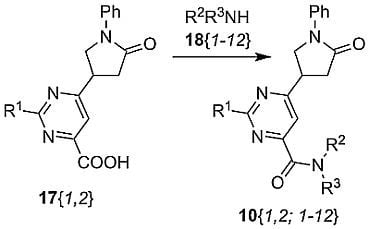
Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides
Abstract
:1. Introduction
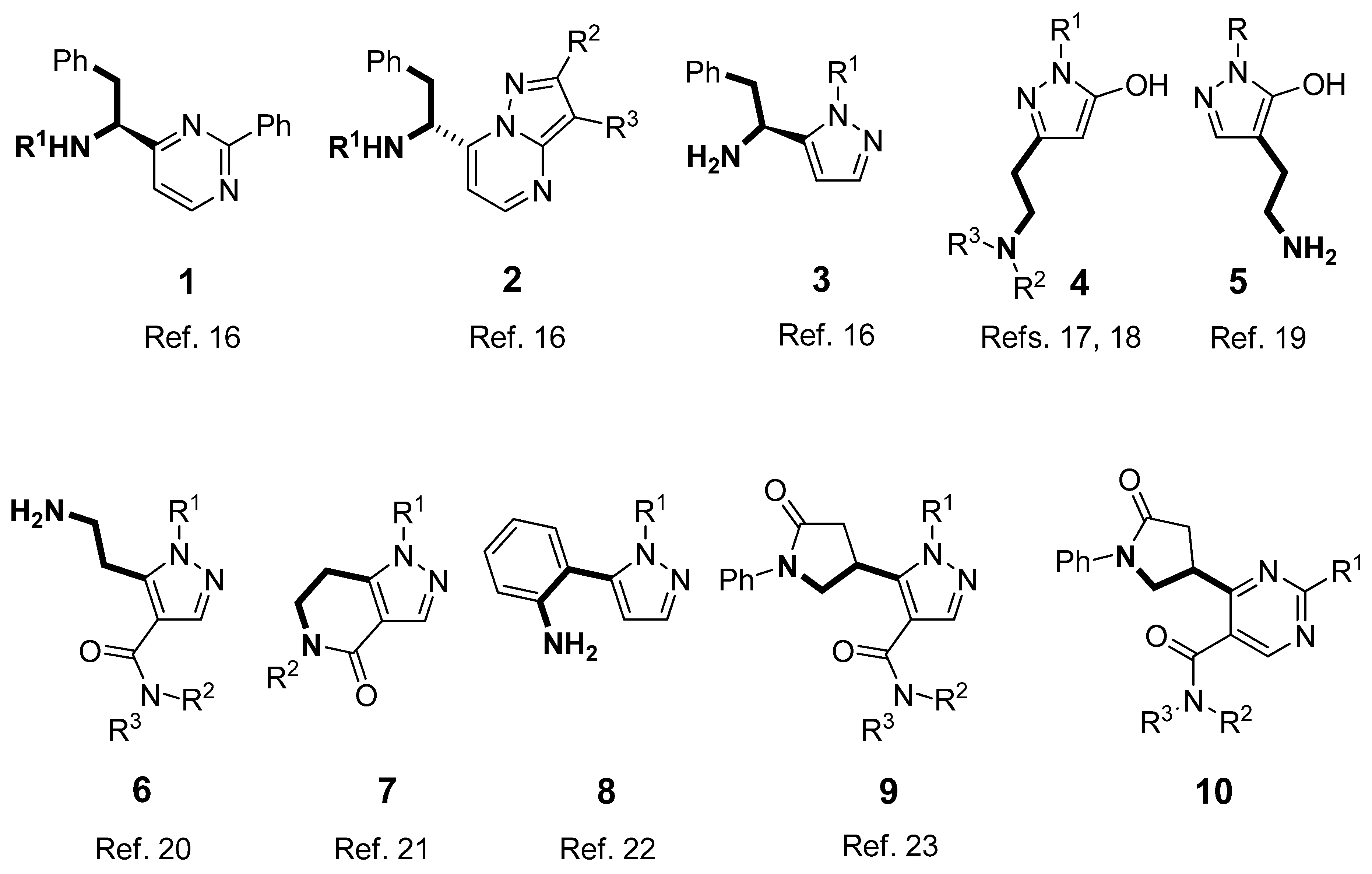
2. Results and Discussion
2.1. Synthesis of Title Compounds 10
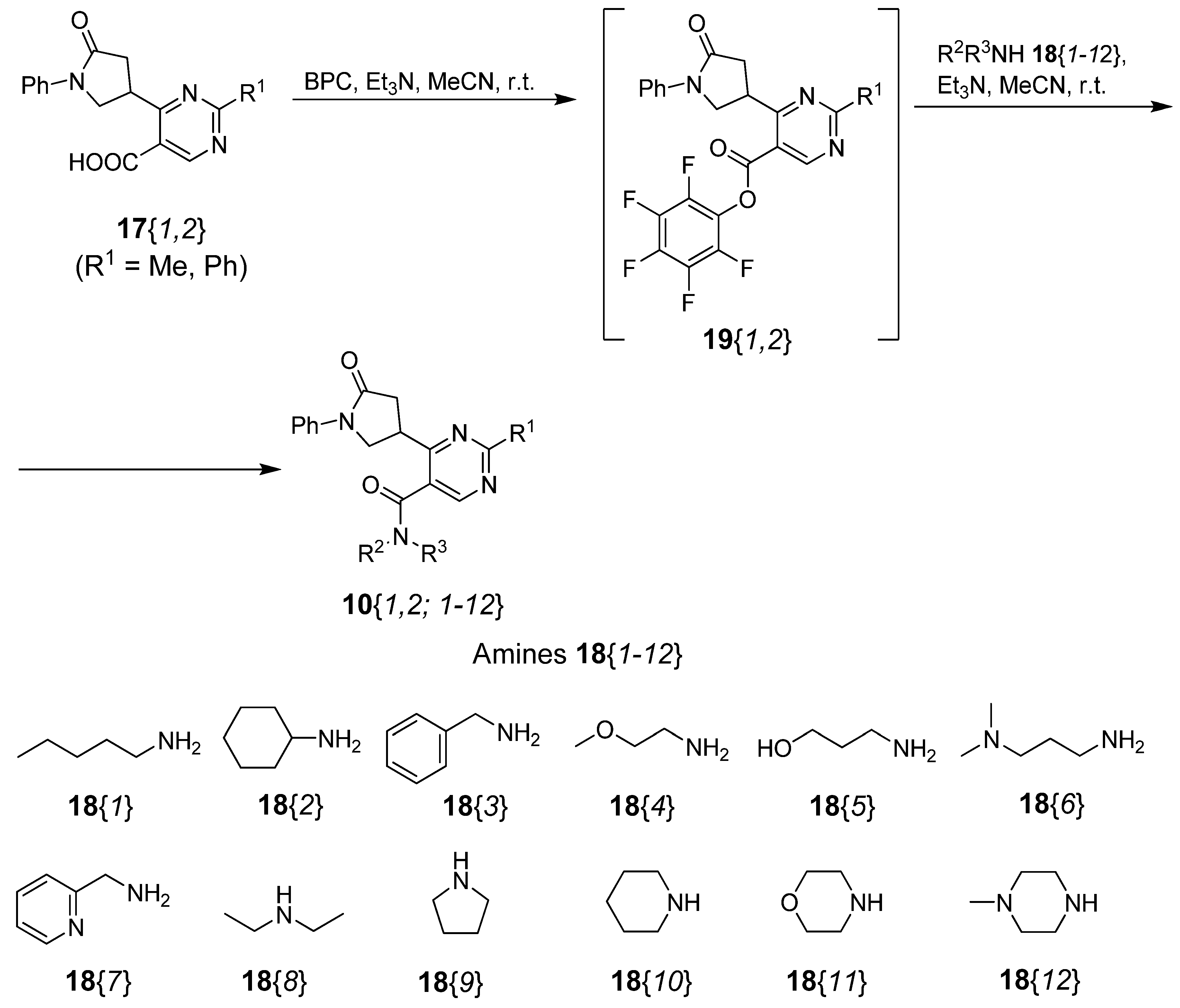
| Compd. | R1 | R2R3NH 18 | Workup [a] | Yield (%) | Purity (%) |
|---|---|---|---|---|---|
| 10{1; 1} | Me | 1-pentylamine 18{1} | B | 85 | 80 [b] |
| 10{1; 2} | Me | cyclohexylamine 18{2} | B | 69 | 100 [b] |
| 10{1; 3} | Me | benzylamine 18{3} | A | 77 | 100 [b,c] |
| 10{1; 4} | Me | 2-methoxyethylamine 18{4} | A | 28 | 100 [b,c] |
| 10{1; 5} | Me | 3-amino-1-propanol 18{5} | B | 94 | 81 [b] |
| 10{1; 6} | Me | 3-dimethylamino-1-propylamine 18{6} | B | 40 | 94 [b] |
| 10{1; 7} | Me | 2-picolylamine 18{7} | B | 76 | 100 [b] |
| 10{1; 8} | Me | diethylamine 18{8} | B | 100 | 100 [b] |
| 10{1; 9} | Me | pyrrolidine 18{9} | B | 79 | 100 [b] |
| 10{1; 10} | Me | piperidine 18{10} | B | 100 | 100 [b] |
| 10{1; 11} | Me | morpholine 18{11} | B | 99 | 100 [b] |
| 10{1; 12} | Me | 4-methylpiperazine 18{12} | B | 100 | 100 [b] |
| 10{2; 1} | Ph | 1-pentylamine 18{1} | A | 100 | 100 [b,c] |
| 10{2; 2} | Ph | cyclohexylamine 18{2} | A | 100 | 86 [b,c] |
| 10{2; 3} | Ph | benzylamine 18{3} | A | 77 | 100 [b,c] |
| 10{2; 4} | Ph | 2-methoxyethylamine 18{4} | A | 65 | 100 [b,c] |
| 10{2; 5} | Ph | 3-amino-1-propanol 18{5} | A | 98 | 84 [b,c] |
| 10{2; 6} | Ph | 3-dimethylamino-1-propylamine 18{6} | A | 71 | 100 [b] |
| 10{2; 7} | Ph | 2-picolylamine 18{7} | A | 89 | 87 [b,c] |
| 10{2; 8} | Ph | diethylamine 18{8} | B | 68 | 88 [b,c] |
| 10{2; 9} | Ph | pyrrolidine 18{9} | B | 100 | 100 [b] |
| 10{2; 10} | Ph | piperidine 18{10} | B | 100 | 100 [b] |
| 10{2; 11} | Ph | morpholine 18{11} | B | 95 | 100 [b] |
| 10{2; 12} | Ph | 4-methylpiperazine 18{12} | B | 100 | 100 [b] |
2.2. Structure Determination
| Compd. | δ (ppm) | 3JH–H (Hz) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-H | 2'-Ha | 2'-Hb | 3'-H | 4'-Ha | 4'-Hb | 2'a-2'b | 2'a-3' | 2'b-3' | 3'-4'a | 3'-4'b | 4'a-4'b | ||
| 16{1} | 9.11 | 4.15 | 4.24 | 4.70 | 2.96 | 3.17 | 9.6 | 6.4 | 8.4 | 9.1 | 7.3 | 16.9 | |
