Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics
Abstract
:1. Introduction
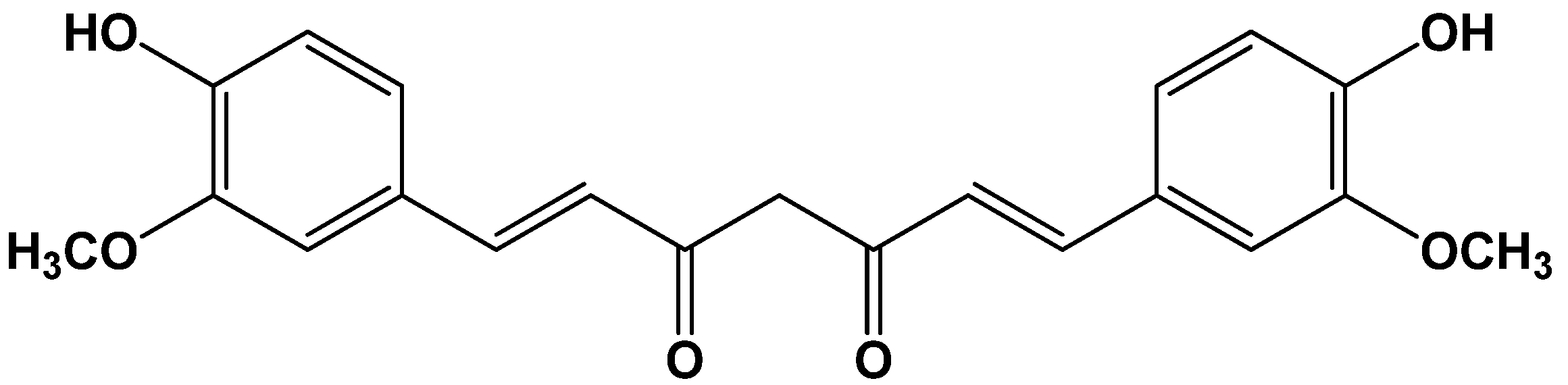
2. Results and Discussion
2.1. Physical Characterization of Liposome Dispersions
| Liposome dispersion | Particle size (nm) | Polydispersity index | Zeta potential (mv) | EE (%,w/w) |
|---|---|---|---|---|
| C-SPC-L | 82.37 ± 2.19 | 0.247 ± 0.028 | −12.88 ± 1.38 | 82.32 ± 3.91 |
| C-EPC-L | 83.13 ± 4.89 | 0.261 ± 0.013 | −11.97 ± 1.92 | 81.59 ± 2.38 |
| C-HSPC-L | 92.42 ± 4.56 | 0.279 ± 0.039 | −10.39 ± 2.67 | 80.77 ± −4.12 |
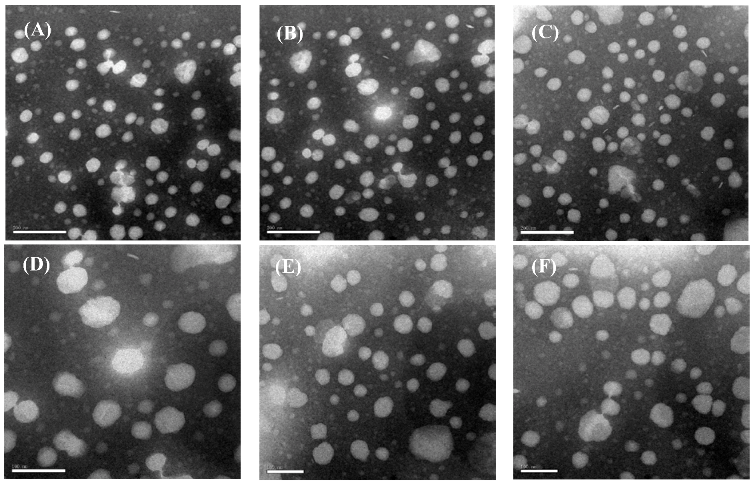
2.2. Liposome Morphology
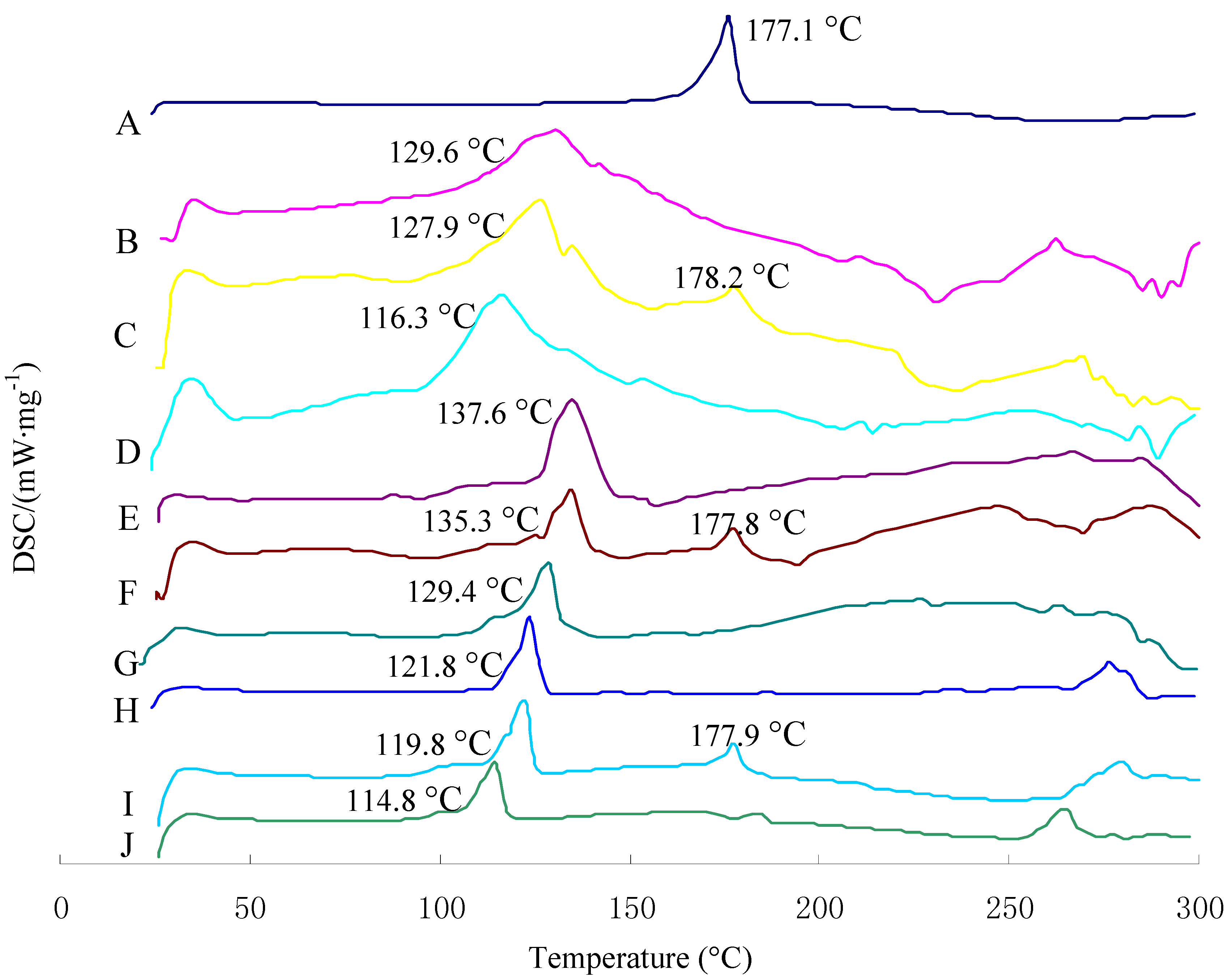
2.3. Differential Scanning Calorimetry Characterization
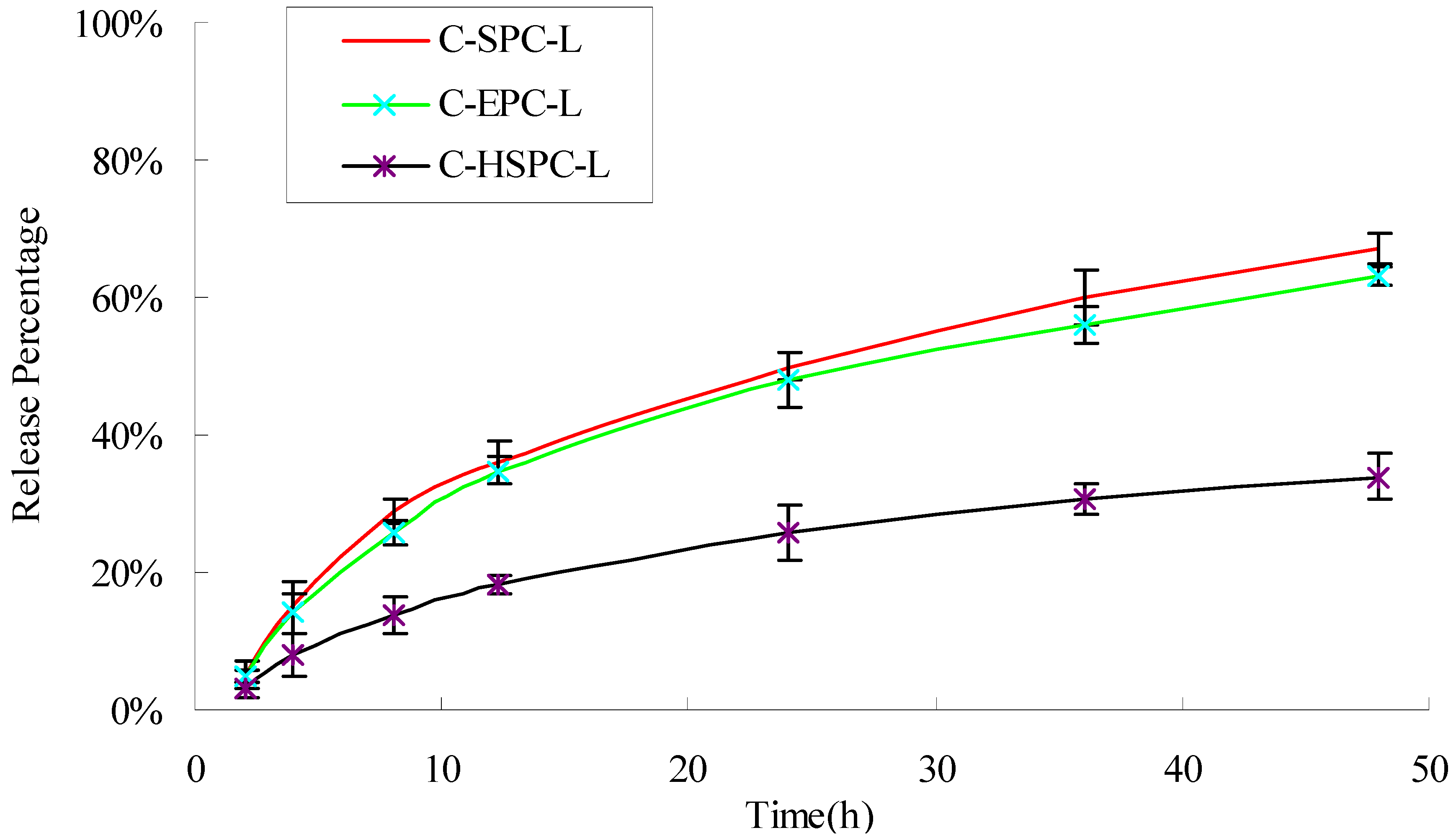
2.4. In Vitro Drug Release
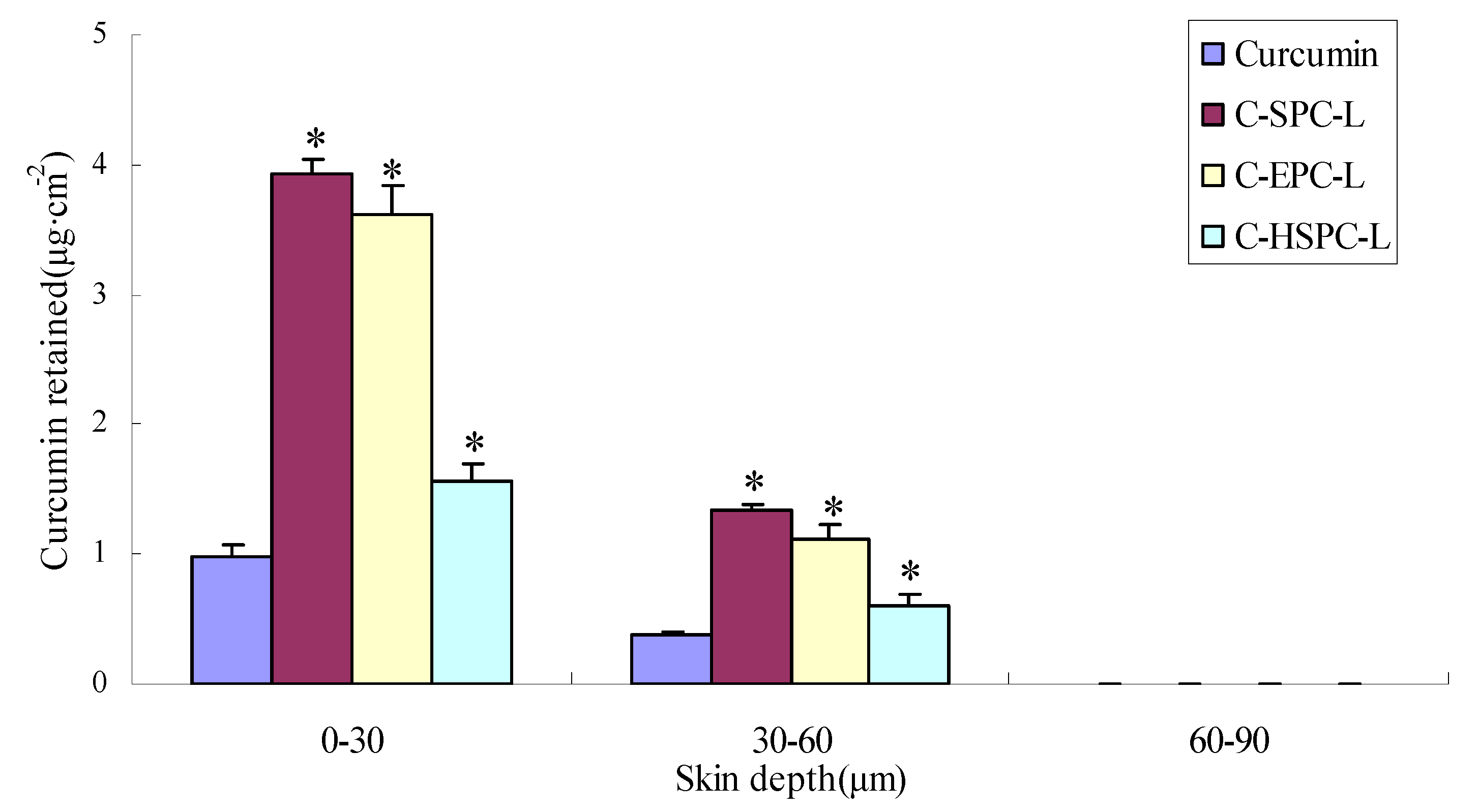
2.5. Skin Penetration
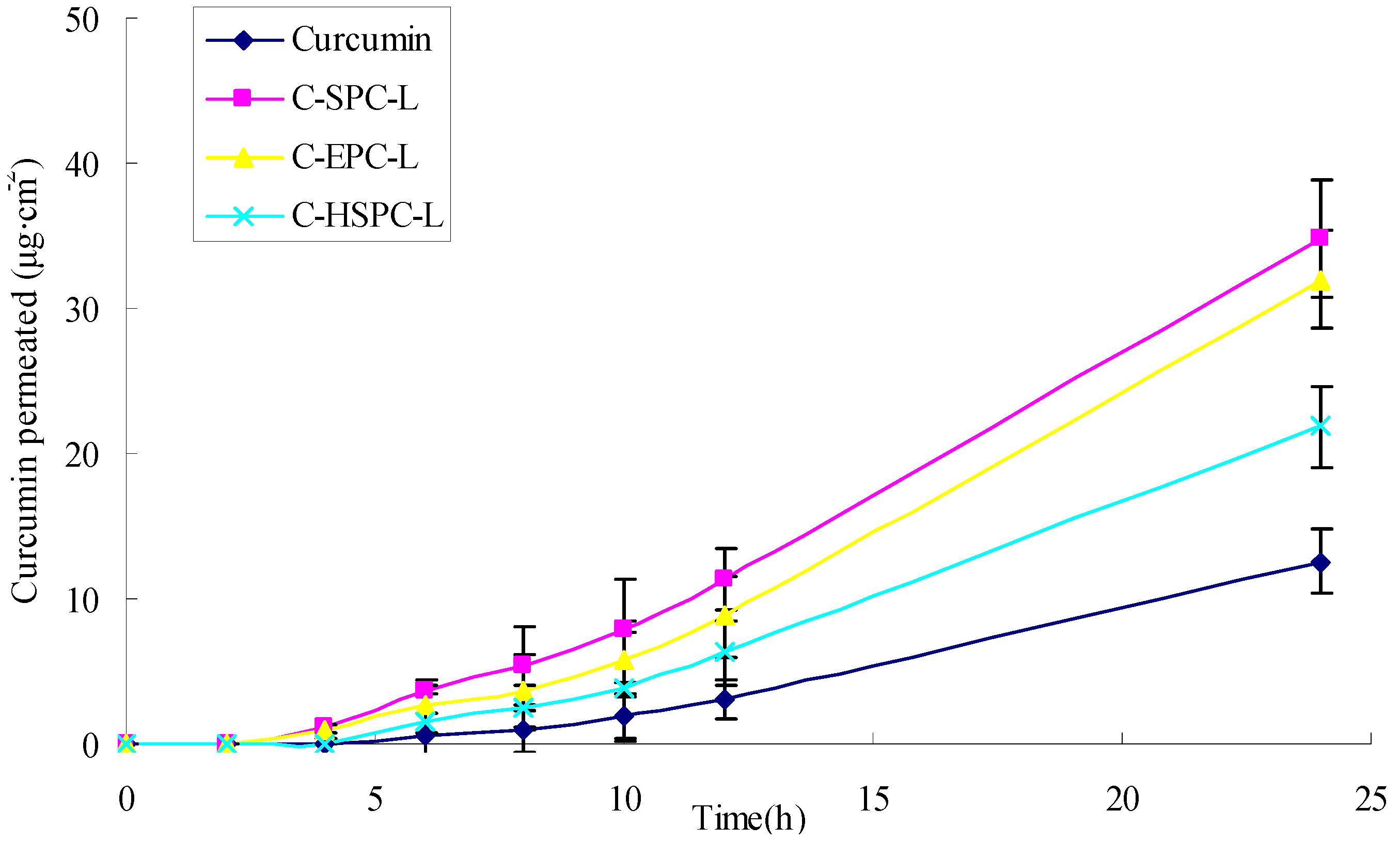
| Sample | Curcumin permeated at 24 h (μg·cm−2) | Curcumin retained in skin at 24 h (μg·cm−2) | Flux (μg·cm−2h−1) |
