Preussianone, a New Flavanone-Chromone Biflavonoid from Garcinia preussii Engl.
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structure Elucidation

 = +3° (c = 0.1, MeOH). The 1H- and 13C-NMR spectra recorded in DMSO-d6 at room temperature (Table 1 and Table 2) showed two sets of signals (in a 1:1 ratio), while in LC-MS only one single peak was observed. The use of a chiral column under the same separation conditions did not show a splitting, either, which could be related to conformationally semi-stable isomeric structures of compound 2, possibly atropo-diastereomers. In order to obtain better resolved signals, the NMR spectra were recorded on a 600-MHz spectrometer at 25 °C and at 80 °C. At this high temperature only one set of signals was obtained suggesting that 2 may adopt different conformations at 25 °C, which are in a rapid equilibrium at 80 °C. From the UV maxima and from the 1H-NMR, 13C-NMR, and HRESIMS data, the base structure of 2 corresponded to 3'',4',4''',5,5'', 5''',7,7''-octahydroxy-biflavanone, which has previously been isolated from Cratoxylum neriifoliu [26]. In order to assign whether the monomers were attached via a C-3/C-6'' or a C-3/C-8'' linkage, 2 was O-methylated and carbon chemical shifts recorded in DMSO-d6 were compared. The permethylated compound displayed eight signals below 57 ppm corresponding to the eight methoxylated aromatic groups. Duddeck et al. showed that in ortho-disubstituted methoxylated C-3/C-6'' biflavonoids, the methoxyl groups appear at low-field with chemical shifts between 59 and 61 ppm.
= +3° (c = 0.1, MeOH). The 1H- and 13C-NMR spectra recorded in DMSO-d6 at room temperature (Table 1 and Table 2) showed two sets of signals (in a 1:1 ratio), while in LC-MS only one single peak was observed. The use of a chiral column under the same separation conditions did not show a splitting, either, which could be related to conformationally semi-stable isomeric structures of compound 2, possibly atropo-diastereomers. In order to obtain better resolved signals, the NMR spectra were recorded on a 600-MHz spectrometer at 25 °C and at 80 °C. At this high temperature only one set of signals was obtained suggesting that 2 may adopt different conformations at 25 °C, which are in a rapid equilibrium at 80 °C. From the UV maxima and from the 1H-NMR, 13C-NMR, and HRESIMS data, the base structure of 2 corresponded to 3'',4',4''',5,5'', 5''',7,7''-octahydroxy-biflavanone, which has previously been isolated from Cratoxylum neriifoliu [26]. In order to assign whether the monomers were attached via a C-3/C-6'' or a C-3/C-8'' linkage, 2 was O-methylated and carbon chemical shifts recorded in DMSO-d6 were compared. The permethylated compound displayed eight signals below 57 ppm corresponding to the eight methoxylated aromatic groups. Duddeck et al. showed that in ortho-disubstituted methoxylated C-3/C-6'' biflavonoids, the methoxyl groups appear at low-field with chemical shifts between 59 and 61 ppm.| No. | Compound (Temp.) | ||
