Protective Effects of Extracts and Flavonoids Isolated from Scutia buxifolia Reissek against Chromosome Damage in Human Lymphocytes Exposed to Hydrogen Peroxide
Abstract
:1. Introduction
2. Results
2.1. HPLC Analysis
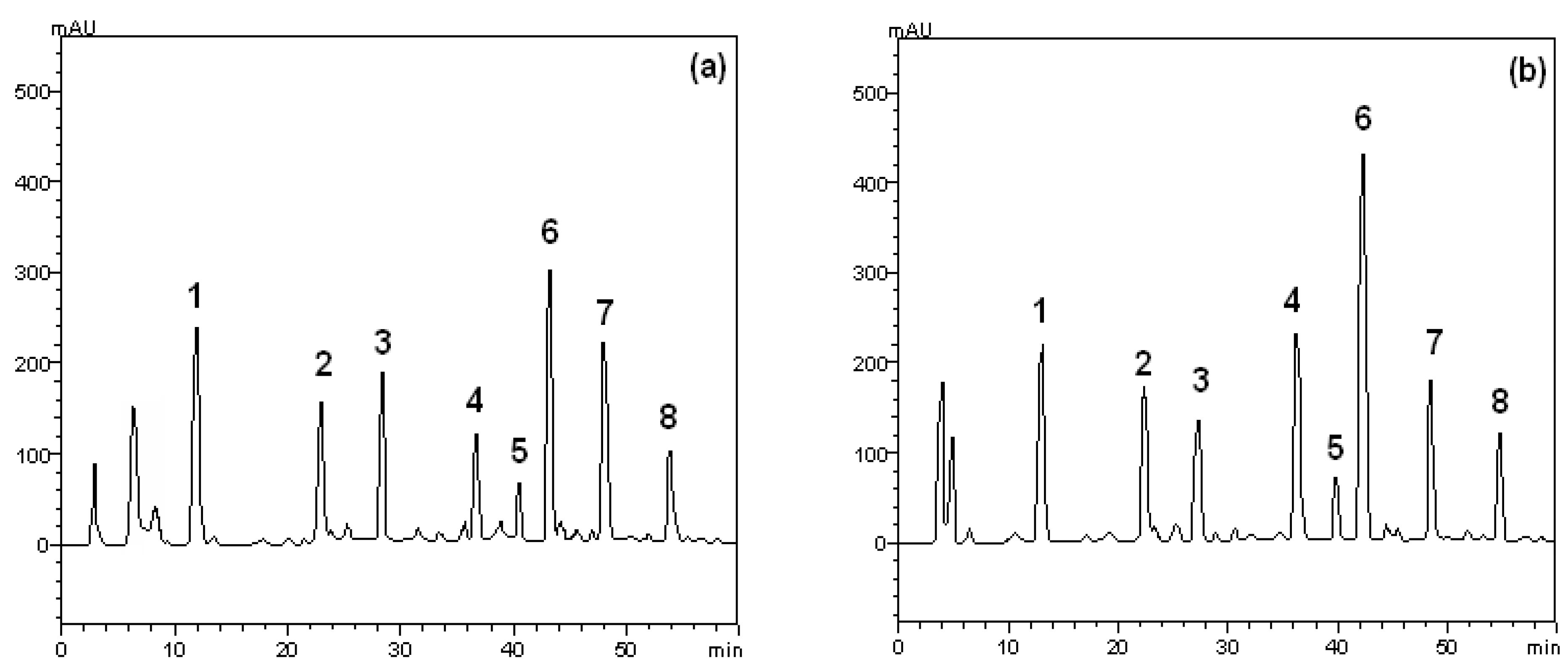
| Compounds | Crude extract | Ethyl acetate fraction | LOD (μg/mL) | LOQ (μg/mL) | |||
|---|---|---|---|---|---|---|---|
| Quantities (mg/g) | Quantities (mg/g) | ||||||
| Gallic acid | 18.1 ± 0.09 a | 34.6 ± 0.10 b | 0.041 | 0.125 | |||
