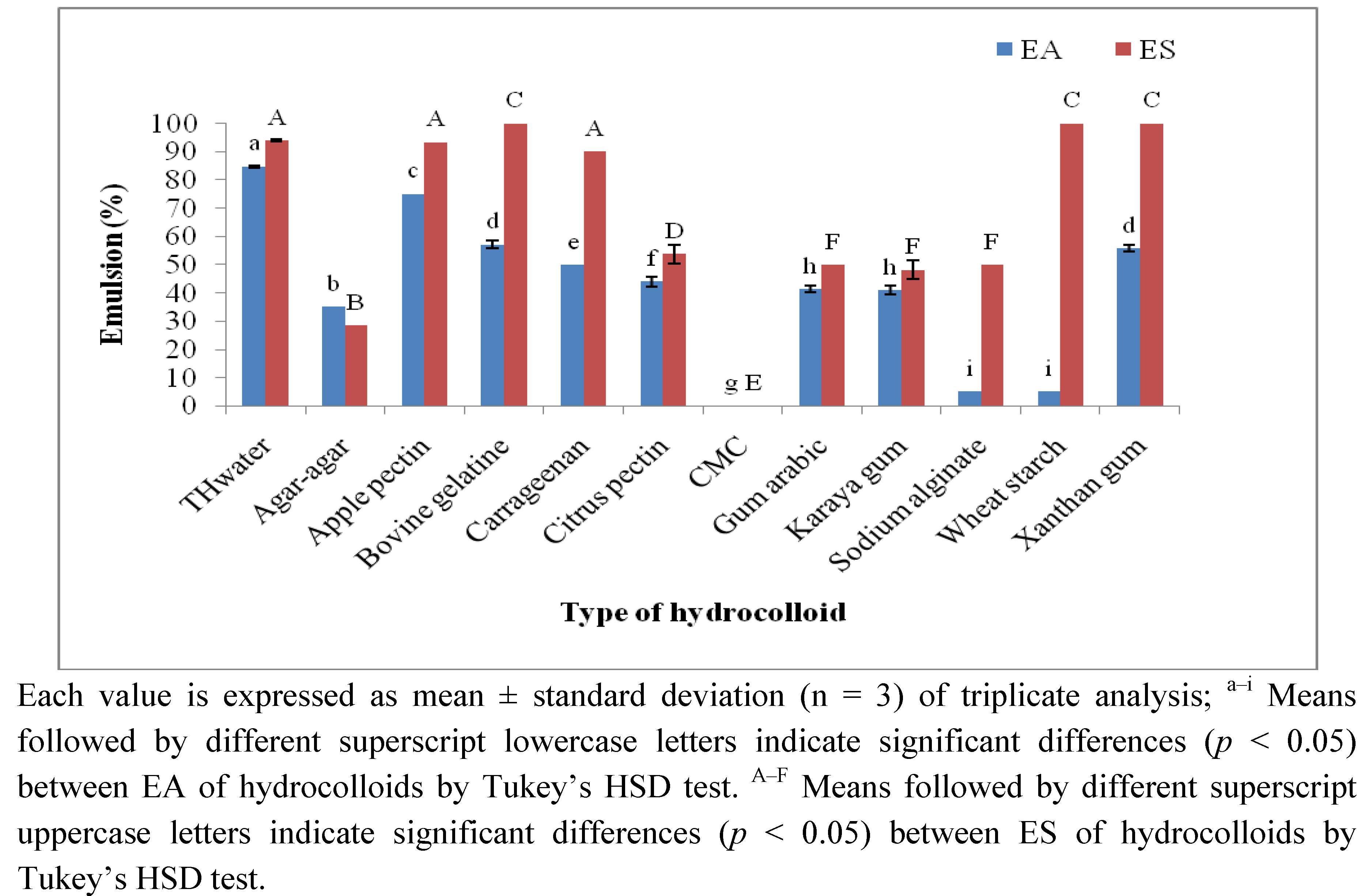
2.3.2. Emulsifying Activity (EA) and Emulsion Stability (ES)
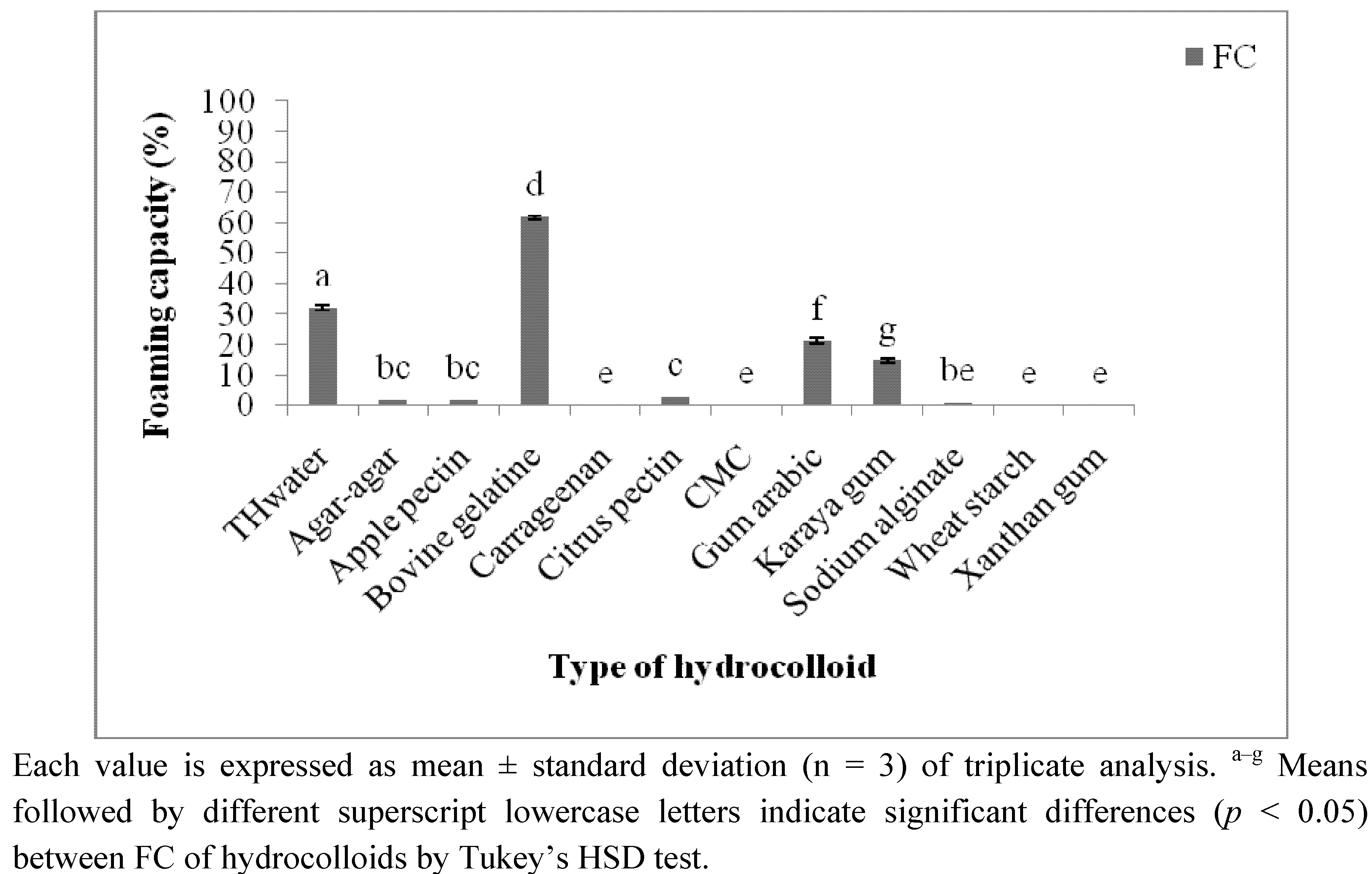
2.3.3. Foaming Capacity (FC) and Foaming Stability (FS)
| Hydrocolloids | Foaming stability (%) at time, t (min) | |||||
|---|---|---|---|---|---|---|
| t = 1 | t = 10 | t = 30 | t = 60 | t = 90 | t = 120 | |
| THwater | 97.59 aA | 91.96 aB | 84.99 aC | 81.51 aD | 79.36 aE | 79.36 aE |
| Agar-agar | 100 bA | 0 bB | 0 bB | 0 bB | 0 bB | 0 bB |
| Apple pectin | 100 bA | 100 cA | 100 cA | 50 cB | 50 cB | 50 cB |
| Bovine gelatine | 90.36 cA | 51.80 dB | 31.57 dC | 23.87 dD | 19.77 dE | 11.01 dF |
| Carrageenan | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Citrus pectin | 66.67dA | 66.67 eA | 66.67 eA | 66.67 eA | 66.67 eA | 66.67 eA |
| CMC | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Gum arabic | 90.46 cA | 82.37 fB | 66.77 eC | 46.40 fD | 28.69 fE | 22.25 fF |
| Karaya gum | 97.62 aA | 85.79 gB | 75.87 fC | 75.87 gC | 63.1 gD | 48.73 gE |
| Sodium alginate | 100 bA | 100 cA | 100 cA | 50 cB | 25 hC | 12.5 hD |
| Wheat starch | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Xanthan gum | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