| 16{2} | 9.28 | 4.16 | 4.36 | 4.78 | 3.04 | 3.29 | 9.7 | 5.4 | 8.1 | 8.9 | 6.2 | 16.9 | |
| 17{1} | 9.05 | 4.00 | 4.23 | 4.56 | 2.92 [a] | 9.8 | 5.4 | 8.5 | [a] | [a] | [a] | ||
| 17{2} | 9.25 | 4.06 | 4.35 | 4.73 | 2.96 | 3.03 | 9.9 | 4.1 | 7.9 | 4.9 | 8.6 | 16.7 | |
| 10{1; 1} | 8.62 | 4.14 | 4.23 | 4.28 | 2.90 | 3.15 | 9.3 | 6.5 | 7.3 | 8.8 | 7.3 | 17.0 | |
| 10{1; 2} | 8.60 | 4.16 | 4.22 | 4.27 | 2.91 | 3.15 | 9.1 | 6.5 | 8.7 | 8.8 | 7.5 | 16.9 | |
| 10{1; 3} | 8.63 | 4.09 | 4.18 | 4.28 | 2.83 | 3.10 | 9.5 | 6.8 | 8.9 | 9.0 | 7.7 | 16.9 | |
| 10{1; 4} | 8.65 | 4.15 | 4.21 | 4.28 | 2.90 | 3.16 | 9.4 | 7.1 | 8.6 | 8.9 | 7.9 | 16.8 | |
| 10{1; 5} | 8.65 | 4.16 | 4.22 | 4.31 | 2.92 | 3.12 | 9.6 | 6.8 | 8.9 | 9.0 | 7.7 | 16.9 | |
| 10{1; 6} | 8.61 | 4.14 | 4.25 | 4.43 | 2.92 | 3.18 | 9.6 | 6.7 | 8.4 | 9.1 | 7.8 | 16.9 | |
| 10{1; 7} | 8.78 | 4.16 | 4.22 | 4.34 | 2.93 | 3.19 | 9.5 | 7.1 | 8.9 | 9.0 | 8.0 | 16.9 | |
| 10{1; 8} | 8.49 | 4.13 | 4.20 | 3.84 | 2.88 | [b] | 8.5 | 8.5 | 8.5 | 8.8 | [a] | 16.7 | |
| 10{1; 9} | 8.56 | 4.17 | 4.21 | 3.96 | 2.90 | 3.17 | 9.5 | 8.4 | 7.6 | 9.0 | 8.7 | 16.9 | |
| 10{1;10} | 8.47 | 4.19 [a] | 3.90 | 2.90 | 3.19 | [a] | [a] | [a] | [a] | [a] | [a] | ||
| 10{1; 11 | 8.48 | 4.19 [a] | 3.91 | 2.90 | 3.18 | [a] | [a] | [a] | [a] | [a] | [a] | ||
| 10{1;12} | 8.47 | 4.17 [a] | 3.89 | 2.90 | 3.19 | [a] | [a] | [a] | [a] | [a] | [a] | ||
| 10{2; 1} | 8.77 | 4.14 | 4.31 | 4.35 | 2.95 | 3.23 | 9.1 | 5.2 | 8.6 | 6.5 | 8.7 | 16.9 | |
| 10{2; 2} | 8.75 | 4.15 | ~4.3 [a] | 2.95 | 3.23 | 9.6 | 4.8 | [a] | 8.8 | 6.5 | 16.9 | ||
| 10{2; 3} | 8.81 | 4.14 | 4.31 | 4.39 | 2.96 | 3.26 | 9.6 | 5.8 | 8.8 | 8.8 | 6.7 | 16.9 | |
| 10{2; 4} | 8.82 | 4.17 | 4.32 | 4.37 | 2.98 | 3.27 | 9.4 | 5.8 | 8.8 | 8.6 | 6.8 | 16.8 | |
| 10{2; 5} | 8.80 | 4.17 | 4.31 | 4.38 | 2.98 | 3.21 | 9.6 | 5.6 | 8.9 | 8.7 | 6.5 | 16.9 | |
| 10{2; 6} | 8.77 | 4.20 | 4.28 | 4.51 | 3.00 | 3.29 | 9.7 | 5.8 | 8.1 | 8.9 | 6.7 | 16.9 | |
| 10{2; 7} | 8.96 | 4.19 | 4.32 | 4.44 | 3.00 | 3.29 | 9.6 | 6.1 | 8.3 | 8.9 | 7.1 | 16.9 | |
| 10{2; 8} | 8.65 | 4.22 | 4.24 | 3.92 | 2.95 | [a] | [a] | [a] | [a] | 8.9 | [a] | 16.9 | |
| 10{2; 9} | 8.72 | 4.24 | 4.27 | 4.06 | 2.97 | 3.28 | 9.7 | 6.8 | 8.2 | 8.9 | 7.8 | 16.9 | |