| Chlorogenic acid | 4.9 ± 0.16 a | 22.1 ± 0.07 b | 0.030 | 0.992 | |||
| Caffeic acid | 7.3 ± 0.04 a | 19.8 ± 0.13 b | 0.026 | 0.079 | |||
| Rutin | 14.2 ± 0.05 a | 48.1 ± 0.18 b | 0.028 | 0.084 | |||
| Isoquercitrin * | 1.5 ± 0.07 a | 6.6 ± 0.04 b | - | - | |||
| Quercitrin * | 26.5 ± 0.03 a | 183.2 ± 0.05 b | - | - | |||
| Quercetin | 42.1 ± 0.11 a | 27.1 ± 0.03 b | 0.033 | 0.100 | |||
| Kaempferol | 5.6 ± 0.02 a | 15.5 ± 0.17 b | 0.021 | 0.063 | |||
2.2. Cell Viability
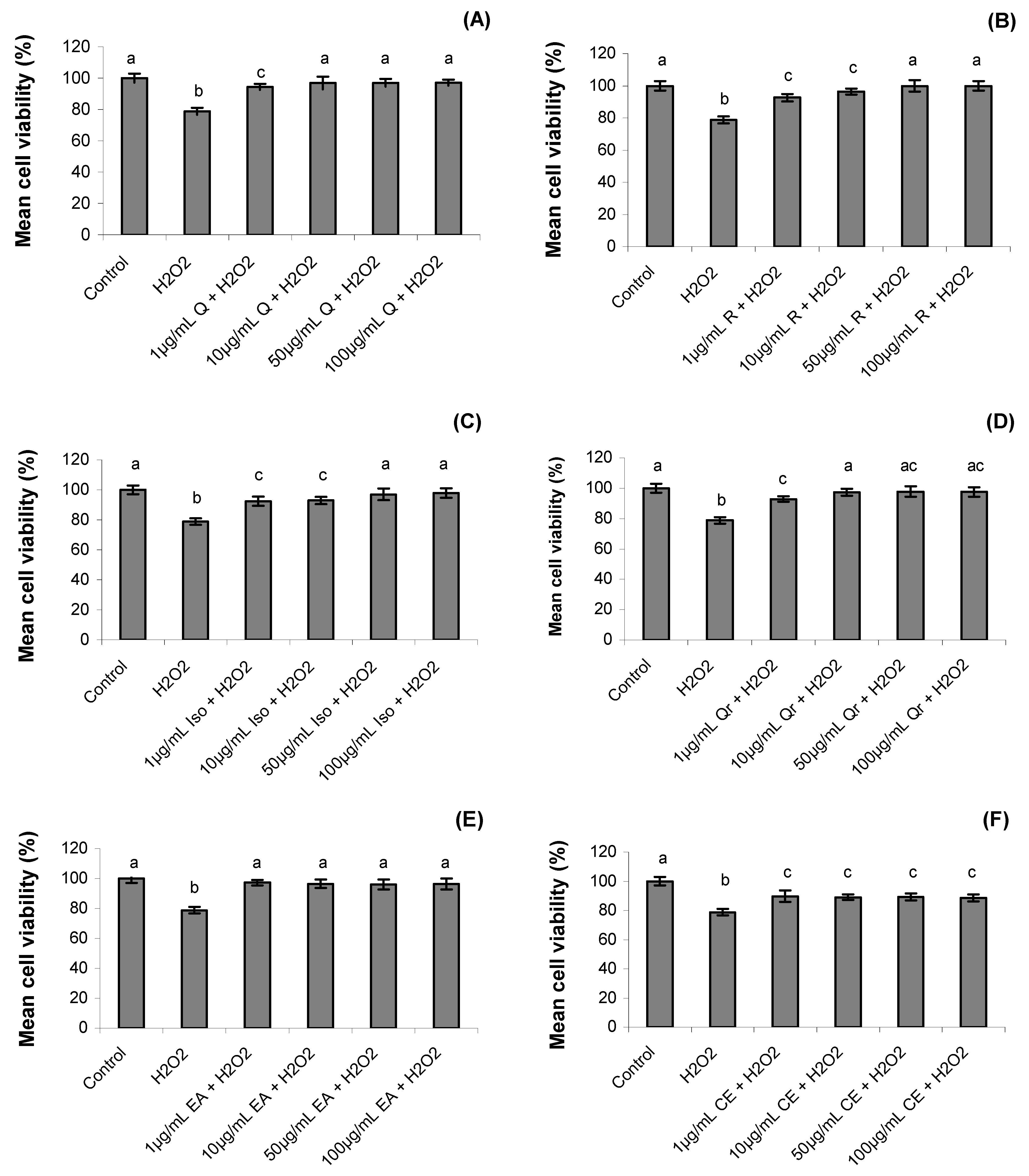
2.3. Mitotic Index
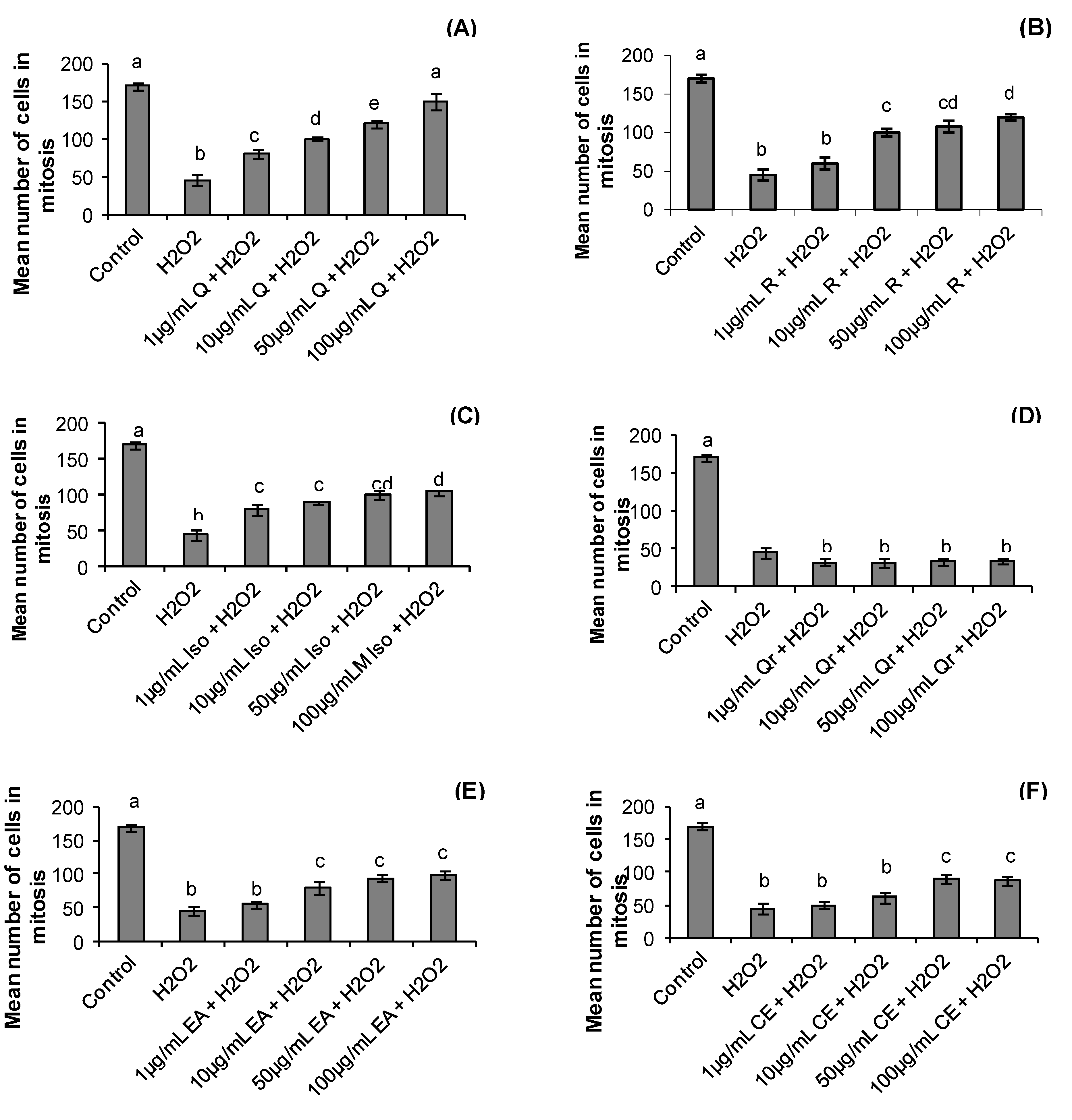
2.4. Chromosomal Instability
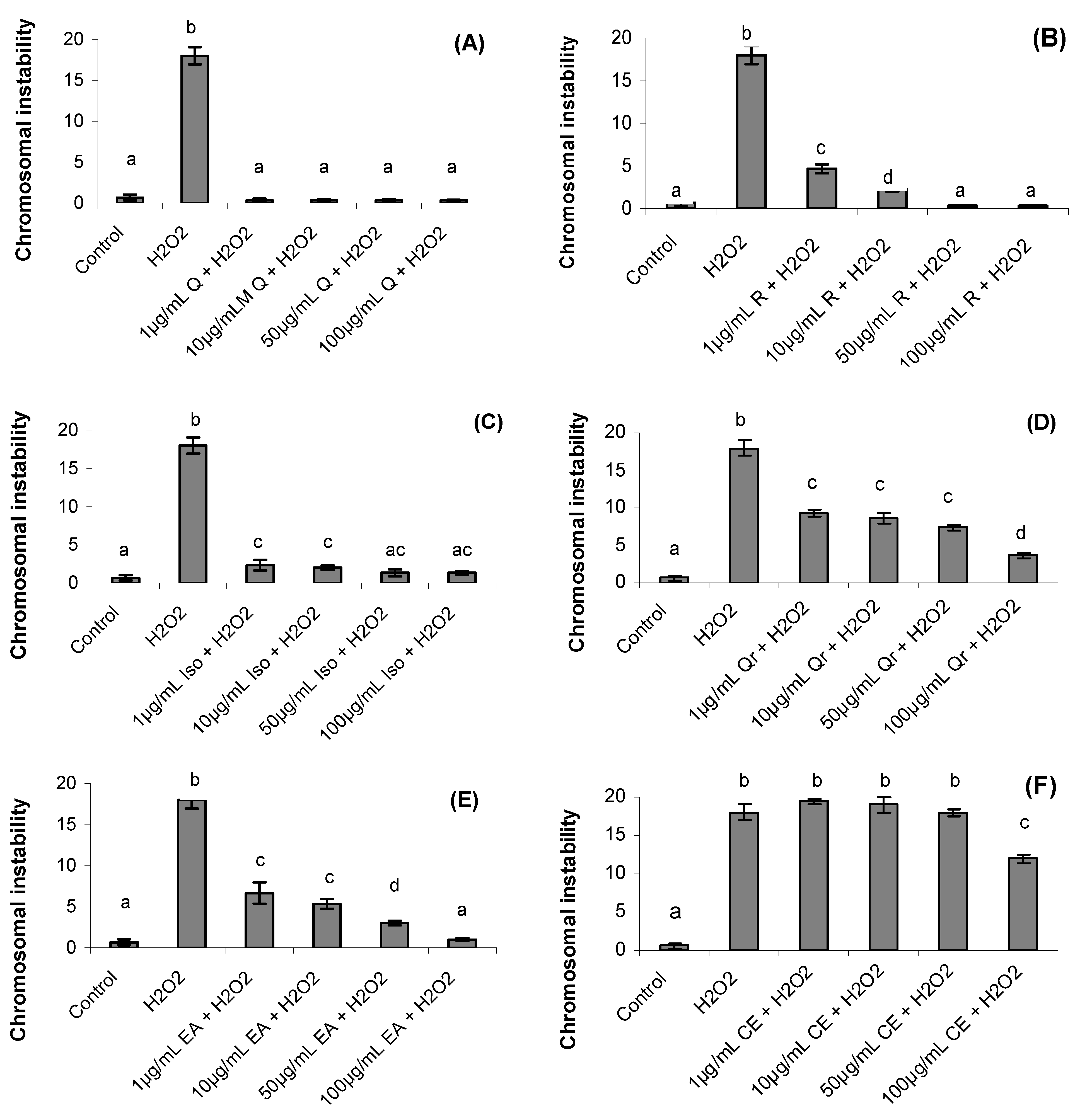
2.5. Genotoxicity Analyzed by Alkaline Comet Assay
| Concentration (50 µg/mL) | Comet Class (0–4) | Index of DNA damage | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Control | 88 ± 3 | 5 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 0.12 a |
