A Sandwich HIV p24 Amperometric Immunosensor Based on a Direct Gold Electroplating-Modified Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Behavior of the Immunosensor
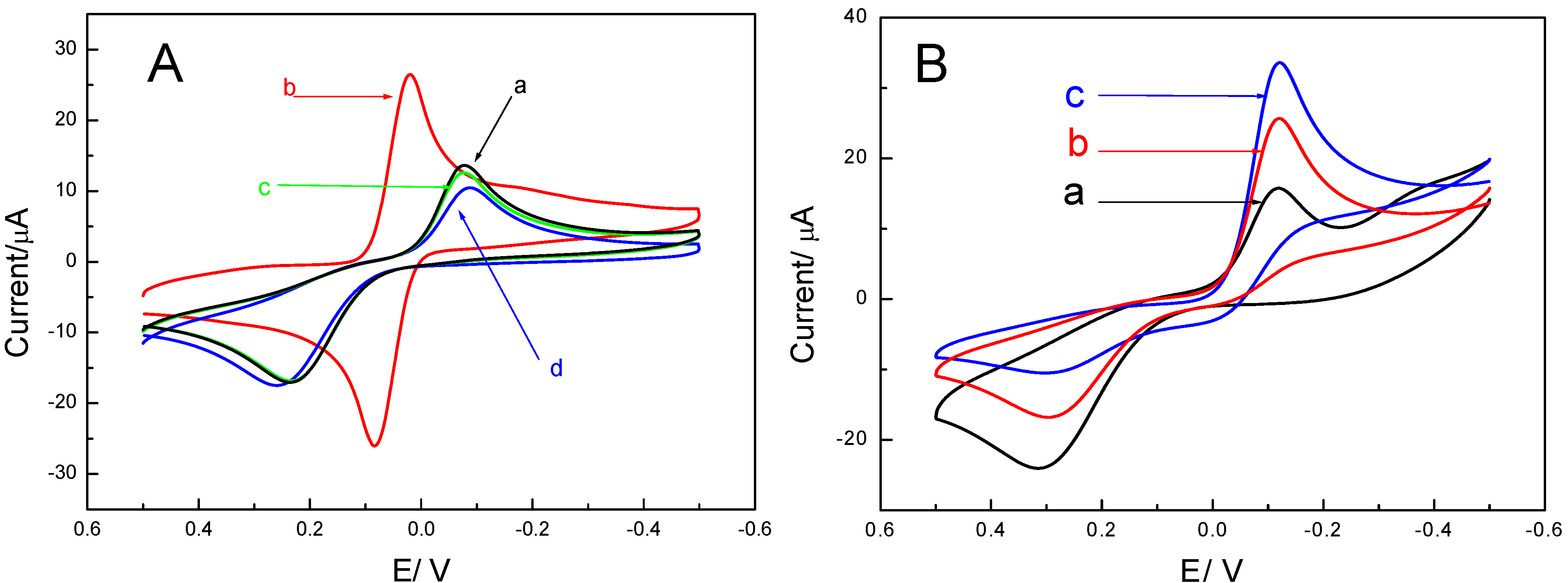
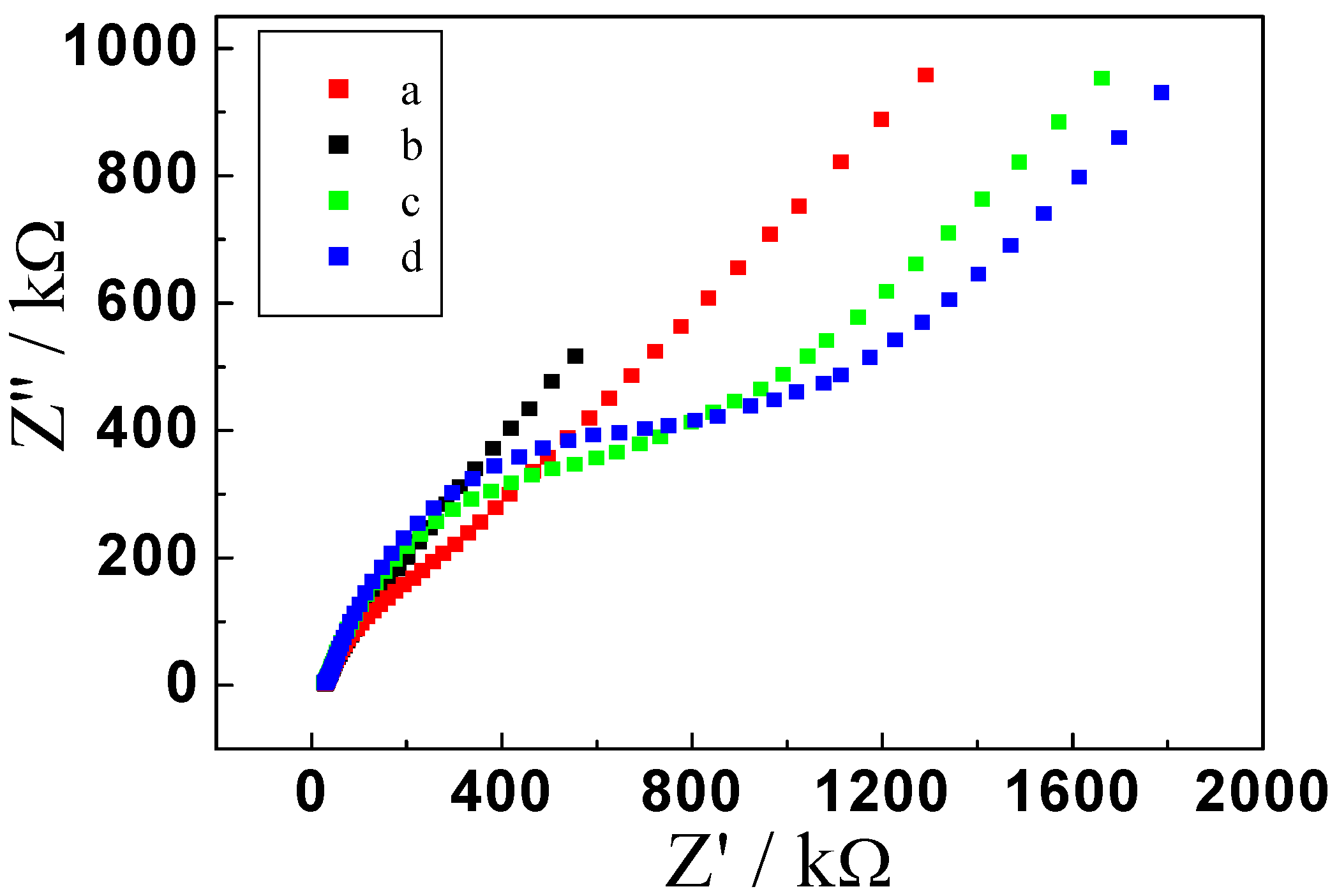
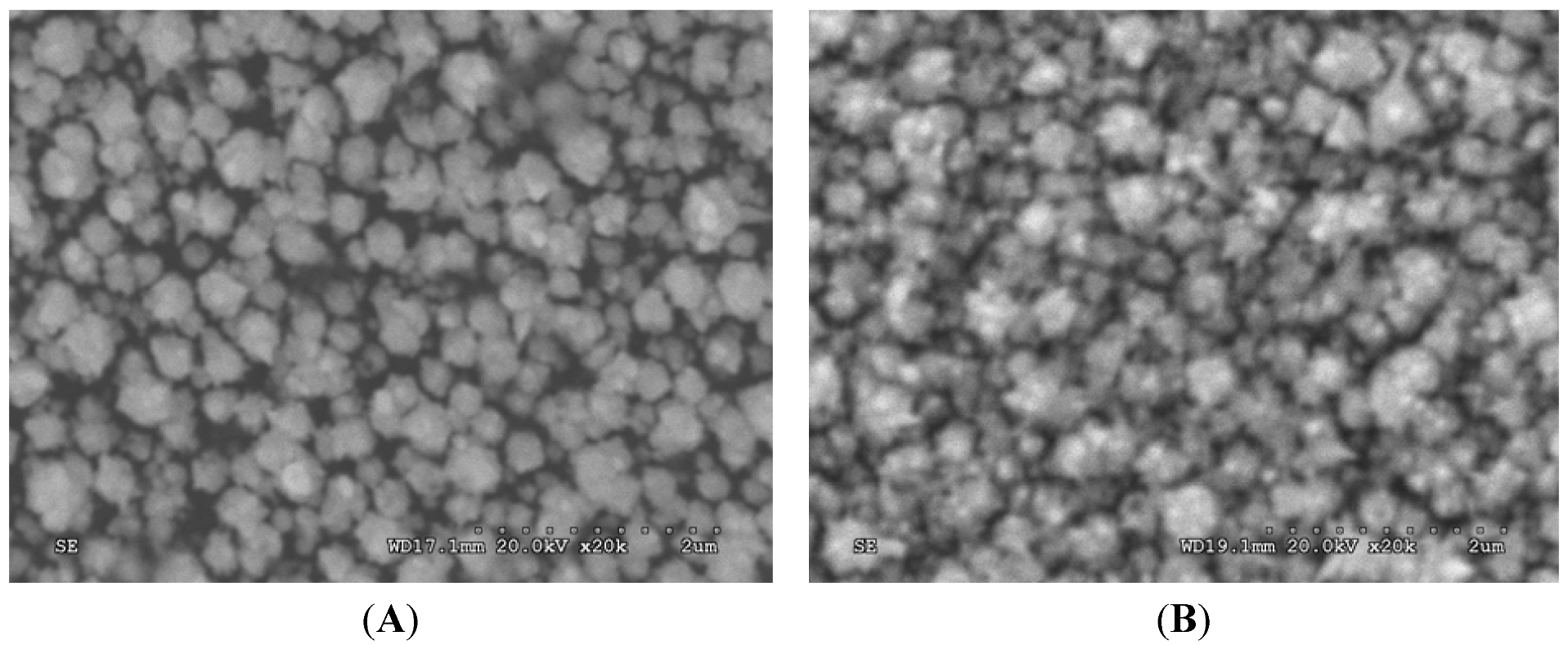
2.2. Optimization of Immunoassay Conditions
2.2.1. Effect of Substrate Concentration on Immunoassay

2.2.2. Effect of Buffer pH on the Sensor Operation

2.2.3. Effects of Incubation Time and Temperature on Sensor Operation
2.2.4. Effect of Electroplating Time on Sensor Operation
2.3. Detection of p24 by the Amperometric Immunosensor
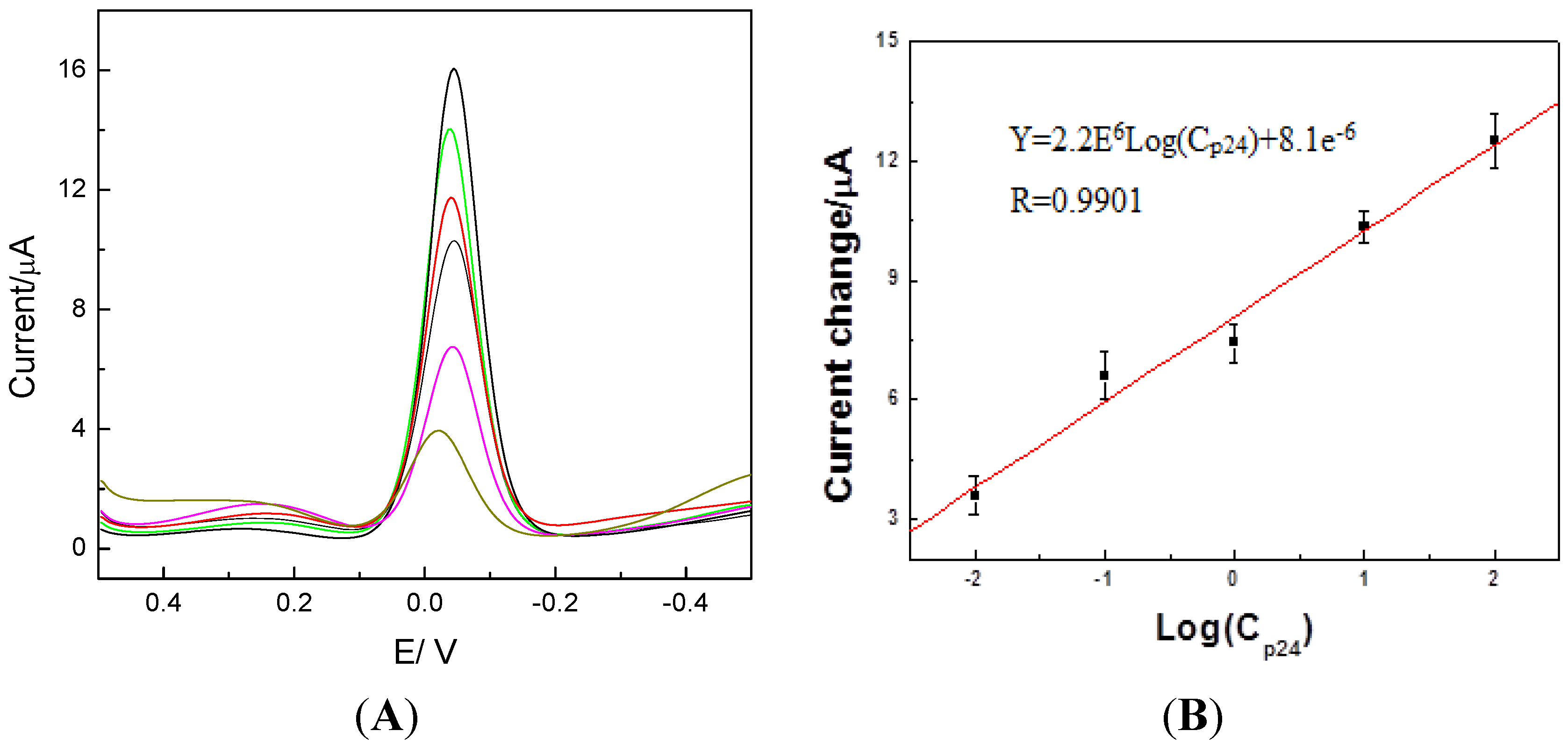
2.4. Reproducibility and Anti-Interference Ability and Stability of the Immunosensor
2.5. Detection of HIV in Samples with the Immunosensor
| Sample | HIV concentration (ng/mL) | ||||
|---|---|---|---|---|---|
| Immunosensor | ELISA | Addition | Result | Recovery rate % a | |
| 1 | 5.3 | 5.2 | 5.0 | 10.2 | 98 |
| 2 | 9.8 | 10.3 | 9.0 | 18.5 | 95.6 |
| 3 | 20.5 | 21.3 | 20.0 | 41.7 | 106 |
3. Experimental
3.1. Instruments and Reagents
3.2. Methods
3.2.1. Preparation of a Sandwich HIV p24 Amperometric Immunosensor Based on Gold Nanoparticle-Modified Electrode
3.2.2. Scanning Electron Microscopy (SEM)
3.2.3. Detection Procedures of the Electrochemical Immunosensor