| 10{2;10} | 8.63 | 4.24 | 4.24 | 3.98 | 2.96 | 3.27 | [a] | [a] | [a] | [a] | [a] | [a] | |
| 10{2;11} | 8.64 | 4.23 | 4.25 | 3.99 | 2.97 | 3.27 | [a] | [a] | [a] | 8.3 | 7.0 | 16.3 | |
| 10{2;12} | 8.64 | 4.25 | 4.25 | 3.98 | 2.98 | 3.29 | [a] | [a] | [a] | [a] | [a] | [a] | |
| Compound | n-hexane:i-PrOH | R t (min) | ||
|---|---|---|---|---|
| Enantiomer A | Enantiomer B | |||
| 10{1; 1} | 50:50 | 4.084 | 5.078 | |
| 10{1; 2} | 50:50 | 9.987 | 16.650 | |
| 10{1; 3} | 50:50 | 8.208 | 12.734 | |
| 10{1; 4} | 50:50 | 5.321 | 7.031 | |
| 10{1; 5} | 50:50 | 3.828 | 4.542 | |
| 10{1; 6} | 50:50 | 5.083 | 5.477 | |
| 10{1; 7} | 50:50 | 7.577 | 8.352 | |
| 10{1; 8} | 50:50 | 5.728 | 6.380 | |
| 10{1; 9} | 50:50 | 6.960 | 9.471 | |
| 10{1; 10} | 50:50 | 5.798 | 7.185 | |
| 10{1; 11} | 50:50 | 8.509 | 9.619 | |
| 10{1; 12} | 50:50 | 7.206 | 7.928 | |
| 10{2; 1} | 50:50 | 4.409 | 5.515 | |
| 10{2; 2} | 50:50 | 4.537 | 5.840 | |
| 10{2; 3} | 50:50 | 11.292 | 29.227 | |
| 10{2; 4} | 50:50 | 6.462 | 7.522 | |
| 10{2; 5} | 80:20 | 14.864 | 18.975 | |
| 10{2; 6} | 50:50 | 6.160 | 19.660 | |
| 10{2; 7} | 50:50 | 10.778 | 12.764 | |
| 10{2; 8} | 50:50 | 5.293 | 24.904 | |
| 10{2; 9} | 50:50 | 9.284 | 10.960 | |
| 10{2; 10} | 50:50 | 7.102 | 8.212 | |
| 10{2; 11} | 80:20 | 14.864 | 18.975 | |
| 10{2; 12} | 50:50 | 14.429 | 19.625 | |
| Compound | MW (g·mol–1) | No. of atoms | CLogP | No. of HBD | No. of HBA | PSA (Ǻ2) |
|---|---|---|---|---|---|---|
| 10{1; 1} | 366 | 53 | 2.82 | 1 | 6 | 74 |
| 10{1; 2} | 378 | 54 | 2.73 | 1 | 6 | 74 |
| 10{1; 3} | 386 | 51 | 2.67 | 1 | 6 | 74 |
| 10{1; 4} | 354 | 48 | 0.90 | 1 | 7 | 83 |
| 10{1; 5} | 354 | 48 | 0.46 | 2 | 7 | 94 |
| 10{1; 6} | 381 | 55 | 1.39 | 1 | 7 | 77 |
| 10{1; 7} | 387 | 50 | 1.17 | 1 | 7 | 86 |
| 10{1; 8} | 352 | 50 | 1.63 | 0 | 6 | 65 |
| 10{1; 9} | 350 | 48 | 1.20 | 0 | 6 | 65 |
| 10{1; 10} | 364 | 51 | 1.76 | 0 | 6 | 65 |
| 10{1; 11} | 366 | 49 | 0.73 | 0 | 7 | 75 |
| 10{1; 12} | 379 | 53 | 1.29 | 0 | 7 | 69 |
| 10{2; 1} | 428 | 60 | 4.42 | 1 | 6 | 74 |
| 10{2; 2} | 440 | 61 | 4.33 | 1 | 6 | 74 |
| 10{2; 3} | 448 | 58 | 4.27 | 1 | 6 | 74 |
| 10{2; 4} | 416 | 55 | 2.50 | 1 | 7 | 83 |