|---|---|---|---|
| 2a (25 °C) | 2b (25 °C) | 2 (80 °C) | |
| H-2 | 5.34 | 5.68 | 5.52 |
| H-3 | 4.66 | 4.44 | 4.57 |
| OH-5 | 12.48 | 12.20 | 12.03 |
| H-6 | 5.88 | 5.88 | 5.83 |
| H-8 | 5.94 | 5.84 | 5.91 |
| H-2' | 7.11 | 7.11 | 7.08 |
| H-3' | 6.76 | 6.64 | 6.72 |
| H-4' | 9.55 | 9.49 | 10.24 |
| H-5' | 6.76 | 6.64 | 6.72 |
| H-6' | 7.11 | 6.64 | 7.08 |
| H-2'' | 4.98 | 4.85 | 4.92 |
| H-3'' | 4.18 | 3.94 | 4.08 |
| OH-5'' | 11.75 | 11.85 | 11.61 |
| H-6'' | 5.88 | 5.88 | 5.92 |
| H-2''' | 6.65 | 6.63 | 6.73 |
| H-3''' | 6.79 | 6.58 | 6.85 |
| H-4''' | 8.99 | 8.99 | 9.11 |
| H-5''' | 8.88 | 8.88 | 8.37 |
| H-6'' | 6.84 | 6.76 | 6.73 |
| No. | Compounds (Temp. °C) | ||
|---|---|---|---|
| 2a (25 °C) | 2b (25 °C) | 2 (80 °C) | |
| C-2 | 81.6 | 81.3 | 81.2 |
| C-3 | 47.2 | 47.1 | 47.2 |
| C-4 | 196.6 | 196.5 | 195.7 |
| C-5 | 163.5 | 163.8 | 162.3 |
| C-6 | 96.0 | 96 | 94.5 |
| C-7 | 166.4 | 166.3 | 165.6 |
| C-8 | 94.8 | 94.9 | 95.7 |
| C-9 | 162.7 | 162.5 | 163.5 |
| C-10 | 101.2 | 101.1 | 101.1 |
| C-1' | 127.8 | 127.9 | 127.7 |
| C-2' | 128.9 | 128.9 | 128.2 |
| C-3' | 115.5 | 118.9 | 114.4 |
| C-4' | 157.8 | 157.6 | 157.2 |
| C-5' | 115.5 | 118.9 | 114.4 |
| C-6' | 128.9 | 128.9 | 128.2 |
| C-2'' | 82.7 | 82.7 | 82.8 |
| C-3'' | 72.3 | 71.9 | 71.9 |
| C-4'' | 197.4 | 197.4 | 196.6 |
| C-5'' | 161.8 | 162.2 | 162.4 |
| C-6'' | 96.0 | 95.8 | 95.4 |
| C-7'' | 162.1 | 161.7 | 164.2 |
| C-8'' | 94.8 | 94.9 | 99.78 |
| C-9'' | 160.1 | 159.4 | 161.6 |
| C-10'' | 100.1 | 99.5 | 100.9 |
| C-1''' | 128.1 | 128.2 | 127.8 |
| C-2''' | 118.9 | 117.3 | 114.8 |
| C-3''' | 115.1 | 115.0 | 114.8 |
| C-4''' | 145.8 | 144.5 | 144.7 |
| C-5''' | 144.9 | 145.3 | 144.7 |
| C-6''' | 115.3 | 115.0 | 114.9 |
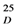 , UV, and 1H-NMR data (Table 1) with those reported in the literature [7,28], compound 2 was assigned as (+)-GB2. In a similar way, compounds 3 and 4 were identified as manniflavanone and (−)-GB1, respectively. Compound 5, named GB2a, with [α]
, UV, and 1H-NMR data (Table 1) with those reported in the literature [7,28], compound 2 was assigned as (+)-GB2. In a similar way, compounds 3 and 4 were identified as manniflavanone and (−)-GB1, respectively. Compound 5, named GB2a, with [α]  = +6° (c = 0.1, MeOH) was found to possess a molecular formula of C30H22O11 (HRESIMS). The 1H- and 13C-NMR data were similar to those previously reported in the literature by several authors [26].