2.4. Functional Groups and Degree of Esterification
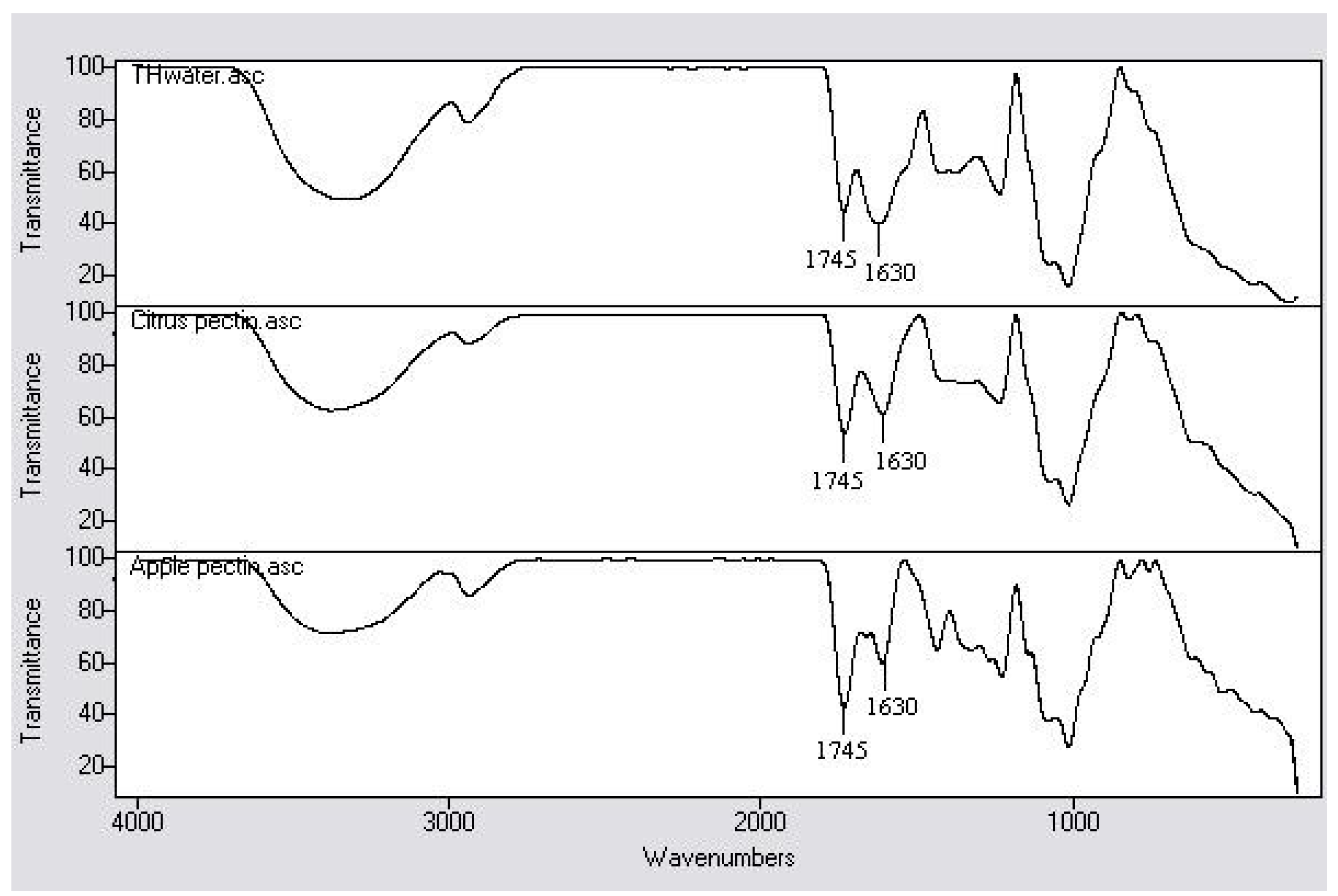
| Hydrocolloid | Degree of esterification (%) |
|---|---|
| THwater | 49.47 a ± 0.23 |
| Citrus pectin | 68.00 b ± 0.19 |
| Apple pectin | 69.64 c ± 0.26 |
2.5. Monosaccharide Composition of Tamarillo Hydrocolloid
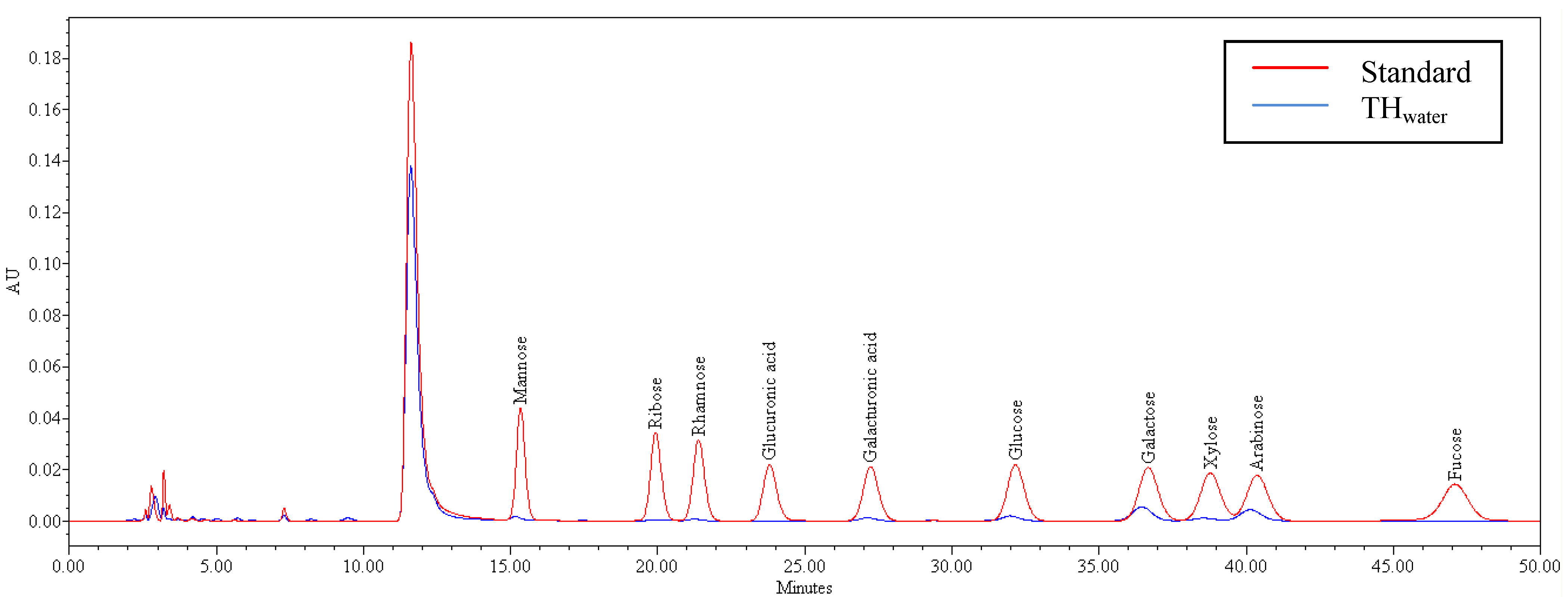
| Monosaccharide | Composition (mol%) a |
|---|---|
| Mannose | Tr b |
| Ribose | tr |
| Rhamnose | tr |
| Glucuronic acid | 0 |
| Galacturonic acid | 0.98 ± 0.28 |
| Glucose | 7.05 ± 0.21 |
| Galactose | 51.63 ± 0.97 |
| Xylose | tr |
| Arabinose | 38.80 ± 0.69 |
| Fucose | 0 |
3. Experimental
3.1. Materials
3.2. Sample Preparation for Hydrocolloid Extraction
3.3. Screening of Various Fruits for High Hydrocolloid Yield
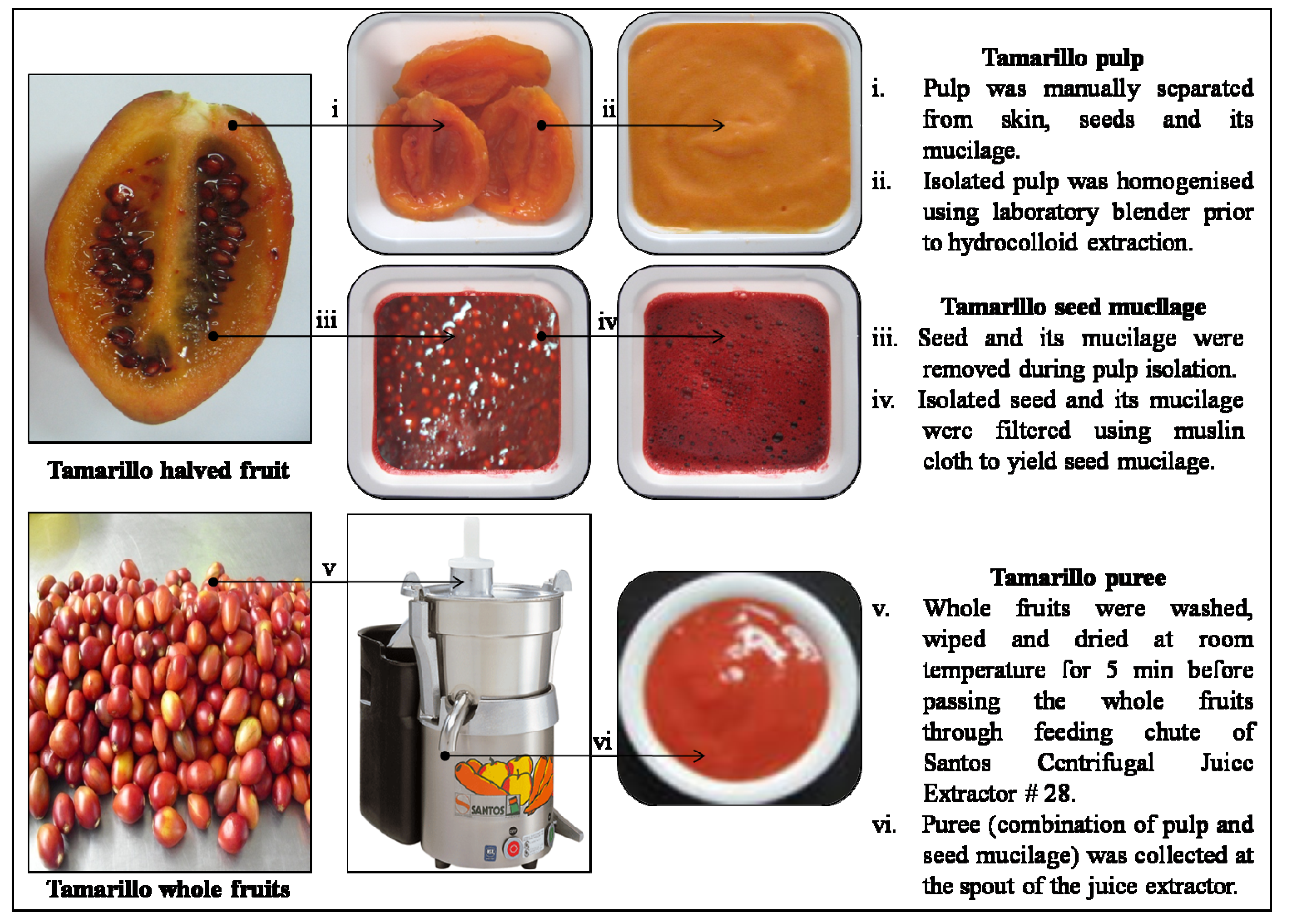

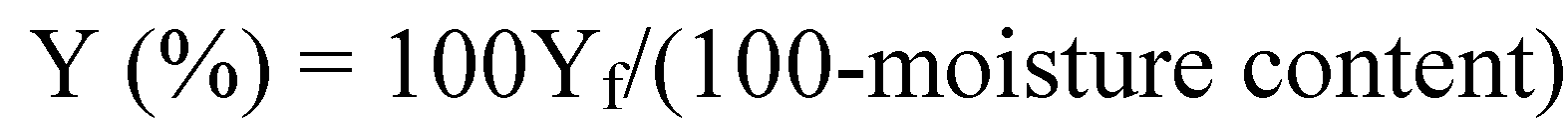
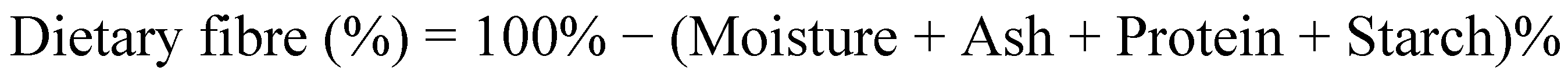
3.5. Functional Characterisation of Tamarillo Hydrocolloid in Comparison to that of Commercial Hydrocolloids
3.5.1. Water-holding Capacity (WHC) and Oil-holding Capacity (OHC)
3.5.2. Emulsifying Activity (EA) and Emulsion Stability (ES)


3.5.3. Foaming capacity (FC) and Foaming Stability (FS)

