Two New Aryltetralin Lignans from the Roots of Dolomiaea souliei
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Identification
 −4.0° (c 0.225, MeOH). The HR-ESI-MS spectrum (m/z 391.13869 [M−H]−, calcd. for 391.13929) indicated the molecular formula of 1 to be C20H24O8. The 1H and 13C-NMR (APT) data of 1 showed the presence of a 1,3,4-trisubstituted benzene moiety [δH: 6.86 (1H, s, H-2'), 6.76 (1H, m, H-5'), 6.78 (1H, m, H-6'); δC: 134.0 (C-1'), 117.0 (C-2'), 148.7 (C-3'), 146.6 (C-4'), 115.5 (C-5'), 126.2 (C-6')], a 1,2,4,5-tetrasubstituted benzene moiety [δH: 6.16 (1H, s, H-3), 6.66 (1H, s, H-6); δC: 126.7 (C-1), 132.7 (C-2), 118.0 (C-3), 145.4 (C-4), 147.7 (C-5), 113.2 (C-6)], two methoxyl groups [δH: 3.76 (3H, s, 3'-OCH3), 3.82 (3H, s, 5-OCH3); δC: 56.6 (3'-OCH3), 56.7 (5-OCH3)] and other aliphatic signals [δH: 4.38 (1H, s, H-7'), 3.49 (1H, d, J = 10.8 Hz, H-9'a), 3.59 (1H, d, J = 10.8 Hz, H-9'b), 2.56 (1H, d, J = 17.4 Hz, H-7a), 3.34 (1H, d, J = 17.4 Hz, H-7b), 3.85 (2H, m, H-9); δC: 48.6 (C-7'), 65.0 (C-9'), 37.0 (C-7), 76.2 (C-8), 68.3 (C-9)]. The NMR signals were assigned with the aid of HSQC and HMBC spectra, and