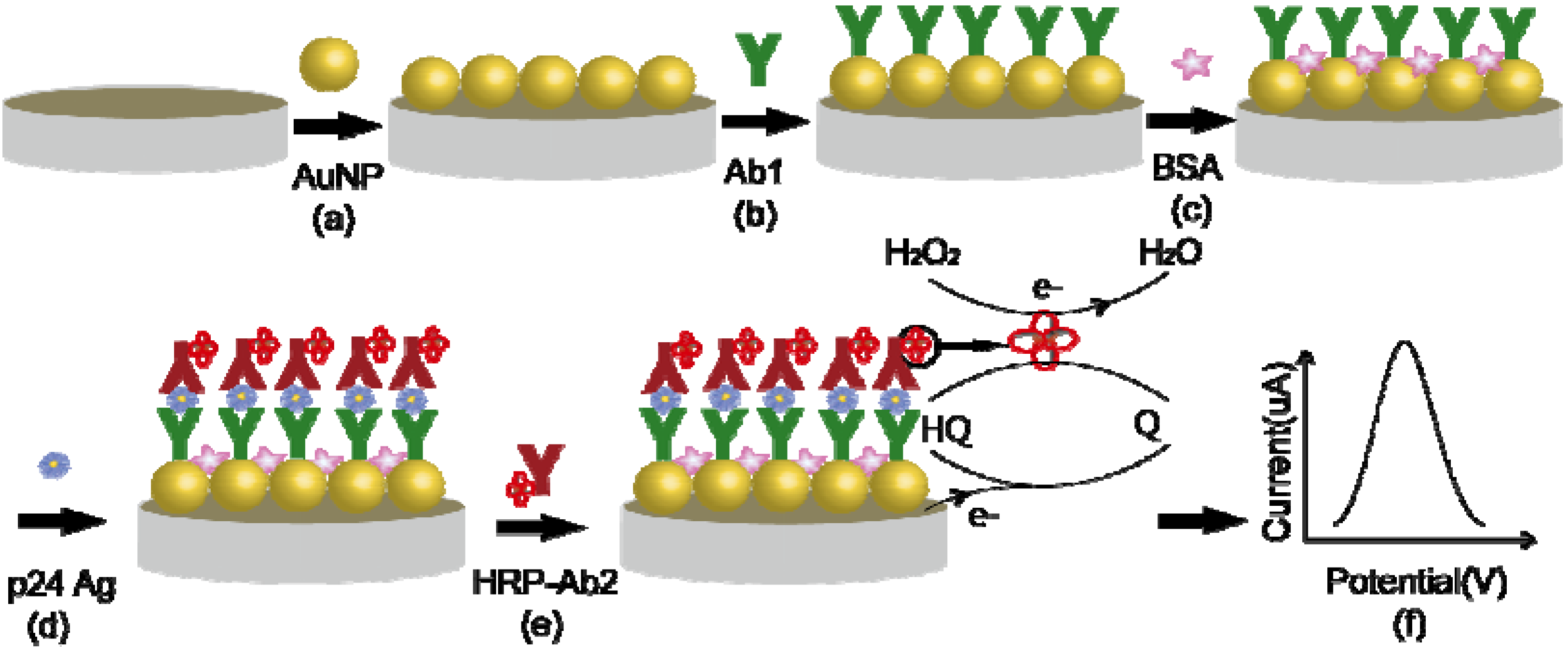
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References and notes
- Gan, N.; Hou, J.; Hu, F.; Zheng, L.; Ni, M.; Cao, Y. An amperometric immunosensor based on a polyelectrolyte/gold magnetic nanoparticle supramolecular assembly-modified electrode for the determination of HIV p24 in serum. Molecules 2010, 15, 5053–5065. [Google Scholar] [CrossRef]
- Zhao, G.; Zhan, X.; Dou, W. A disposable immunosensor for shigella flexneri based on multiwalled carbon nanotube/sodium alginate composite electrode. Anal. Biochem. 2011, 408, 53–58. [Google Scholar]
- Huang, K.J.; Niu, D.J.; Xie, W.Z.; Wang, W. A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes-chitosans nanocomposite film modified glassy carbon electrode. Anal. Chim. Acta 2010, 659, 102–108. [Google Scholar]
- Che, X.; Yuan, R.; Chai, Y.; Li, J.; Song, Z.; Wang, J. Amperometric immunosensor for the determination of [alpha]-1-fetoprotein based on multiwalled carbon nanotube-silver nanoparticle composite. J. Colloid Interf. Sci. 2010, 345, 174–180. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, K.; Zhou, J.; Xuan, J.; Yan, W.; Jiang, L.P.; Zhu, J.J. Electrochemical immunosensor based on colloidal carbon sphere array. Biosens. Bioelectron. 2010, 25, 1130–1136. [Google Scholar] [CrossRef]