| 10{2; 5} | 416 | 55 | 2.06 | 2 | 7 | 94 |
| 10{2; 6} | 443 | 62 | 2.99 | 1 | 7 | 77 |
| 10{2; 7} | 449 | 57 | 2.77 | 1 | 7 | 86 |
| 10{2; 8} | 414 | 57 | 3.22 | 0 | 6 | 65 |
| 10{2; 9} | 412 | 55 | 2.80 | 0 | 6 | 65 |
| 10{2; 10} | 426 | 58 | 3.36 | 0 | 6 | 65 |
| 10{2; 11} | 428 | 56 | 2.33 | 0 | 7 | 75 |
| 10{2; 12} | 441 | 60 | 2.89 | 0 | 7 | 69 |
3. Experimental
3.1. General Methods
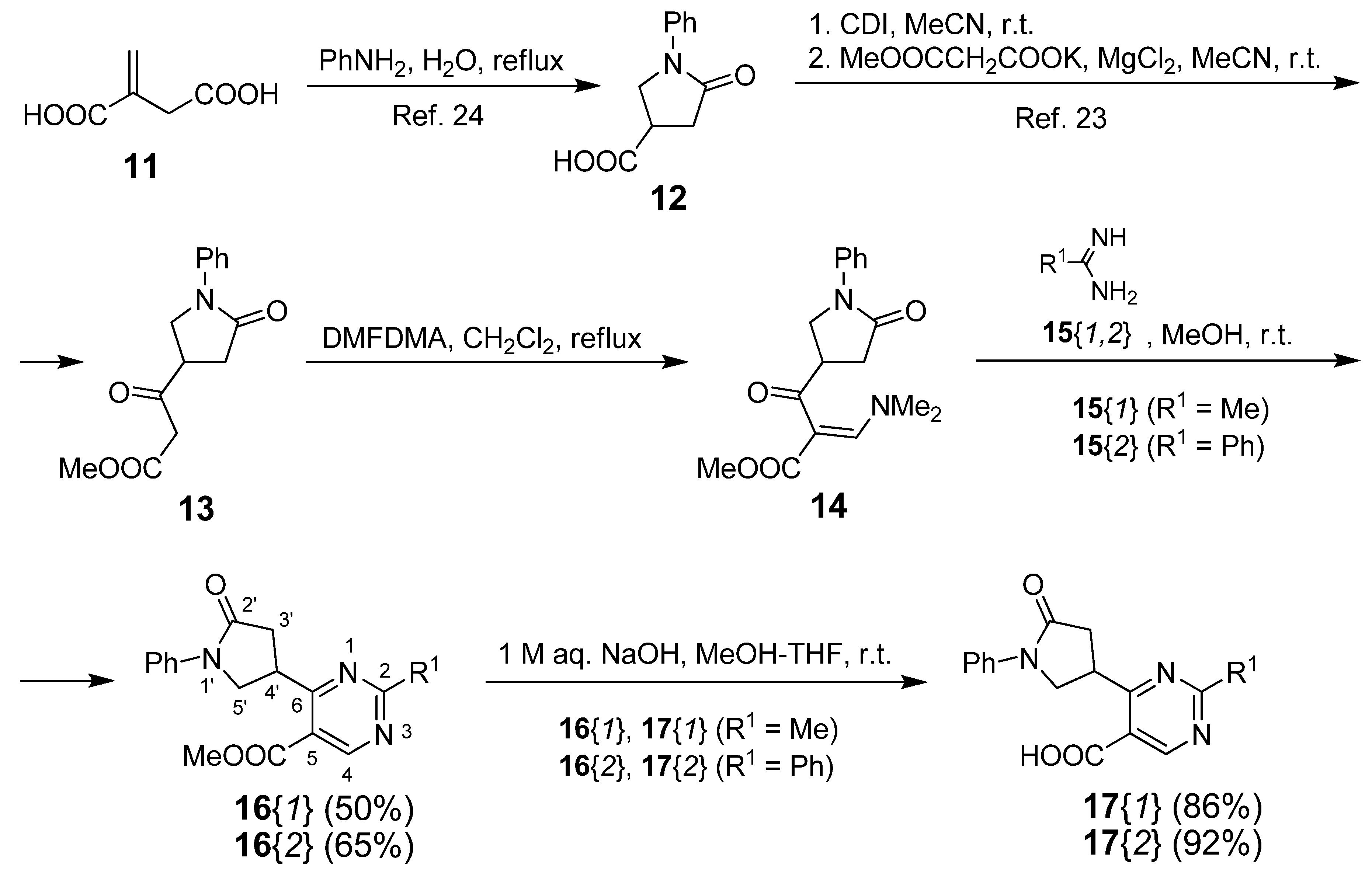
3.2. Synthesis of Methyl 3-(Dimethylamino)-2-(5-oxo-1-phenylpyrrolidine-3-carbonyl)acrylate (14)
3.3. Synthesis of Methyl 2-Methyl-6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylate (16{1})
3.4. Synthesis of Methyl 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxylate (16{2})
3.5. Synthesis of 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxylic Acid (17{1})
3.6. Synthesis of 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxylic Acid (17{2})
3.7. Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides 10{1,2; 1–12}
3.7.1. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-pentylpyrimidine-5-carboxamide (10{1; 1})
3.7.2. N-Cyclohexyl-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 2})
3.7.3. N-Benzyl-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 3})
3.7.4. N-(2-Methoxyethyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 4})
3.7.5. N-(3-Hydroxypropyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 5})
3.7.6. N-(3-Dimethylaminopropyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 6})
3.7.7. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-((pyridin-2-yl)methyl)pyrimidine-5-carboxamide (10{1; 7})
3.7.8. N,N-(Diethyl)-2-methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamide (10{1; 8})
3.7.9. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(pyrrolidin-1-yl)pyrimidine-5-carboxamide (10{1; 9})
3.7.10. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(piperidin-1-yl)pyrimidine-5-carboxamide (10{1; 10})
3.7.11. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(morpholin-4-yl)pyrimidine-5-carboxamide (10{1; 11})
3.7.12. 2-Methyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-N-(4-methylpiperazin-1-yl)pyrimidine-5-carboxamide (10{1; 12})