cross-peaks observed in the HMBC (H-2'/C-1', C-3', C-7'; 3'-OCH3/C-3'; H-5°/C-3', C-4'; H-6'/C-4'; H-7'/C-2', C-6', C-3; H-9'/C-7', C-8', C-8; H-3/C-7', C-2, C-4; 5-OCH3/C-5; H-6/C-1, C-5, C-7; H-7/C-8', C-6, C-8; H-9/C-8', C-7, C-8) indicated that 1 resembled the structure of (+)-cycloolivil [5].
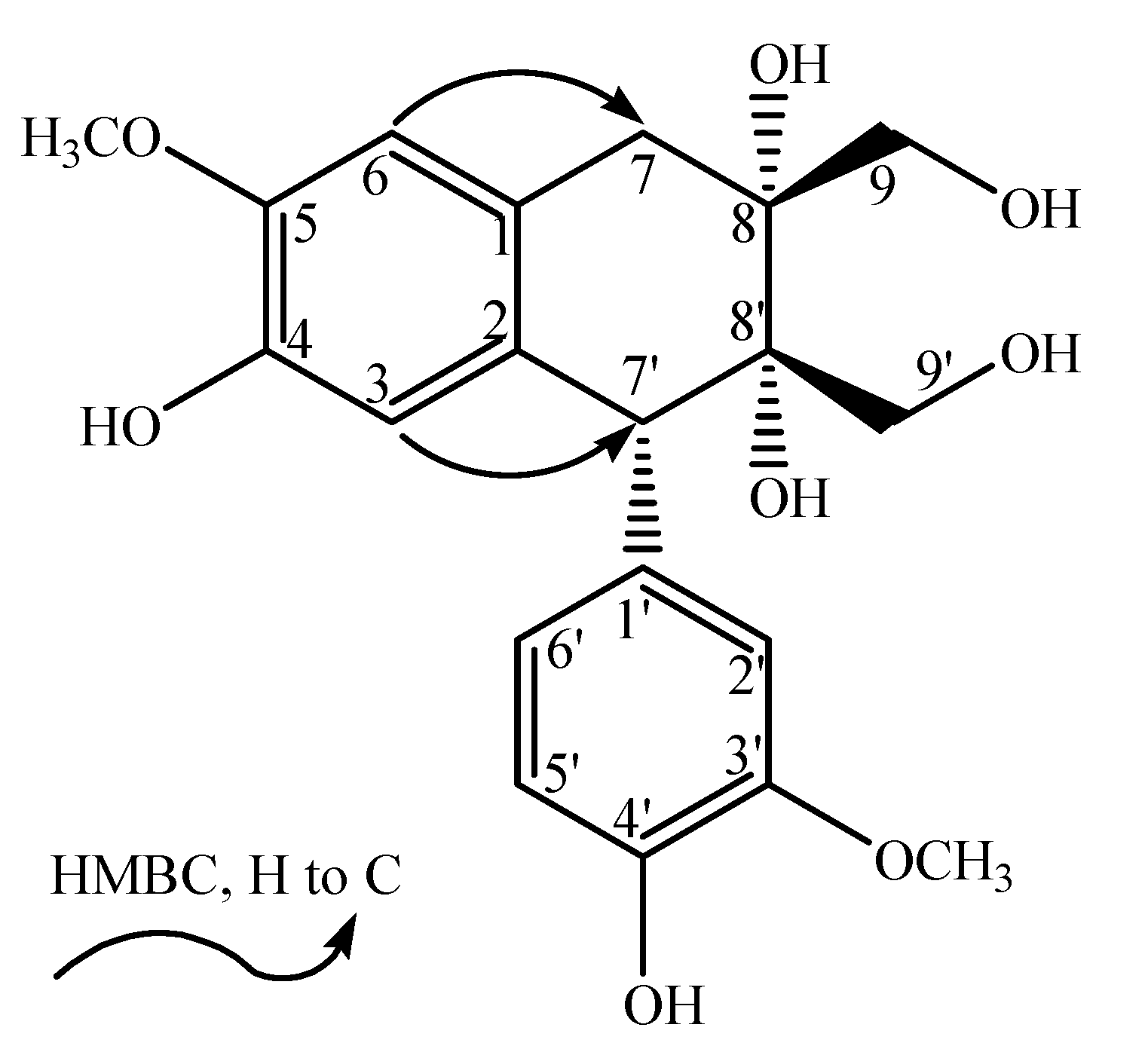
−4.0° (c 0.225, MeOH). The HR-ESI-MS spectrum (m/z 391.13869 [M−H]−, calcd. for 391.13929) indicated the molecular formula of 1 to be C20H24O8. The 1H and 13C-NMR (APT) data of 1 showed the presence of a 1,3,4-trisubstituted benzene moiety [δH: 6.86 (1H, s, H-2'), 6.76 (1H, m, H-5'), 6.78 (1H, m, H-6'); δC: 134.0 (C-1'), 117.0 (C-2'), 148.7 (C-3'), 146.6 (C-4'), 115.5 (C-5'), 126.2 (C-6')], a 1,2,4,5-tetrasubstituted benzene moiety [δH: 6.16 (1H, s, H-3), 6.66 (1H, s, H-6); δC: 126.7 (C-1), 132.7 (C-2), 118.0 (C-3), 145.4 (C-4), 147.7 (C-5), 113.2 (C-6)], two methoxyl groups [δH: 3.76 (3H, s, 3'-OCH3), 3.82 (3H, s, 5-OCH3); δC: 56.6 (3'-OCH3), 56.7 (5-OCH3)] and other aliphatic signals [δH: 4.38 (1H, s, H-7'), 3.49 (1H, d, J = 10.8 Hz, H-9'a), 3.59 (1H, d, J = 10.8 Hz, H-9'b), 2.56 (1H, d, J = 17.4 Hz, H-7a), 3.34 (1H, d, J = 17.4 Hz, H-7b), 3.85 (2H, m, H-9); δC: 48.6 (C-7'), 65.0 (C-9'), 37.0 (C-7), 76.2 (C-8), 68.3 (C-9)]. The NMR signals were assigned with the aid of HSQC and HMBC spectra, and cross-peaks observed in the HMBC (H-2'/C-1', C-3', C-7'; 3'-OCH3/C-3'; H-5°/C-3', C-4'; H-6'/C-4'; H-7'/C-2', C-6', C-3; H-9'/C-7', C-8', C-8; H-3/C-7', C-2, C-4; 5-OCH3/C-5; H-6/C-1, C-5, C-7; H-7/C-8', C-6, C-8; H-9/C-8', C-7, C-8) indicated that 1 resembled the structure of (+)-cycloolivil [5].  −16.3° (c 0.24, MeOH). The HR-ESI-MS spectrum (m/z 391.13897 [M−H]−, calcd. for 391.13929) indicated the molecular formula of 2 to be C20H24O8. The NMR signals of 2 were assigned with the aid of HSQC and HMBC spectra and by comparison with the signals of 1. The spectroscopic data of 2 suggested that it was another aryltetralin-type lignin, exhibiting an identical skeleton of 1.
−16.3° (c 0.24, MeOH). The HR-ESI-MS spectrum (m/z 391.13897 [M−H]−, calcd. for 391.13929) indicated the molecular formula of 2 to be C20H24O8. The NMR signals of 2 were assigned with the aid of HSQC and HMBC spectra and by comparison with the signals of 1. The spectroscopic data of 2 suggested that it was another aryltetralin-type lignin, exhibiting an identical skeleton of 1.