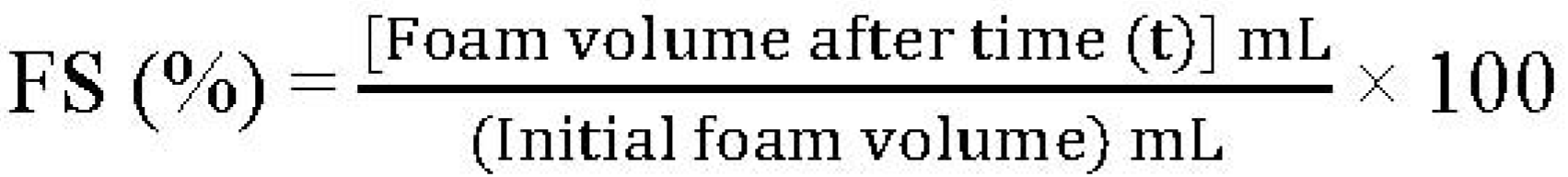
3.6. Functional Groups and Degree of Esterification Determination Using FT-IR Spectroscopy
3.7. Monosaccharide Profiling Using HPLC
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloid. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Harris, P.J.; Smith, B.G. Plant cell walls and cell-wall polysaccharides: Structures, properties and uses in food products. Int. J. Food Sci. Technol. 2006, 41, 129–143. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Introduction to food hydrocolloids. In Handbook of Hydrocolloids; Phillips, G.O, Williams, P.A., Eds.; Woodhead Publishing Ltd: Cambridge, UK, 2000; pp. 1–19. [Google Scholar]
- Walter, R.H. Classifications. In Polysaccharide Dispersions; Academic Press: New York, NY, USA, 1997; pp. 157–180. [Google Scholar]
- Funami, T. Next target for food hydrocolloid studies: Texture design of foods using hydrocolloid technology. Food Hydrocolloid. 2011, 25, 1904–1914. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N.; Gorinstein, S.; Martín-Belloso, O. Characterisation of peach dietary fibre concentrate as a food ingredient. Food Chem. 1999, 65, 175–181. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N.; Martín-Belloso, O. Influence of fruit dietary fibre addition on physical and sensorial properties of strawberry jams. J. Food Eng. 1999, 41, 13–21. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohyd. Polym. 2010, 82, 454–459. [Google Scholar] [CrossRef]
- Sanchez-Alonso, I.; Borderias, A.J. Technological effect of red grape antioxidant dietary fibre added to minced fish muscle. Int. J. Food Sci. Technol. 2008, 43, 1009–1018. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q.; He, Y.; He, X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int. J. Biol. Macromol. 2010, 47, 356–360. [Google Scholar] [CrossRef]
- Acosta-Quezada, P.G.; Martínez-Laborde, J.B.; Prohens, J. Variation among tree tomato (Solanum betaceum Cav.) accessions from different cultivar groups: Implications for conservation of genetic resources and breeding. Genet. Resour. Crop Evol. 2010. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. HPLC–PDA–MS/MS of anthocyanins and carotenoids from Dovyalis and Tamarillo fruits. J. Agric. Food Chem. 2007, 55, 9135–9141. [Google Scholar] [CrossRef]
- Ordonez, R.M.; Vattuone, M.A.; Isla, M.I. Changes in carbohydrate content and related enzyme activity during Cyphomandra betaceae (Cav.) Sendtn. fruit maturation. Postharvest Biol. Tec. 2005, 35, 293–301. [Google Scholar] [CrossRef]