- Wei, Q.; Mao, K.; Wu, D.; Dai, Y.; Yang, J.; Du, B.; Yang, M.; Li, H. A novel label-free electrochemical immunosensor based on graphene and thionine nanocomposite. Sens. Actuators B Chem. 2010, 149, 314–318. [Google Scholar] [CrossRef]
- Wei, Q.; Xiang, Z.; He, J.; Wang, G.; Li, H.; Qian, Z.; Yang, M. Dumbbell-like Au-Fe3O4 nanoparticles as label for the preparation of electrochemical immunosensors. Biosens. Bioelectron. 2010, 26, 627–631. [Google Scholar] [CrossRef]
- Wei, Q.; Xin, X.; Du, B.; Wu, D.; Han, Y.; Zhao, Y.; Cai, Y.; Li, R.; Yang, M.; Li, H. Electrochemical immunosensor for norethisterone based on signal amplification strategy of graphene sheets and multienzyme functionalized mesoporous silica nanoparticles. Biosens. Bioelectron. 2010, 26, 723–729. [Google Scholar] [CrossRef]
- Chen, H.; Xi, F.; Gao, X.; Chen, Z.; Lin, X. Bienzyme bionanomultilayer electrode for glucose biosensing based on functional carbon nanotubes and sugar-lectin biospecific interaction. Anal. Biochem. 2010, 403, 36–42. [Google Scholar]
- Yang, M.; Javadi, A.; Li, H.; Gong, S. Ultrasensitive immunosensor for the detection of cancer biomarker based on graphene sheet. Biosens. Bioelectron. 2010, 26, 560–565. [Google Scholar] [CrossRef]