3.7.13. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-N-pentyl-1-phenylpyrimidine-5-carboxamide (10{2; 1})
3.7.14. N-Cyclohexyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 2})
3.7.15. N-Benzyl-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 3})
3.7.16. N-(2-Methoxyethyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 4})
3.7.17. N-(3-Hydroxypropyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 5})
3.7.18. N-(3-Dimethylaminopropyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 6})
3.7.19. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenyl-N-((pyridin-2-yl)methyl)pyrimidine-5-carboxamide (10{2; 7})
3.7.20. N,N-(Diethyl)-6-(5-oxo-1-phenylpyrrolidin-3-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 8})
3.7.21. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenyl-N-(pyrrolidin-1-yl)pyrimidine-5-carboxamide (10{2; 9})
3.7.22. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-2-phenyl-N-(piperidin-1-yl)pyrimidine-5-carboxamide (10{2; 10})
3.7.23. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-N-(morhpolin-4-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 11})
3.7.24. 6-(5-Oxo-1-phenylpyrrolidin-3-yl)-N-(4-methylpiperazin-1-yl)-2-phenylpyrimidine-5-carboxamide (10{2; 12})
4. Conclusions
Acknowledgements
References and Notes
- Patrick, G.L. An Introduction to Medicinal Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 2009; pp. 1–752. [Google Scholar]
- Takahashi, T.; Miyazawa, M. N-Caffeoyl serotonin as selective COX-2 inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 2494–2496. [Google Scholar] [CrossRef]
- Zefirova, O.N.; Baranova, T.Y.; Lyssenko, K.A.; Zefirov, N.A.; Zyk, N.V.; Vassiliev, P.M.; Yakovlev, D.S.; Spasov, A.A. Synthesis and biological testing of conformationally restricted serotonin analogues with bridgehead moieties. Mendeleev Commun. 2012, 22, 75–77. [Google Scholar] [CrossRef]
- Bonner, L.A.; Laban, U.; Chemel, B.R.; Juncosa, J.I.; Lill, M.A.; Watts, V.J.; Nichols, D.E. Mapping the catehol binding site in dopamine D1 receptors: Synthesis and evaluation of two parallel series of bicyclic dopamine analogues. ChemMedChem 2011, 6, 1024–1040. [Google Scholar] [CrossRef]