- Mertz, C.; Gancel, A.-L.; Gunata, Z.; Alter, P.; Dhuique-Mayer, C.; Vaillant, F.; Perez, A.M.; Ruales, J.; Brat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009, 22, 381–387. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Polysaccharides from the pulp of cupuassu (Theobroma grandiflorum): Structural characterization of a pectic fraction. Carbohyd. Polym. 2009, 77, 72–79. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohyd. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Yuliarti, O.; Goh, K.; Matia-Merino, L.; Mawson, J.; Drummond, L.; Brennan, C.S. Int. J. Food Sci. Technol. 2008, 43, 2268–2277. [CrossRef]
- Brewer, M.S. Reducing the fat content in ground beef without sacrificing quality: A review. Meat Sci. 2012, 91, 385–395. [Google Scholar] [CrossRef]
- Sandrou, D.K.; Arvanitoyannis, I.S. Low-fat/calorie foods: Current state and perspectives. Crit. Rev. Food Sci. 2010, 40, 427–447. [Google Scholar]
- Alfredo, V.-O.; Gabriel, R.-R.; Luis, C.G.; David, B.A. Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.). LTW-Food Sci. Technol. 2009, 42, 168–173. [Google Scholar] [CrossRef]
- Naji, S.; Razavi, S.M.A.; Karazhiyan, H. Effect of thermal treatments on functional properties of cress seed (Lepidium sativum) and xanthan gums: A comparative study. Food Hydrocolloid. 2012, 28, 75–81. [Google Scholar] [CrossRef]
- Liu, L.; Cao, J.; Huang, J.; Cai, Y.; Yao, J. Extraction of pectins with different degrees of esterification from mulberry branch bark. Bioresource Technol. 2010, 101, 3268–3273. [Google Scholar] [CrossRef]
- Chatjigakis, A.K.; Pappas, C.; Proxenia, N.; Kalantzi, O.; Rodis, P.; Polissiou, M. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohyd. Polym. 1998, 37, 395–408. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin–a review. Crit. Rev. Food Sci. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- May, C.D. Pectins. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Ltd: Cambridge, UK, 2000; pp. 169–188. [Google Scholar]
- Yang, B.; Jiang, Y.; Zhao, M.; Chen, F.; Wanga, R.; Chen, Y.; Zhang, D. Structural characterisation of polysaccharides purified from longan (Dimocarpus longan Lour.) fruit pericarp. Food Chem. 2009, 115, 609–614. [Google Scholar] [CrossRef]