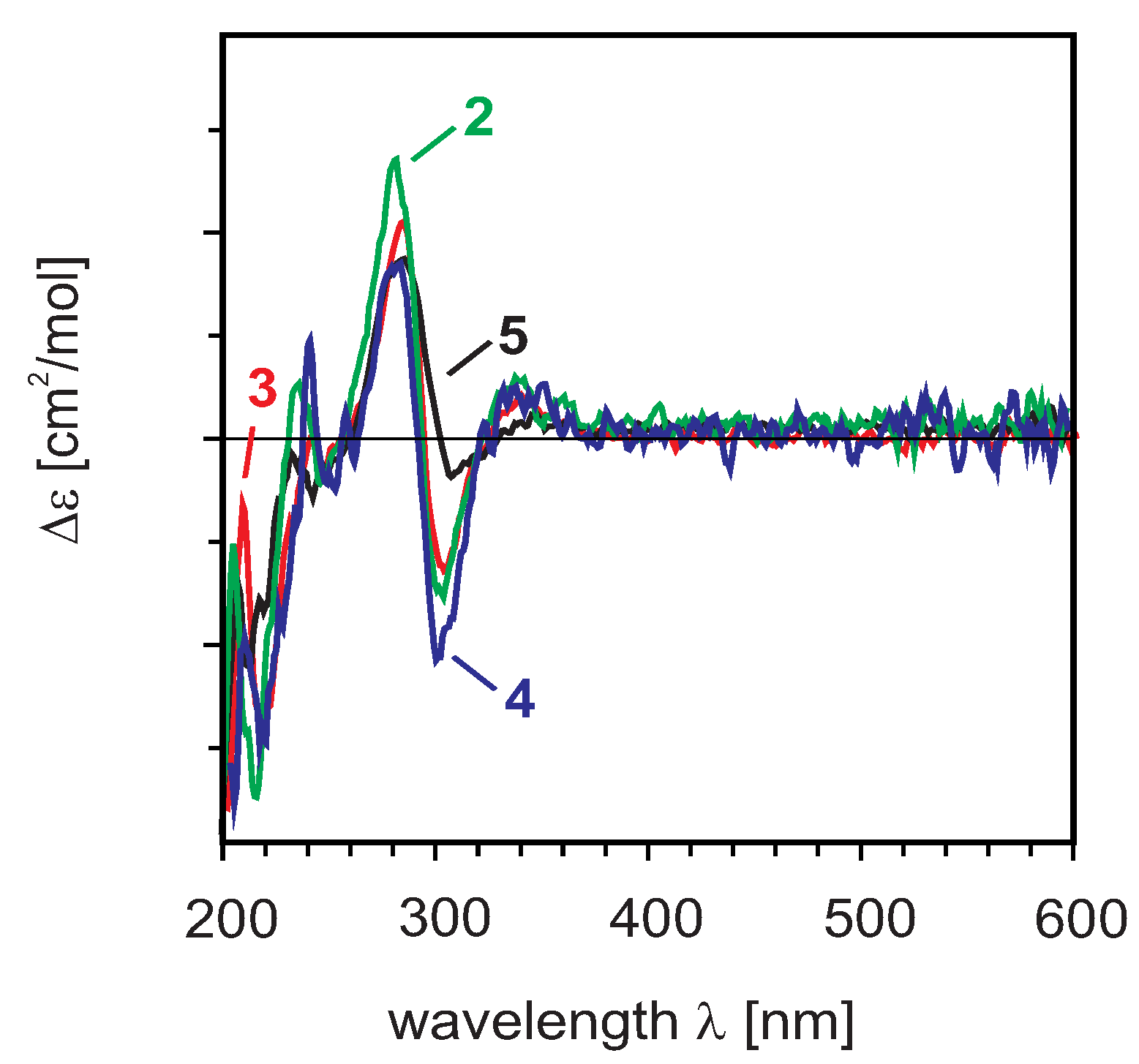
= +6° (c = 0.1, MeOH) was found to possess a molecular formula of C30H22O11 (HRESIMS). The 1H- and 13C-NMR data were similar to those previously reported in the literature by several authors [26].2.2. Absolute Configurations of Compounds 2–5
2.3. Biological Activity
| Compounds | E. coli | P. aeruginosa | S. aureus | E. faecalis |
|---|---|---|---|---|
| 2 (+)-GB2 | >512 | >512 | >512 | >512 |
| 3 manniflavanone | >512 | >512 | 256 | >512 |
| Extract | >128 | >512 | Nt | Nt |
| Gentamycin | 1.0 | 1.0 | 1.0 | 16.0 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Preussianone
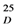 +48.7 (c 0.1, MeOH); UV (MeOH) λmax (logε) nm 258 (4.05), 291 (4.00), 326 (3.58), 386 (2.76); IR (crystal) νmax cm−1 3,080, 2,259, 2,129, 1,616, 1,506, 1,436, 1,363, 1,207, 1,131, 974. Negative-HRESIMS m/z 479.0589 [M-H]− (calcd for C24H15O11 479.0614); CD (MeOH; c = 0.003472 mol/L): ∆ε = 208 (−2.18), 236 (1.75), 296 (−3.56) cm2/mol. For 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6), see Table 4.