- Tang, J.; Su, B.; Tang, D.; Chen, G. Conductive carbon nanoparticles-based electrochemical immunosensor with enhanced sensitivity for [alpha]-1-fetoprotein using irregular-shaped gold nanoparticles-labeled enzyme-linked antibodies as signal improvement. Biosens. Bioelectron. 2010, 25, 2657–2662. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Vaque, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive electrochemical immunosensor for oral cancer biomarker il-6 using carbon nanotube forest electrodes and multilabel amplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef]
- Yang, G.; Chang, Y.; Yang, H.; Tan, L.; Wu, Z.; Lu, X.; Yang, Y. The preparation of reagentless electrochemical immunosensor based on a nano-gold and chitosan hybrid film for human chorionic gonadotrophin. Anal. Chim. Acta 2009, 644, 72–77. [Google Scholar] [CrossRef]
- Ahirwal, G.K.; Mitra, C.K. Gold nanoparticles based sandwich electrochemical immunosensor. Biosens. Bioelectron. 2010, 25, 2016–2020. [Google Scholar] [CrossRef]
- Ran, X.-Q.; Yuan, R.; Chai, Y.-Q.; Hong, C.-L.; Qian, X.-Q. A sensitive amperometric immunosensor for alpha-fetoprotein based on carbon nanotube/DNA/thi/nano-Au modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2010, 79, 421–426. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Davis, T.P.; Gooding, J.J. Electrochemical impedance immunosensor based on gold nanoparticles and aryl diazonium salt functionalized gold electrodes for the detection of antibody. Biosens. Bioelectron. 2011, 26, 3660–3665. [Google Scholar] [CrossRef]