- Zlotos, D.P.; Attia, M.I.; Julius, J.; Sethi, S.S.; Witt-Enderby, P.A. 2-[(2,3-Dihydro-1H-indol-1-yl)methyl]melatonin analogues: A novel class of MT2-Selective melatonin receptor antagonists. J. Med. Chem. 2009, 52, 826–833. [Google Scholar] [CrossRef]
- Dolle, R.E. Solid-phase Synthesis of Heterocyclic Systems (Heterocycles Containing One Heteroatom). In Handbook of Combinatorial Chemistry. Drugs, Catalysts, Materials; Nicolaou, K.C., Hanko, R., Hartwig, W., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; Volume 2, pp. 725–742. [Google Scholar]
- Pernerstorfer, J. Molecular Design and Combinatorial Compound Libraries. In Handbook of Combinatorial Chemistry. Drugs, Catalysts, Materials; Nicolaou, K.C., Hanko, R., Hartwig, W., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; Volume 2, pp. 725–742. [Google Scholar]
- Dolle, R.E.; le Bourdonnec, B.; Morales, G.A.; Moriarty, K.J.; Salvino, J.M. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2007. J. Comb. Chem. 2008, 10, 753–802. [Google Scholar] [CrossRef]
- Dolle, R.E.; le Bourdonnec, B.; Goodman, A.J.; Morales, G.A.; Thomas, C.J.; Zhang, W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2008. J. Comb. Chem. 2009, 11, 739–790. [Google Scholar] [CrossRef]
- Dolle, R.E.; le Bourdonnec, B.; Worm, K.; Morales, G.A.; Thomas, C.J.; Zhang, W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2009. J. Comb. Chem. 2010, 12, 765–806. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Synthesis of heterocycles from alkyl 3-(dimethylamino)propenoates and related enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef]
- Svete, J. Ex-chiral pool enaminones in the synthesis of functionalised heterocycles. Monatsh. Chem. 2004, 135, 629–641. [Google Scholar] [CrossRef]
- Svete, J. Utilisation of chiral enaminones and azomethine imines in the synthesis of functionalised pyrazoles. ARKIVOC 2006, vii, 35–56. [Google Scholar]
- Bevk, D.; Svete, J.; Stanovnik, B. Enaminones and Related Compounds in the Synthesis of Pyrazoles. In Modern Approaches to the Synthesis of O- and N-Heterocycles; Research Signpost: Kerala, India, 2007; Volume 3, pp. 73–88. [Google Scholar]