+48.7 (c 0.1, MeOH); UV (MeOH) λmax (logε) nm 258 (4.05), 291 (4.00), 326 (3.58), 386 (2.76); IR (crystal) νmax cm−1 3,080, 2,259, 2,129, 1,616, 1,506, 1,436, 1,363, 1,207, 1,131, 974. Negative-HRESIMS m/z 479.0589 [M-H]− (calcd for C24H15O11 479.0614); CD (MeOH; c = 0.003472 mol/L): ∆ε = 208 (−2.18), 236 (1.75), 296 (−3.56) cm2/mol. For 1H-NMR (DMSO-d6) and 13C-NMR (DMSO-d6), see Table 4.| No. | C | H |
|---|---|---|
| 2 | 82.9 | 4.95 (d, 1H, 10.7 Hz) |
| 3 | 71.6 | 4.43 (d, 1H, 10.7 Hz) |
| 4 | 198.0 | - |
| 5-OH | 162.8 | 11.90 |
| 6 | 95.74 | 6.08 (s, 1H) |
| 7 | 165.1 | - |
| 8 | 98.2 | - |
| 9 | 160.6 | - |
| 10 | 100.4 | - |
| 1' | 127.9 | - |
| 2' | 115.0 | 6.67 (brs, 1H) |
| 3' | 144.7 | - |
| 4' | 145.6 | - |
| 5' | 119.0 | 6.67 (brs, 1H) |
| 6' | 115.1 | 6.80 (brs, 1H) |
| 2'' | 156.5 | 8.13 (s, 1H) |
| 3'' | 115.1 | - |
| 4'' | 179.8 | |
| 5''-OH | 161.6 | 12.70 |
| 6'' | 98.9 | 6.19 (d, 1H, 2.1 Hz) |
| 7''-OH | 164.2 | - |
| 8'' | 93.7 | 6.34 (d, 1H, 2.1 Hz) |
| 9'' | 157.5 | - |
| 10'' | 104.3 | - |
3.5. Antimicrobial Assay
4. Conclusions
Acknowledgments
References and Notes
- Williams, C.A.; Harborne, J.B. Biflavonoids. In Methods in Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: San Diego, CA, USA, 1989; pp. 357–388. [Google Scholar]
- Kim, H.; Park, H.; Son, K.; Chang, H.; Kang, S. Biochemical pharmacology of biflavonoids: Implications for anti-inflammatory action. Arch. Pharm. Res. 2008, 31, 265–273. [Google Scholar] [CrossRef]
- Iwu, M.M.; Igboko, O.A. Biflavonoid constituents of Garcinia kola roots. Fitoterapia 1990, 61, 178–181. [Google Scholar]
- Geiger, H.; Quinn, C. Biflavonoids. In The Flavonoids; Harborne, J.B., Mabry, T.J., Mabry, H., Eds.; Chapman & Hall: London, UK, 1975; pp. 692–742. [Google Scholar]
- Geiger, H.; Quinn, C. Biflavonoids. In The Flavonoids; Harborne, J.B., Mabry, T.J., Eds.; Chapman & Hall: London, UK, 1982; pp. 505–534. [Google Scholar]
- Ferreira, D.; Slade, D.; Marais, J.P.J. Bi-, Tri-, Tetra-, Penta-, and Hexaflavonoids. In The Flavonoids: Chemistry, Biochemistry and Applications; Øyvind, M.A., Markham, K.R., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 1101–1124. [Google Scholar]
- Amellal, M.; Bronner, C.; Briancon, F.; Haag, M.; Anton, R.; Landry, Y. Inhibition of mast-cell histamine-release by flavonoids and biflavonoids. Planta Med. 1985, 51, 16–20. [Google Scholar] [CrossRef]
- Geiger, H.; Quinn, C. Biflavonoids. In The Flavonoids; Harborne, J.B., Ed.; Chapman & Hall: London, UK, 1988; pp. 99–124. [Google Scholar]
- Braide, V.B. Anti-inflammatory effect of Kolaviron, bio-flavonoid extract of Garcinia kola. Fitoterapia 1993, 64, 433–436. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–282. [Google Scholar]
- Kaikabo, A.A.; Eloff, J.N. Antibacterial activity of two biflavonoids from Garcinia livingstonei leaves against Mycobacterium smegmatis. J. Ethnopharmacol. 2011, 138, 253–255. [Google Scholar] [CrossRef]
- Louh, G.N.; Lannang, A.M.; Mbazoa, C.D.; Tangmouo, J.G.; Komguem, J.; Castilho, P.; Ngninzeko, F.N.; Qamar, N.; Lontsi, D.; Choudhary, M.I.; et al. Polyanxanthone A, B and C, three xanthones from the wood trunk of Garcinia polyantha Oliv. Phytochemistry 2008, 69, 1013–1017. [Google Scholar]