|---|---|---|---|
| Curcumin solution | 12.56 ± 2.77 | 1.49 ± 0.18 | 0.59 ± 0.11 |
| C-SPC-L | 34.84 ± 4.33 | 5.66 ± 0.43 | 1.45 ± 0.31 |
| C-EPC-L | 31.97 ± 3.37 | 5.23 ± 0.35 | 1.34 ± 0.24 |
| C-HSPC-L | 21.87 ± 2.93 | 3.32 ± 0.21 | 0.96 ± 0.18 |
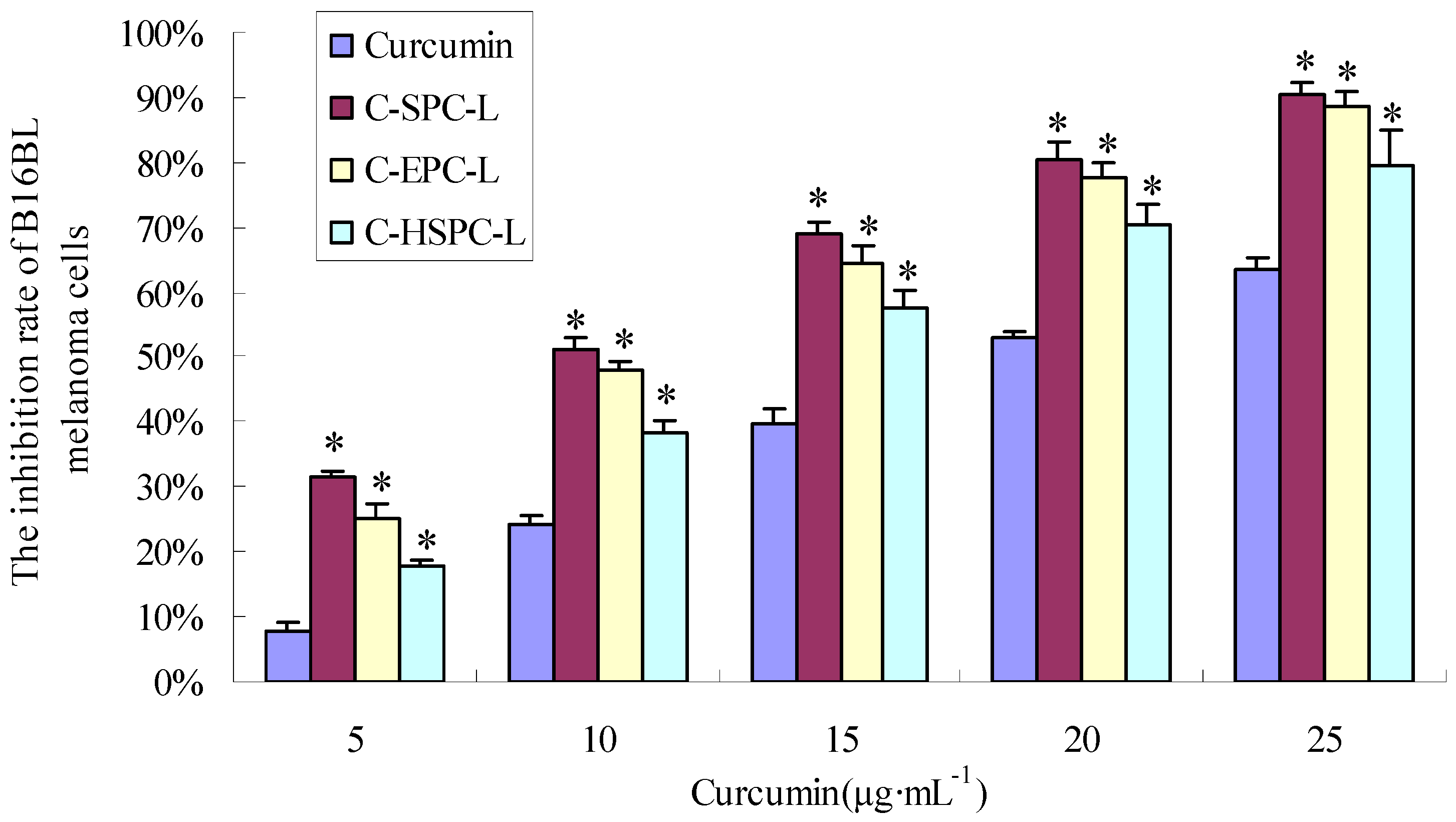
2.6. Cytotoxicity
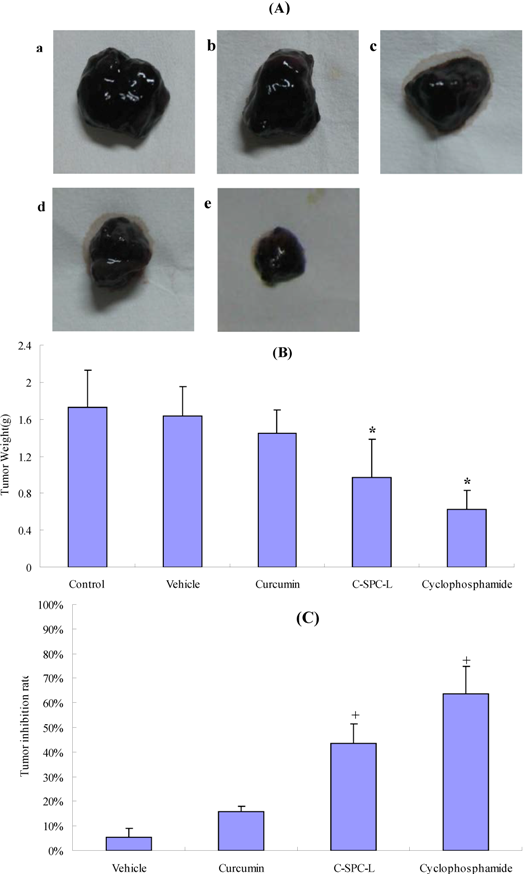
2.7. Effects on Tumor Growth
3. Experimental
3.1. Chemicals
3.2. Animals and Cell Lines
3.3. Liposome Preparation
| Formulation | Curcumin (mg) | SPC (mg) | EPC (mg) | HSPC (mg) | Cholesterol (mg) | Tween-80 (mg) | PBS pH 6.5 (mL) |
|---|---|---|---|---|---|---|---|
| C-SPC-L | 2 | 40 | - | - | 5 | 5 | 1 |
| C-EPC-L | 2 | - | 40 | - | 5 | 5 | 1 |
| C-HSPC-L | 2 | - | - | 40 | 5 | 5 | 1 |
3.4. Photon Correlation Spectroscopy
3.5. Determination of Encapsulation Efficiency

3.6. Transmission Electron Microscopy
3.7. Differential Scanning Calorimetry
3.8. In Vitro Drug Release
3.9. Skin Preparation
3.10. Skin-Penetration Experiment