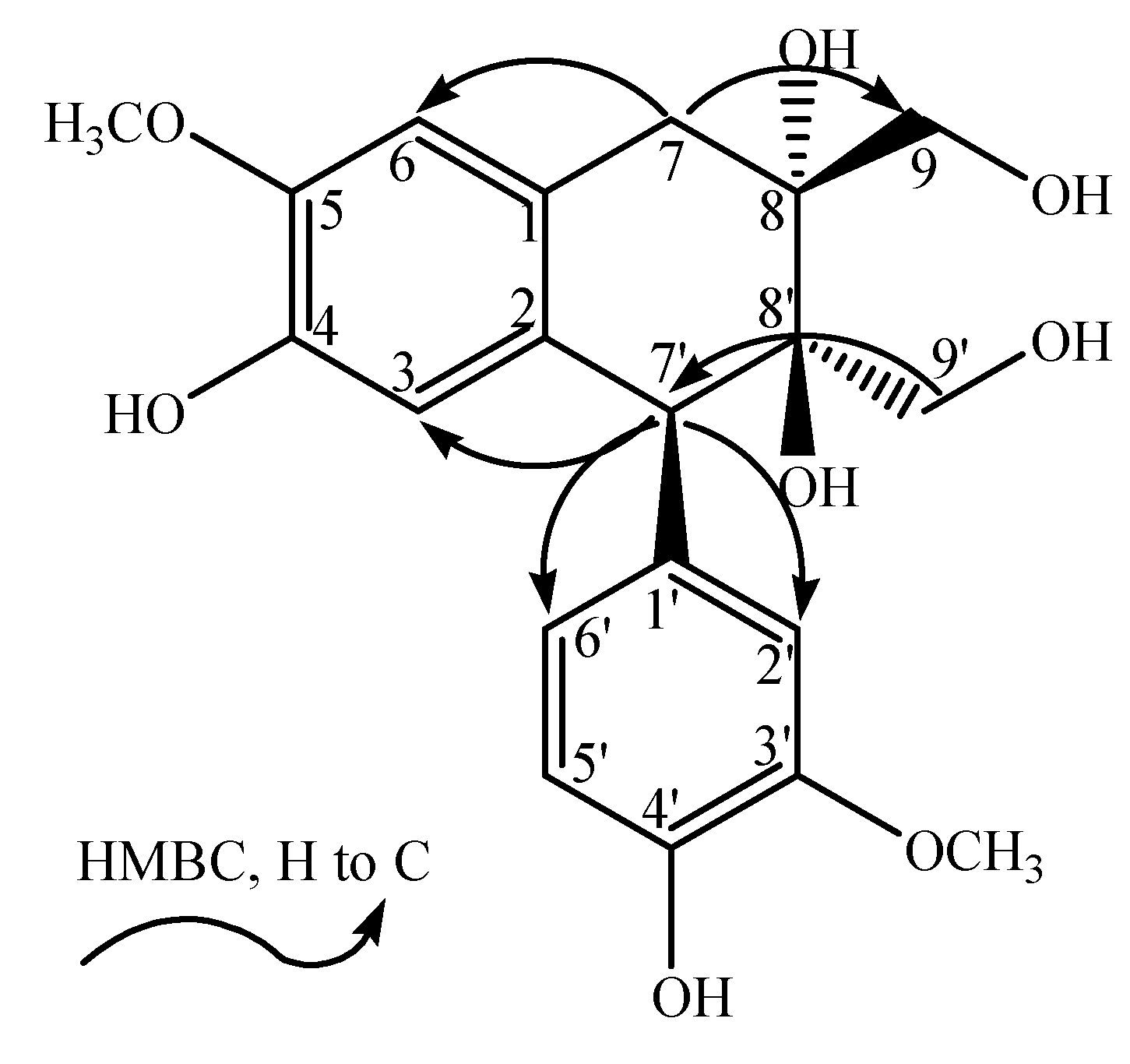
2.2. Cytotoxic Activity
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
 : −4.0° (c 0.225, MeOH); UV λmax (log ε) nm (MeOH): 207 (4.46), 283 (3.60); CD nm (Δε) (c 1.28 × 10−3 mol/L, MeOH): 290 (−1.8), 271 (+0.5), 237 (+0.7); IR νmax cm−1 (KBr): 3392, 2928, 1647, 1516, 1445, 1383, 1261, 1126, 1100, 1033, 798, 762, 652, 601; 1H-NMR (CD3OD, 600 MHz) δ: 6.86 (1H, s, H-2'), 3.76 (3H, s, 3'-OCH3), 6.76 (1H, m, H-5'), 6.78 (1H, m, H-6'), 4.38 (1H, s, H-7'), 3.49 (1H, d, J = 10.8 Hz, H-9'a), 3.59 (1H, d, J = 10.8 Hz, H-9'b), 6.16 (1H, s, H-3), 3.82 (3H, s, 5-OCH3), 6.66 (1H, s, H-6), 2.56 (1H, d, J = 17.4 Hz, H-7a), 3.34 (1H, d, J = 17.4 Hz, H-7b), 3.85 (2H, m, H-9); 13C-NMR (CD3OD, 150 MHz) δ: 134.0 (C-1'), 117.0 (C-2'), 148.7 (C-3'), 56.6 (3'-OCH3), 146.6 (C-4'), 115.5 (C-5'), 126.2 (C-6'), 48.6 (C-7'), 75.1 (C-8'), 65.0 (C-9'), 126.7 (C-1), 132.7 (C-2), 118.0 (C-3), 145.4 (C-4), 147.7 (C-5), 56.7 (5-OCH3), 113.2 (C-6), 37.0 (C-7), 76.2 (C-8), 68.3 (C-9).