| H2O2 | 42 ± 5 | 10 ± 2 | 5 ± 1 | 12 ± 2 | 29 ± 1 | 0.56 b |
| Crude extract | 53 ± 3 | 17 ± 2 | 11 ± 2 | 13 ± 3 | 6 ± 1 | 0.47 bc |
| Ethyl acetate fraction | 62 ± 6 | 12 ± 2 | 8 ± 1 | 14 ± 2 | 4 ± 1 | 0.38 c |
| Quercetin | 77 ± 4 | 13 ± 1 | 4 ± 1 | 3 ± 1 | 3 ± 1 | 0.23 a |
| Quercitrin | 46 ± 3 | 11 ± 2 | 8 ± 1 | 16 ± 3 | 20 ± 2 | 0.55 b |
| Isoquercitrin | 62 ± 2 | 10 ± 3 | 9 ±1 | 10 ± 2 | 9 ± 1 | 0.38 c |
| Rutin | 66 ± 5 | 16 ± 2 | 8 ± 1 | 5 ± 1 | 8 ± 1 | 0.37 c |
3. Discussion
4. Experimental
4.1. Chemicals, Apparatus and General Procedures
4.2. Plant Collection and Extraction
4.3. Quantification of Phenolics and Flavonoids Compounds by HPLC-DAD
4.4. Treatments
4.5. Blood Collection and Lymphocyte Culture
4.6. Cell Viability
4.7. Single Cell Gel Electrophoresis (Comet Assay)
4.8. Mitotic Index and Chromosomal Instability in Human Lymphocytes
4.9. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds and extracts are available from the authors.