- Wei, Q.; Li, R.; Du, B.; Wu, D.; Han, Y.; Cai, Y.; Zhao, Y.; Xin, X.; Li, H.; Yang, M. Multifunctional mesoporous silica nanoparticles as sensitive labels for immunoassay of human chorionic gonadotropin. Sens. Actuators B Chem. 2010, 153, 256–260. [Google Scholar]
- Zhuo, Y.; Yi, W.-J.; Lian, W.-B.; Yuan, R.; Chai, Y.-Q.; Chen, A.; Hu, C.-M. Ultrasensitive electrochemical strategy for nt-probnp detection with gold nanochains and horseradish peroxidase complex amplification. Biosens. Bioelectron. 2011, 26, 2188–2193. [Google Scholar] [CrossRef]
- Ning, N.; Nai, L.; Tian, L.; Zheng, L.; Jun, N. A non-enzyme amperometric immunosensor for rapid determination of human immunodeficiency virus p24 based on magnetism controlled carbon nanotubes modified printed electrode. Chin. J. Anal. Chem. 2010, 38, 1556–1562. [Google Scholar] [CrossRef]
- Teeparuksapun, K.; Hedstrom, M.; Wong, E.Y.; Tang, S.; Hewlett, I.K.; Mattiasson, B. Ultrasensitive detection of HIV-1 p24 antigen using nanofunctionalized surfaces in a capacitive immunosensor. Anal. Chem. 2010, 82, 8406–8411. [Google Scholar]