: −4.0° (c 0.225, MeOH); UV λmax (log ε) nm (MeOH): 207 (4.46), 283 (3.60); CD nm (Δε) (c 1.28 × 10−3 mol/L, MeOH): 290 (−1.8), 271 (+0.5), 237 (+0.7); IR νmax cm−1 (KBr): 3392, 2928, 1647, 1516, 1445, 1383, 1261, 1126, 1100, 1033, 798, 762, 652, 601; 1H-NMR (CD3OD, 600 MHz) δ: 6.86 (1H, s, H-2'), 3.76 (3H, s, 3'-OCH3), 6.76 (1H, m, H-5'), 6.78 (1H, m, H-6'), 4.38 (1H, s, H-7'), 3.49 (1H, d, J = 10.8 Hz, H-9'a), 3.59 (1H, d, J = 10.8 Hz, H-9'b), 6.16 (1H, s, H-3), 3.82 (3H, s, 5-OCH3), 6.66 (1H, s, H-6), 2.56 (1H, d, J = 17.4 Hz, H-7a), 3.34 (1H, d, J = 17.4 Hz, H-7b), 3.85 (2H, m, H-9); 13C-NMR (CD3OD, 150 MHz) δ: 134.0 (C-1'), 117.0 (C-2'), 148.7 (C-3'), 56.6 (3'-OCH3), 146.6 (C-4'), 115.5 (C-5'), 126.2 (C-6'), 48.6 (C-7'), 75.1 (C-8'), 65.0 (C-9'), 126.7 (C-1), 132.7 (C-2), 118.0 (C-3), 145.4 (C-4), 147.7 (C-5), 56.7 (5-OCH3), 113.2 (C-6), 37.0 (C-7), 76.2 (C-8), 68.3 (C-9). : −16.3° (c 0.24, MeOH); UV λmax (log ε) nm (MeOH): 210 (4.8), 284 (3.99); CD nm (Δε) (c 2.55 × 10−3 mol/l, MeOH): 291 (+3.1), 273 (−1.2), 230 (+1.9); IR νmax cm−1 (KBr): 3419, 2954, 1652, 1520, 1456, 1373, 1260, 1127, 1097, 1033, 803, 773, 645, 597; 1H-NMR (CD3OD, 600 MHz) δ: 6.78 (1H, s, H-2'), 3.78 (3H, s, 3'-OCH3), 6.72 (1H, d, J = 8.4 Hz, H-5'), 6.63 (1H, m, H-6'), 4.06 (1H, s, H-7'), 3.55 (1H, d, J = 10.2 Hz, H-9'a), 3.96 (1H, d, J = 10.2 Hz, H-9'b), 6.30 (1H, s, H-3), 3.83 (3H, s, 5-OCH3), 6.67 (1H, s, H-6), 3.02 (2H, m, H-7), 3.34 (1H, d, J = 11.4 Hz, H-9a), 3.91 (1H, d, J = 11.4 Hz, H-9b); 13C-NMR (CD3OD, 150 MHz) δ: 133.3 (C-1'), 117.0 (C-2'), 148.4 (C-3'), 56.6 (3'-OCH3), 146.7 (C-4'), 115.5 (C-5'), 125.7 (C-6'), 56.3 (C-7'), 77.1 (C-8'), 67.0 (C-9'), 127.5 (C-1), 131.4 (C-2), 117.6 (C-3), 145.8 (C-4), 148.2 (C-5), 56.6 (5-OCH3), 112.9 (C-6), 39.2 (C-7), 77.6 (C-8), 67.7 (C-9).