- Chen, J.-J.; Ting, C.-W.; Hwang, T.-L.; Chen, I.-S. Benzophenone derivatives from the fruits of Garcinia multiflora and their anti-inflammatory activity. J. Nat. Prod. 2009, 72, 253–258. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Anderson, H.; Flavin, M.T.; Pai, Y.-H.S.; Mata-Greenwood, E.; Pengsuparp, T.; Pezzuto, J.M.; Schinazi, R.F.; Hughes, S.H.; Chen, F.-C. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997, 60, 884–888. [Google Scholar] [CrossRef]
- Bouquet, A. Féticheurs et Médecines Traditionnelles du Congo (Brazzaville); Office de la Recherche Scientifique et Technique Outre-Mer O.R.S.T.O.M.: Paris, France, 1969; pp. 132–133. [Google Scholar]
- Visser, L.E. Plantes Médicinales de la Côte d'Ivoire; Mededelingen Landbouwhogeschool Wageningen: Wageningen, The Netherlands, 1975; p. 54. [Google Scholar]
- Cotterill, P.J.; Scheinmann, F.; Stenhouse, I.A. Extractives from Gurriferae 34. Kolaflavanone, a new biflavanone from nuts of Garcinia kola HECKEL-applications of C-13 nuclear magnetic-resonance in elucidation of structures of flavonoids. J. Chem. Soc. Perkin Trans. 1 1978, 1978, 532–539. [Google Scholar]
- Jackson, B.; Locksley, H.D.; Scheinmann, F.; Wolstenholme, W.A. Extractives from guttiferae. Part XXII. The isolation and structure of four novel biflavanones from the heartwoods of Garcinia buchananii Baker and Garcinia. eugeniifolia Wall. J. Chem. Soc. C 1971, 1971, 3791–3804. [Google Scholar]
- Markham, K.R.; Sheppard, C.; Geiger, H. 13C-NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry 1987, 26, 3335–3337. [Google Scholar] [CrossRef]
- Chari, V.M.; Ilyas, M.; Wagner, H.; Neszmélyi, A.; Fa-Ching, C.; Li-Kuang, C.; Yu-Chin, L.; Yu-Meei, L. 13C-NMR spectroscopy of biflavanoids. Phytochemistry 1977, 16, 1273–1278. [Google Scholar]
- Terashima, K.; Kondo, Y.; Aqil, M.; Waziri, M.; Niwa, M. A study of biflavanones from the stems of Garcinia kola (Guttiferae). Heterocycles 1999, 50, 283–290. [Google Scholar] [CrossRef]
- Slade, D.; Ferreira, D.; Marais, J.P.J. Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 2005, 66, 2177–2215. [Google Scholar] [CrossRef]
- Gaffield, W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: Determination of aglycone chirality in flavanone glycosides. Tetrahedron 1970, 26, 4093–4108. [Google Scholar] [CrossRef]
- Hosoi, S.; Shimizu, E.; Ohno, K.; Yokosawa, R.; Kuninaga, S.; Coskun, M.; Sakushima, A. Structural studies of zoospore attractants from Trachelospermum jasminoides var. pubescens: Taxifolin 3-O-glycosides. Phytochem. Anal. 2006, 17, 20–24. [Google Scholar] [CrossRef]
- Ansari, W.H.; Rahman, W.; Barraclough, D.; Maynard, R.; Scheinmann, F. Biflavonoids and a flavanone chromone from leaves of Garcinia dulsis (Roxb) Kurz. J. Chem. Soc. Perkin Trans. 1 1976, 1976, 1458–1463. [Google Scholar]
- Kumar, V.; Brecht, V.; Frahm, A.W. Conformational analysis of the biflavanoid GB2 and a polyhydroxylated flavanone-chromone of Cratoxylum neriifolium. Planta Med. 2004, 70, 646–651. [Google Scholar] [CrossRef]