- Ramirez-Truquea, C.; Esquivela, P.; Carleb, R. Neutral sugar profile of cell wall polysaccharides of pitaya (Hylocereus sp.) fruits. Carbohyd. Polym. 2011, 83, 1134–1138. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Shiga, T.M.; Lajolo, F. Non-starch polysaccharide composition of two cultivars of banana (Musa acuminata L.: Cvs Mysore and Nanicao). Carbohyd. Polym. 2008, 71, 26–31. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Horwitz, W., Latimer, G.W., Jr., Eds.; AOAC International: Gaithersburg, MD, USA, 2005; Volume 18th ed. [Google Scholar]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.F. Hydration properties of dietary fiber and resistant starch: A European collaborative study. LTW-Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Chau, C.F.; Cheung, P.C.K.; Wong, Y.S. Functional properties of protein concentrate from three Chinese indigenous legume seeds. J. Agric. Food Chem. 1997, 45, 2500–2503. [Google Scholar] [CrossRef]
- Coffman, C.W.; Garcia, V.V. Functional properties and amino acid content of a protein isolate from mung bean flour. J. Food Technol. 1977, 12, 473–484. [Google Scholar]
- Lv, Y.; Yang, X.; Zhao, Y.; Ruan, Y.; Ying, Y.; Wang, Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.W.; Zhu, S.; Yin, H.P.; Wang, M.; Tang, J. Carbohyd. Polym. 2010, 82, 629–635. [CrossRef]