3.11. HPLC Assay
3.12. Data Analysis of Skin Penetration
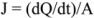
3.13. MTT Assay
3.14. Pharmacodynamic Evaluation
3.15. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Not available.
References
- Shang, Y.J.; Jin, X.L.; Shang, X.L.; Tang, J.J.; Liu, G.Y.; Dai, F.; Qian, Y.P.; Fan, G.J.; Liu, Q.; Zhou, B. Antioxidant capacity of curcumin-directed analogues: Structure-activity relationship and influence of microenvironment. Food Chem. 2010, 119, 1435–1442. [Google Scholar]
- Lee, K.H.; Aziz, F.H.A.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200. [Google Scholar] [CrossRef]
- Tang, H.D.; Murphy, C.J.; Zhang, B.; Shen, Y.Q.; van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Curcumin polymers as anticancer conjugates. Biomaterials 2010, 31, 7139–7149. [Google Scholar]
- Hua, W.F.; Fu, Y.S.; Liao, Y.J.; Xia, W.J.; Chen, Y.C.; Zeng, Y.X.; Kung, H.F.; Xie, D. Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur. J. Pharmacol. 2010, 637, 16–21. [Google Scholar] [CrossRef]
- Lekha Nair, K.; Thulasidasan, A.K.T.; Deepa, G.; Anto, R.J.; Vinod Kumar, G.S. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier original. Int. J. Pharm. 2012, 425, 44–52. [Google Scholar] [CrossRef]
- Wena, Y.D.; Ho, Y.L.; Shiau, R.J.; Yeh, J.K.; Wua, J.Y.; Wanga, W.L.; Chiou, S.J. Synergistic antitumor effect of curcumin and dinitrosyl iron complexes for against melanoma cells. J. Organomet. Chem. 2010, 695, 352–359. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Dhillon, N.; Wolff, R.A.; Abbruzzese, J.L.; Hong, D.S.; Camachi, L.H.; Li, L. Phase II clinical trial of curcumin in patients with advanced pancreatic cancer. J. Clin. Oncol. 2006, 24, 14151. [Google Scholar]
- Shi, S.; Manjul, M. Comparative bioavailability of curcumin, turmeric and BiocurcumaxTM in traditional vehicles using non-everted rat intestinal sac model. J. Funct. Foods 2010, 2, 60–65. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Jiang, Y.; Li, J.; Di, Y.; Ni, Q.; Fu, D. Liposome based delivery systems in pancreatic cancer treatment: From bench to bedside. Cancer Treat. Rev. 2011, 37, 633–642. [Google Scholar] [CrossRef]
- Muthu, M.S.; Singh, S. Targeted nanomedicines: Effective treatment and brain disorders. Nanomedicine 2009, 4, 105–118. [Google Scholar] [CrossRef]
- Seth, A.K.; Misra, A.; Umrigar, D. Topical liposomal gel of idoxuridine for the treatment of herpes simplex: Pharmaceutical and clinical implications. Pharm. Dev. Technol. 2004, 9, 277–289. [Google Scholar]
- Mura, P.; Maestrelli, F.; Gonzalez-Rodrıguez, M.L.; Michelacci, I.; Ghelardini, C.; Rabasco, A.M. Development, characterization and in vivo evaluation of benzocaine-loaded liposomes. Eur. J. Pharm. Biopharm. 2007, 67, 86–95. [Google Scholar] [CrossRef]
- Nina, D.C.; Dietrich, S.; Volker, A.; Alfred, F. Development of liposomes containing ethanol for skin delivery of temoporfin: Characterization and in vitro penetration studies. Colloids Surf. B 2009, 74, 114–122. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Sainz, M.C.; Chantres, J.R.; Elorza, B.; Elorza, M.A. DSC study of action of phenylbutazone on phospholipid phase transitions. Int. J. Pharm. 1993, 91, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Lin, A.H.; Chen, Z.P.; Wang, W.; Zhang, T.; Cai, H.; Cai, B.C. Ammonium sulfate gradient loading of brucine into liposomes: Effect of phospholipid composition on entrapment efficiency and physicochemical properties in vitro. Drug Dev. Ind. Pharm. 2010, 36, 245–253. [Google Scholar] [CrossRef]
- Sebastian, Z.; Wolfgang, P.; Jiirgen, L. Interaction of phosphatidylcholine liposomes with the human stratum corneum. BBA Biomembranes 1995, 1237, 176–182. [Google Scholar] [CrossRef]
- Makiko, F.J.; Kumi, S.; Yoichi, W.; Mitsuo, M. Effect of phosphatidylcholine on skin permeation of indomethacin from gel prepared with liquid paraffin and hydrogenated phospholipid. J. Pharm. Pharmacol. 2001, 222, 57–64. [Google Scholar]
- Kien, X.N.; Hiroshi, U.; Toshinori, S.; Huong, T.B.; Ryoichi, K. Enhanced release of chitosanase from streptomyces griseus through direct interaction of liposome with cell membrane under heat stress. J. Biosci. Bioeng. 2008, 106, 602–605. [Google Scholar] [CrossRef]
- Marilena, C.; Maria, G.C.; Stefania, B.; Stefania, B.; Donatella, P.; Franco, A.; Domenicoantonio, R.; Sebastiano, F.; Massimo, F.; Diego, R. Cytotoxic effects of Gemcitabine-loaded liposomes in human anaplastic thyroid carcinoma cells. BMC Cancer 2004, 4, 63. [Google Scholar] [CrossRef]
- Deshiikan, S.R.; Papadopoulos, K.D. Modified booth equation for the calculation of zeta potential. Colloid Polym. Sci. 1998, 276, 117–124. [Google Scholar] [CrossRef]
- El-Maghraby, G.M.; Williams, A.C.; Barry, B.W. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J. Pharm. Pharmacol. 2001, 53, 1069–1077. [Google Scholar]
- Liu, C.H.; Chang, F.Y. Development and characterization of eucalyptol microemulsions for topic delivery of curcumin. Chem. Pharm. Bull. 2011, 59, 172–178. [Google Scholar] [CrossRef]
- Song, J.; Shi, F.; Zhang, Z.H.; Zhu, F.X.; Xue, J.; Tan, X.B.; Zhang, L.Y.; Jia, X.B. Formulation and evaluation of celastrol-loaded liposomes. Molecules 2011, 16, 7880–7892. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics. Molecules 2012, 17, 5972-5987. https://doi.org/10.3390/molecules17055972
Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics. Molecules. 2012; 17(5):5972-5987. https://doi.org/10.3390/molecules17055972
Chicago/Turabian StyleChen, Yan, Qingqing Wu, Zhenghai Zhang, Ling Yuan, Xuan Liu, and Lei Zhou. 2012. "Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics" Molecules 17, no. 5: 5972-5987. https://doi.org/10.3390/molecules17055972
APA StyleChen, Y., Wu, Q., Zhang, Z., Yuan, L., Liu, X., & Zhou, L. (2012). Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics. Molecules, 17(5), 5972-5987. https://doi.org/10.3390/molecules17055972