- Duddeck, H.; Snatzke, G.; Yemul, S.S. 13C-NMR and CD of some 3,8''-biflavanoids from Garcinia species and of related flavanone. Phytochemistry 1978, 17, 1369–1973. [Google Scholar] [CrossRef]
- Crichton, E.G.; Waterman, P.G. Manniflavanone, a new 3,8-linked flavanone dimer from the stem bark of Garcinia mannii. Phytochemistry 1979, 18, 1553–1557. [Google Scholar] [CrossRef]
- Ferrari, J.; Terreaux, C.; Kurtán, T.; Szikszai-Kiss, A.; Antus, S.; Msonthi, J.D.; Hostettmann, K. Isolation and on-Line LC/CD analysis of 3,8'-linked biflavonoids from Gnidia involucrata. Helv. Chim. Acta 2003, 86, 2768–2778. [Google Scholar] [CrossRef]
- Ding, Y.; Xing-Cong, L.; Ferreira, D. Theoretical calculation of electronic circular dichroism of the rotationally restricted 3,8"-biflavonoid morelloflavone. J. Org. Chem. 2007, 72, 9010–9017. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Kapingu, M.C.; Moshi, M.J.; Machumi, F.; Apers, S.; Cos, P.; Ferreira, D.; Marais, J.P.J.; Vanden Berghe, D.; Maes, L.; et al. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J. Nat. Prod. 2006, 69, 369–372. [Google Scholar]
- Li, X.-C.; Joshi, A.S.; Tan, B.; ElSohly, H.N.; Walker, L.A.; Zjawiony, J.K.; Ferreira, D. Absolute configuration, conformation, and chiral properties of flavanone-(3 → 8'')-flavone biflavonoids from Rheedia acuminata. Tetrahedron 2002, 58, 8709–8717. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar]
- Eloff, J.N. A Sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Sonnenbichler, J.; Madubunyi, I.; Scheer, H. Stereochemistry of 2-hydroxybiflavanonols from Garcinia kola nuts. Z. Naturforsch. 1987, 42c, 855–857. [Google Scholar]
- Sample Availability: Samples of compounds 1–3 and 5 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Messi, B.B.; Ndjoko-Ioset, K.; Hertlein-Amslinger, B.; Lannang, A.M.; Nkengfack, A.E.; Wolfender, J.-L.; Hostettmann, K.; Bringmann, G. Preussianone, a New Flavanone-Chromone Biflavonoid from Garcinia preussii Engl. Molecules 2012, 17, 6114-6125. https://doi.org/10.3390/molecules17056114
Messi BB, Ndjoko-Ioset K, Hertlein-Amslinger B, Lannang AM, Nkengfack AE, Wolfender J-L, Hostettmann K, Bringmann G. Preussianone, a New Flavanone-Chromone Biflavonoid from Garcinia preussii Engl. Molecules. 2012; 17(5):6114-6125. https://doi.org/10.3390/molecules17056114
Chicago/Turabian StyleMessi, Bernadette Biloa, Karine Ndjoko-Ioset, Barbara Hertlein-Amslinger, Alain Meli Lannang, Augustin E. Nkengfack, Jean-Luc Wolfender, Kurt Hostettmann, and Gerhard Bringmann. 2012. "Preussianone, a New Flavanone-Chromone Biflavonoid from Garcinia preussii Engl." Molecules 17, no. 5: 6114-6125. https://doi.org/10.3390/molecules17056114
APA StyleMessi, B. B., Ndjoko-Ioset, K., Hertlein-Amslinger, B., Lannang, A. M., Nkengfack, A. E., Wolfender, J.-L., Hostettmann, K., & Bringmann, G. (2012). Preussianone, a New Flavanone-Chromone Biflavonoid from Garcinia preussii Engl. Molecules, 17(5), 6114-6125. https://doi.org/10.3390/molecules17056114