- Branson, B.M. The future of HIV testing. J. Acquir. Immune Defic. Syndr. 2010, 55 (Suppl. 2), S102–S105. [Google Scholar] [CrossRef]
- Gerasimov, J.Y.; Lai, R.Y. An electrochemical peptide-based biosensing platform for HIV detection. Chem. Commun. (Camb.) 2010, 46, 395–397. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, L.; Jia, L.; Li, B.; Situ, B.; Liu, Q.; Wang, Q.; Gan, N. A Sandwich HIV p24 Amperometric Immunosensor Based on a Direct Gold Electroplating-Modified Electrode. Molecules 2012, 17, 5988-6000. https://doi.org/10.3390/molecules17055988
Zheng L, Jia L, Li B, Situ B, Liu Q, Wang Q, Gan N. A Sandwich HIV p24 Amperometric Immunosensor Based on a Direct Gold Electroplating-Modified Electrode. Molecules. 2012; 17(5):5988-6000. https://doi.org/10.3390/molecules17055988
Chicago/Turabian StyleZheng, Lei, Liyong Jia, Bo Li, Bo Situ, Qinlan Liu, Qian Wang, and Ning Gan. 2012. "A Sandwich HIV p24 Amperometric Immunosensor Based on a Direct Gold Electroplating-Modified Electrode" Molecules 17, no. 5: 5988-6000. https://doi.org/10.3390/molecules17055988
APA StyleZheng, L., Jia, L., Li, B., Situ, B., Liu, Q., Wang, Q., & Gan, N. (2012). A Sandwich HIV p24 Amperometric Immunosensor Based on a Direct Gold Electroplating-Modified Electrode. Molecules, 17(5), 5988-6000. https://doi.org/10.3390/molecules17055988