- Stanovnik, B.; Grošelj, U. Dialkyl acetone-1,3-dicarboxylates and their mono- and bis(dimethylamino) methylidene derivatives in the synthesis of heterocyclic systems. Adv. Heterocycl. Chem. 2010, 100, 145–174. [Google Scholar] [CrossRef]
- Pirc, S.; Bevk, D.; Golobič, A.; Stanovnik, B.; Svete, J. Transformation of amino acids into nonracemic 1-(heteroaryl)ethanamines by the enamino ketone methodology. Helv. Chim. Acta 2006, 89, 30–44. [Google Scholar] [CrossRef]
- Kralj, D.; Grošelj, U.; Meden, A.; Dahmann, G.; Stanovnik, B.; Svete, J. A simple synthesis of 4-(2-aminoethyl)-5-hydroxy-1H-pyrazoles. Tetrahedron 2007, 63, 11213–11222. [Google Scholar] [CrossRef]
- Kralj, D.; Novak, A.; Dahmann, G.; Grošelj, U.; Meden, A.; Svete, J. One-pot parallel solution-phase synthesis of 1-substituted 4-(2-aminoethyl)-1H-pyrazol-5-ols. J. Comb. Chem. 2008, 10, 664–670. [Google Scholar] [CrossRef]
- Grošelj, U.; Kralj, D.; Wagger, J.; Dahmann, G.; Stanovnik, B.; Svete, J. Synthesis of 3-(2-aminoethyl)-5-hydroxy-1H-pyrazole derivatives. ARKIVOC 2012, iii, 49–65. [Google Scholar]
- Kralj, D.; Friedrich, M.; Grošelj, U.; Kiraly-Potpara, S.; Meden, A.; Wagger, J.; Dahmann, G.; Stanovnik, B.; Svete, J. A synthesis of 1-substituted 5-[2-(acylamino)ethyl]-1H-pyrazole-4-carboxamides. Tetrahedron 2009, 65, 7151–7162. [Google Scholar]
- Žerovnik, D.; Grošelj, U.; Kralj, D.; Malavašič, Č.; Bezenšek, J.; Dahmann, G.; Stare, K.; Meden, A.; Stanovnik, B.; Svete, J. Synthesis of 1,5,6,7-tetrahydro-4H-pyrazolo[4,3-c]pyridin-4-ones as conformationally constrained pyrazole analogues of histamine. Synthesis 2010, 3363–3373. [Google Scholar]
- Janjić, M.; Prebil, R.; Grošelj, U.; Kralj, D.; Malavašič, Č.; Golobič, A.; Stare, K.; Dahmann, G.; Stanovnik, B.; Svete, J. A simple synthesis of 5-(2-aminophenyl)-1H-pyrazoles. Helv. Chim. Acta 2011, 94, 1703–1717. [Google Scholar] [CrossRef]
- Perdih, P.; Baškovč, J.; Dahmann, G.; Grošelj, U.; Kočar, D.; Novak, A.; Stanovnik, B.; Svete, J. Parallel synthesis of 1-substituted 5-(5-oxopyrrolidin-3-yl)-1H-pyrazole-4-carboxamides. Synthesis 2011, 2822–2832. [Google Scholar]
- Paytash, P.L.; Sparrow, E.; Gathe, J.C. The reaction of itaconic acid with primary amines. J. Am. Chem. Soc. 1950, 72, 1415–1416. [Google Scholar] [CrossRef]