: −16.3° (c 0.24, MeOH); UV λmax (log ε) nm (MeOH): 210 (4.8), 284 (3.99); CD nm (Δε) (c 2.55 × 10−3 mol/l, MeOH): 291 (+3.1), 273 (−1.2), 230 (+1.9); IR νmax cm−1 (KBr): 3419, 2954, 1652, 1520, 1456, 1373, 1260, 1127, 1097, 1033, 803, 773, 645, 597; 1H-NMR (CD3OD, 600 MHz) δ: 6.78 (1H, s, H-2'), 3.78 (3H, s, 3'-OCH3), 6.72 (1H, d, J = 8.4 Hz, H-5'), 6.63 (1H, m, H-6'), 4.06 (1H, s, H-7'), 3.55 (1H, d, J = 10.2 Hz, H-9'a), 3.96 (1H, d, J = 10.2 Hz, H-9'b), 6.30 (1H, s, H-3), 3.83 (3H, s, 5-OCH3), 6.67 (1H, s, H-6), 3.02 (2H, m, H-7), 3.34 (1H, d, J = 11.4 Hz, H-9a), 3.91 (1H, d, J = 11.4 Hz, H-9b); 13C-NMR (CD3OD, 150 MHz) δ: 133.3 (C-1'), 117.0 (C-2'), 148.4 (C-3'), 56.6 (3'-OCH3), 146.7 (C-4'), 115.5 (C-5'), 125.7 (C-6'), 56.3 (C-7'), 77.1 (C-8'), 67.0 (C-9'), 127.5 (C-1), 131.4 (C-2), 117.6 (C-3), 145.8 (C-4), 148.2 (C-5), 56.6 (5-OCH3), 112.9 (C-6), 39.2 (C-7), 77.6 (C-8), 67.7 (C-9).3.5. Bioassays
4. Conclusions
Acknowledgements
References and Notes
- Editoria Committee of Flora of China. In Flora of China; Science Press: Beijing, China; Volume 78, p. 141, Chapter 2.
- Zhou, L.Z.; Jiang, J.H.; Li, Y.P.; Chen, Y.G. Chemical and bioactive studies on Tibetan medicines plants of Vladimiria genus. Yunnan Chem. Tech 2010, 37, 57–62. [Google Scholar]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Ethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef]
- Madhuri, K.; Elango, K.; Ponnusankar, S. Saussurea lappa (Kuth root): Review of its traditional uses, phytochemistry and pharmacology. Orient. Pharm. Exp. Med. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Ghogomu-Tih, R.; Bodo, B.; Nyasse, B.; Sondengam, B.L. Isolation and identification of (−)-olivil and (+)-cycloolivil from Stereospermum kunthianum. Planta Med. 1985, 51, 464. [Google Scholar] [CrossRef]
- Klyne, W.; Stevenson, R.; Swan, J. Optical rotatory dispersion. Part XXVIII. The absolute configuration of otobain and derivatives. J. Chem. Soc. C 1966, 893–896. [Google Scholar]
- Sugiyama, M.; Nagayama, E.; Kikuchi, M. Lignan and phenylpropanoid glycosides from Osmanthus asiaticus. Phytochemistry 1993, 33, 1215–1219. [Google Scholar] [CrossRef]
- Lv, M.; Xu, H. Recent advances in semisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: An update (2008–2010). Mini-Rev. Med. Chem. 2011, 11, 901–909. [Google Scholar] [CrossRef]
- Sample Availability: Samples of dolomiaeasin A and dolomiaeasin B are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, H.; He, C.; Peng, Y.; Zhang, S.; Chen, X.; Xiao, P. Two New Aryltetralin Lignans from the Roots of Dolomiaea souliei. Molecules 2012, 17, 5544-5549. https://doi.org/10.3390/molecules17055544
Wei H, He C, Peng Y, Zhang S, Chen X, Xiao P. Two New Aryltetralin Lignans from the Roots of Dolomiaea souliei. Molecules. 2012; 17(5):5544-5549. https://doi.org/10.3390/molecules17055544
Chicago/Turabian StyleWei, Hua, Chunnian He, Yong Peng, Sen Zhang, Xiaoguang Chen, and Peigen Xiao. 2012. "Two New Aryltetralin Lignans from the Roots of Dolomiaea souliei" Molecules 17, no. 5: 5544-5549. https://doi.org/10.3390/molecules17055544
APA StyleWei, H., He, C., Peng, Y., Zhang, S., Chen, X., & Xiao, P. (2012). Two New Aryltetralin Lignans from the Roots of Dolomiaea souliei. Molecules, 17(5), 5544-5549. https://doi.org/10.3390/molecules17055544