- Samples Availability: Samples of tamarillo are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gannasin, S.P.; Ramakrishnan, Y.; Adzahan, N.M.; Muhammad, K. Functional and Preliminary Characterisation of Hydrocolloid from Tamarillo (Solanum betaceum Cav.) Puree. Molecules 2012, 17, 6869-6885. https://doi.org/10.3390/molecules17066869
Gannasin SP, Ramakrishnan Y, Adzahan NM, Muhammad K. Functional and Preliminary Characterisation of Hydrocolloid from Tamarillo (Solanum betaceum Cav.) Puree. Molecules. 2012; 17(6):6869-6885. https://doi.org/10.3390/molecules17066869
Chicago/Turabian StyleGannasin, Sri Puvanesvari, Yogeshini Ramakrishnan, Noranizan Mohd. Adzahan, and Kharidah Muhammad. 2012. "Functional and Preliminary Characterisation of Hydrocolloid from Tamarillo (Solanum betaceum Cav.) Puree" Molecules 17, no. 6: 6869-6885. https://doi.org/10.3390/molecules17066869
APA StyleGannasin, S. P., Ramakrishnan, Y., Adzahan, N. M., & Muhammad, K. (2012). Functional and Preliminary Characterisation of Hydrocolloid from Tamarillo (Solanum betaceum Cav.) Puree. Molecules, 17(6), 6869-6885. https://doi.org/10.3390/molecules17066869