References
- Nsimba, R.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 231–255. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Mošovska, S.; Mikulašova, M.; Brindzova, L.; Valik, L.; Mikušova, L. Genotoxic and antimutagenic activities of extracts from pseudocereals in the Salmonella mutagenicity assay. Food Chem. Toxicol. 2010, 48, 1483–1487. [Google Scholar] [CrossRef]
- Hayder, N.; Abdelwahed, A.; Kilani, S.; Ben Ammar, R.; Mahmoud, A.; Ghedira, K.; Chekir-Ghedira, L. Antimutagenic and free radical scavenging activities of extracts from (Tunisian) Myrtus communis. Mutat. Res. 2004, 564, 89–95. [Google Scholar] [CrossRef]
- Kilani, S.; Ben Ammar, R.; Abdelwahed, A.; Hayder, N.; Mahmoud, A.; Ben Chibani, J.; Chekir-Ghedira, L.; Ghedira, K. Evaluation of the antimutagenic and antiradical potentials of extracts from the tubers of (Tunisian) Cyperus rotundus. Environ. Toxicol. Chem. 2005, 87, 415–425. [Google Scholar] [CrossRef]
- Yilmaz, O.; Keser, S.; Tuzcu, M.; Cetintas, B. Resveratrol (trans-3,40,5- trihydroxystilbene) decreases lipid peroxidation level and protects antioxidant capacity in sera and erythrocytes of old female Wistar rats induced by the kidney carcinogen potassium bromate. Environ. Toxicol. Pharm. 2007, 24, 79–85. [Google Scholar] [CrossRef]
- Bhouri, W.; Derbel, S.; Skandrani, I.; Boubaker, J.; Bouhlel, I.; Sghaier, M.B.; Kilani, S.; Mariotte, A.M.; Dijoux-Franca, M.G.; Ghedira, K.; Chekir-Ghedira, L. Study of genotoxic, antigenotoxic and antioxidant activities of the digallic acid isolated from Pistacia lentiscus fruits. Toxicol. In Vitro 2010, 24, 509–515. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2010, 84, 551–562. [Google Scholar]
- Bhattacharya, S. Natural antimutagens: A review. Res. J. Med. Plant 2011, 5, 116–126. [Google Scholar] [CrossRef]
- Wasicky, R.; Wasicky, M.; Joachimovits, R. Erstuntersuchungen na Coronilha—Scutia buxifolia Reissek. Planta Med. 1964, 12, 13–25. [Google Scholar] [CrossRef]
- Morel, A.F.; Maldaner, G.; Ilha, V.; Bissau, F.; Silva, U.F.; Dalcol, II. Cyclopeptide alkaloids from Scutia buxifolia Reiss and their antimicrobial activity. Phytochemistry 2005, 66, 2571–2576. [Google Scholar] [CrossRef]
- Boligon, A.A.; Janovik, V.; Frohlich, J.K.; Spader, T.B.; Froeder, A.L.F.; Alves, S.H.; Athayde, M.L. Antimicrobial and cytotoxic activities of leaves, twigs and stem bark of Scutia buxifolia Reissek. Nat. Prod. Res. 2011. [Google Scholar] [CrossRef]
- Boligon, A.A.; Agertt, V.; Janovik, V.; Cruz, R.C.; Campos, M.M.A.; Guillaume, D.; Athayde, M.L.; dos Santos, A.R.S. Antimycobacterial activity of the fractions and compounds from Scutia buxifolia. Br. J. Pharm. 2012, 22, 45–52. [Google Scholar]
- Boligon, A.A.; Pereira, R.P.; Feltrin, A.C.; Machado, M.M.; Janovik, V.; Rocha, J.B.T.; Athayde, M.L. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss. Bioresour. Technol. 2000, 100, 6592–6598. [Google Scholar]
- Wilms, L.C.; Hollman, P.C.H.; Boots, A.W.; Kleinjans, J.C.S. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat. Res. 2005, 582, 155–162. [Google Scholar] [CrossRef]
- Duthie, S.J.; Collins, A.R.; Duthie, G.G.; Dobson, V.L. Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidized pyrimidines) in human lymphocytes. Mutat. Res. 2007, 393, 223–231. [Google Scholar]
- Duthie, S.J.; Johnson, W.; Dobson, V.L. The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimdines) and growth in human cells. Mutat. Res. 2007, 390, 141–151. [Google Scholar]
- Min, K.; Ebeler, S.E. Quercetin inhibits hydrogen peroxide-induced DNA damage and enhances DNA repair in Caco-2 cells. Food Chem. Toxicol. 2009, 47, 2716–2722. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.-L.; Catteau, J.-P.; Pommery, J.; Wallet, J.-C.; Gaydou, E.M. Antioxidant properties of hydroxy- flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- Obermeier, M.T.; White, R.E.; Yang, C.S. Effects of bioflavonoids on hepatic P450 activities. Xenobiotica 1995, 25, 575–584. [Google Scholar] [CrossRef]
- Laghari, A.H.; Memon, S.; Nelofar, A.; Khan, K.M.; Yasmin, A. Determination of free phenolic acids and antioxidant activity of methanolic extracts obtained from fruits and leaves of Chenopodium album. Food Chem. 2011, 126, 1850–1855. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2 (R1). 2005. Available online: http://www.ich.org accessed (accessed on 24 April 2012).