- Harwood, L.M.; Moody, C.J. ‘Dry Flash’ Column Chromatography. In Experimental Organic Chemistry, Principles and Practice; Blackwell Science: Oxford, UK, 1989; pp. 185–188. [Google Scholar]
- Harwood, L.M. Dry-Column” Flash Chromatography. Aldrichimica Acta 1985, 18, 25–25. [Google Scholar]
- Since satisfactory results were obtained with evapotative workup and DFCC, the use of scavenging reagents such as solid supported tosyl chloride or propionyl chloride was not explored. Besides, covalent binding of scavenging reagents to products containing hydroxy and amino functions would probably make the isolation of products more difficult.
- The above method is applicable for the synthesis of libraries of racemic compounds 10 for primary testing and screening. However, for a larger scale synthesis of certain enantiomerically pure final products 10, a modified ‘chiral pool’ synthesis of non-racemic 10 utilizing enantiomerically pure starting compound 12 should be developed.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswandhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 16{1,2}, 17{1,2}, and 10{1,2; 1-12} are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Črček, B.; Baškovč, J.; Grošelj, U.; Kočar, D.; Dahmann, G.; Stanovnik, B.; Svete, J. Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides. Molecules 2012, 17, 5363-5384. https://doi.org/10.3390/molecules17055363
Črček B, Baškovč J, Grošelj U, Kočar D, Dahmann G, Stanovnik B, Svete J. Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides. Molecules. 2012; 17(5):5363-5384. https://doi.org/10.3390/molecules17055363
Chicago/Turabian StyleČrček, Bojana, Jernej Baškovč, Uroš Grošelj, Drago Kočar, Georg Dahmann, Branko Stanovnik, and Jurij Svete. 2012. "Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides" Molecules 17, no. 5: 5363-5384. https://doi.org/10.3390/molecules17055363
APA StyleČrček, B., Baškovč, J., Grošelj, U., Kočar, D., Dahmann, G., Stanovnik, B., & Svete, J. (2012). Parallel Synthesis of 2-Substituted 6-(5-Oxo-1-phenylpyrrolidin-3-yl)pyrimidine-5-carboxamides. Molecules, 17(5), 5363-5384. https://doi.org/10.3390/molecules17055363