- Burow, M.E.; Weldon, C.B.; Tang, Y.; Navar, G.L.; Krajewski, S.; Reed, J.C.; Hammond, T.G.; Clejan, S.; Backman, B.S. Differences in susceptibility to tumour necrosis factor alpha-induced apoptosis among MCF-7 breast cancer cell variants. Cancer Res. 1998, 58, 4940–4946. [Google Scholar]
- Singh, N.; McCoy, M.; Tice, R.; Schneider, E. A simple technique for quantification of low levels of DNA damage in individuals cells. Exp. Cell Res. 1995, 175, 184–191. [Google Scholar]
- Tice, R.R.; Agurell, D.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Hartmann, A.; Agurell, E.; Beevers, C.; Brendler-Schwaab, S.; Burlinson, B.; Clay, P.; Collins, A.; Smith, G.; Speit, G.; Thybaud, V.; Tice, R.R. Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis 2003, 18, 45–51. [Google Scholar] [CrossRef]
- Speit, G.; Hanelt, S.; Helbig, R.; Seidel, A.; Hartmann, A. Detection of DNA effects in human cells with the comet assay and their relevance formutagenesis. Toxicol. Lett. 1996, 88, 91–98. [Google Scholar]
- Yunis, J.J. High resolution of human chromosomes. Science 2006, 1976, 1268–1270. [Google Scholar]
- Rusak, G.; Piantanida, I.; Masic, L.; Kapuralinc, K.; Durgo, K.; Kopjar, N. Spectrophotometric analysis of flavonoid-DNA interactions and DNA damaging/protecting and cytotoxic potential of flavonoids in human peripheral blood lymphocytes. Chem.-Biol. Int. 2011, 188, 181–189. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boligon, A.A.; Sagrillo, M.R.; Machado, L.F.; De Souza Filho, O.; Machado, M.M.; Da Cruz, I.B.M.; Athayde, M.L. Protective Effects of Extracts and Flavonoids Isolated from Scutia buxifolia Reissek against Chromosome Damage in Human Lymphocytes Exposed to Hydrogen Peroxide. Molecules 2012, 17, 5757-5769. https://doi.org/10.3390/molecules17055757
Boligon AA, Sagrillo MR, Machado LF, De Souza Filho O, Machado MM, Da Cruz IBM, Athayde ML. Protective Effects of Extracts and Flavonoids Isolated from Scutia buxifolia Reissek against Chromosome Damage in Human Lymphocytes Exposed to Hydrogen Peroxide. Molecules. 2012; 17(5):5757-5769. https://doi.org/10.3390/molecules17055757
Chicago/Turabian StyleBoligon, Aline Augusti, Michele Rorato Sagrillo, Luiz Filipe Machado, Olmiro De Souza Filho, Michel Mansur Machado, Ivana Beatrice Manica Da Cruz, and Margareth Linde Athayde. 2012. "Protective Effects of Extracts and Flavonoids Isolated from Scutia buxifolia Reissek against Chromosome Damage in Human Lymphocytes Exposed to Hydrogen Peroxide" Molecules 17, no. 5: 5757-5769. https://doi.org/10.3390/molecules17055757
APA StyleBoligon, A. A., Sagrillo, M. R., Machado, L. F., De Souza Filho, O., Machado, M. M., Da Cruz, I. B. M., & Athayde, M. L. (2012). Protective Effects of Extracts and Flavonoids Isolated from Scutia buxifolia Reissek against Chromosome Damage in Human Lymphocytes Exposed to Hydrogen Peroxide. Molecules, 17(5), 5757-5769. https://doi.org/10.3390/molecules17055757




