Simultaneous Determination of Salidroside and Its Aglycone Metabolite p-Tyrosol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
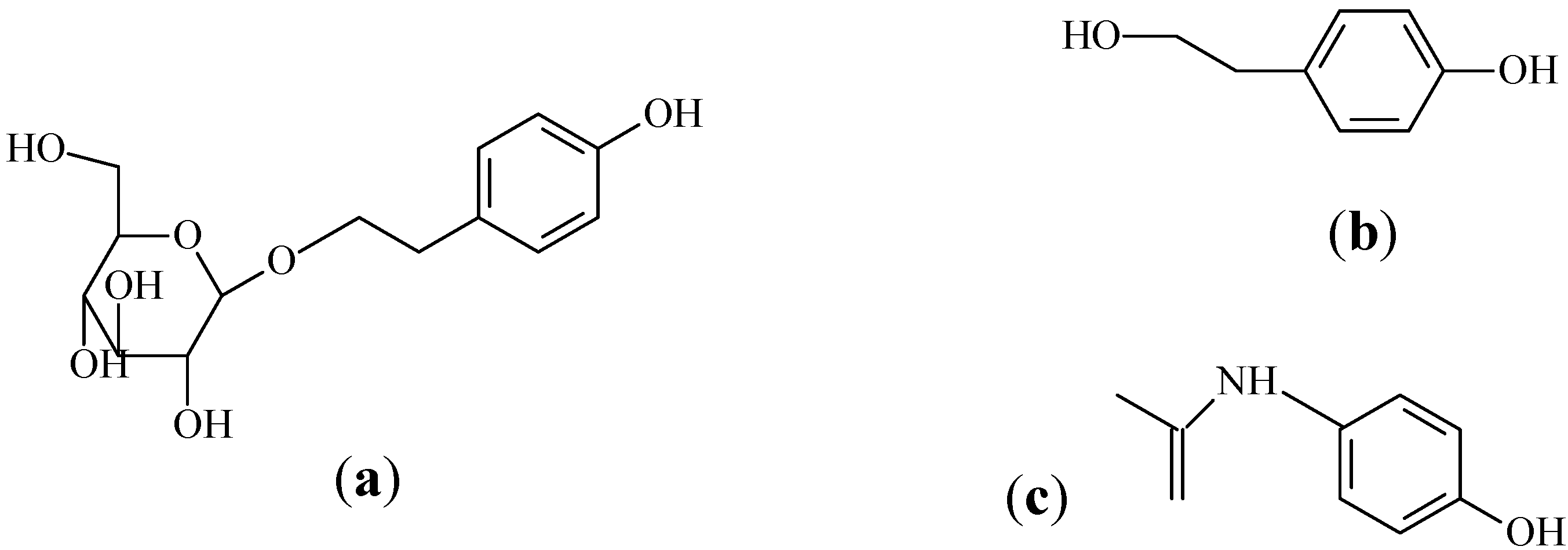
2. Results and Discussion
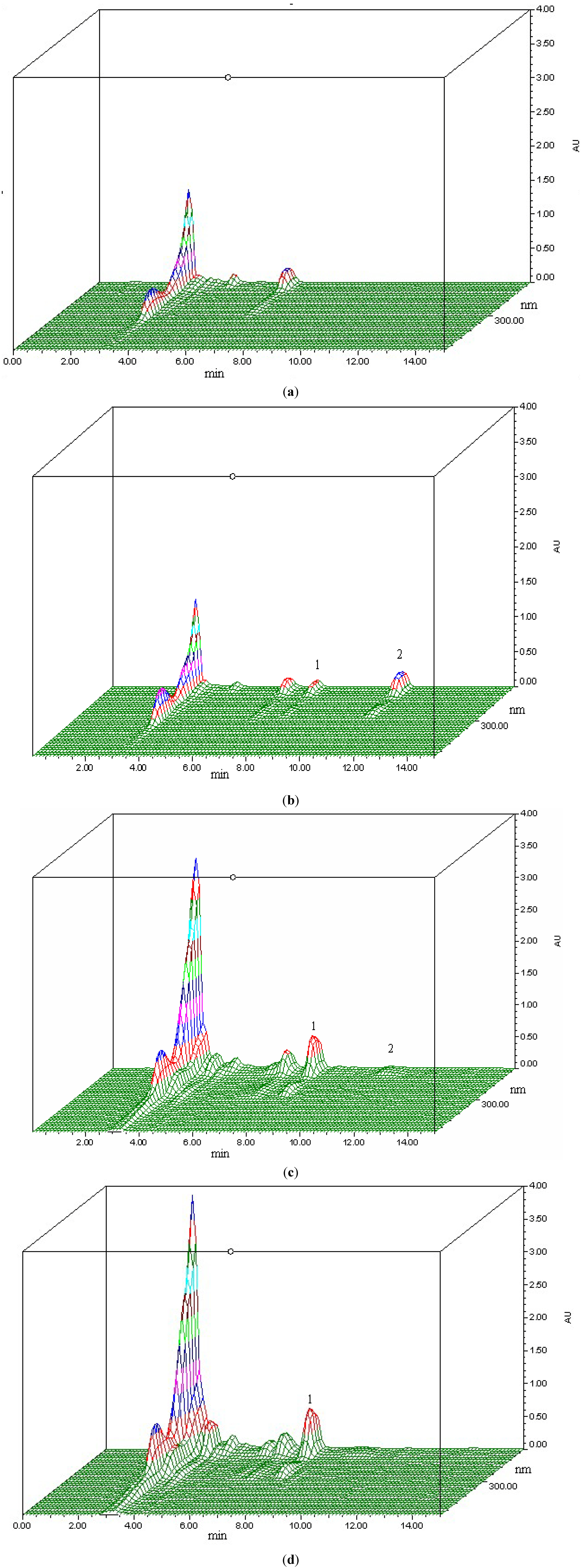
2.1. Identification of Salidroside and p-Tyrosol by HPLC-PDA and LC-MS/MS
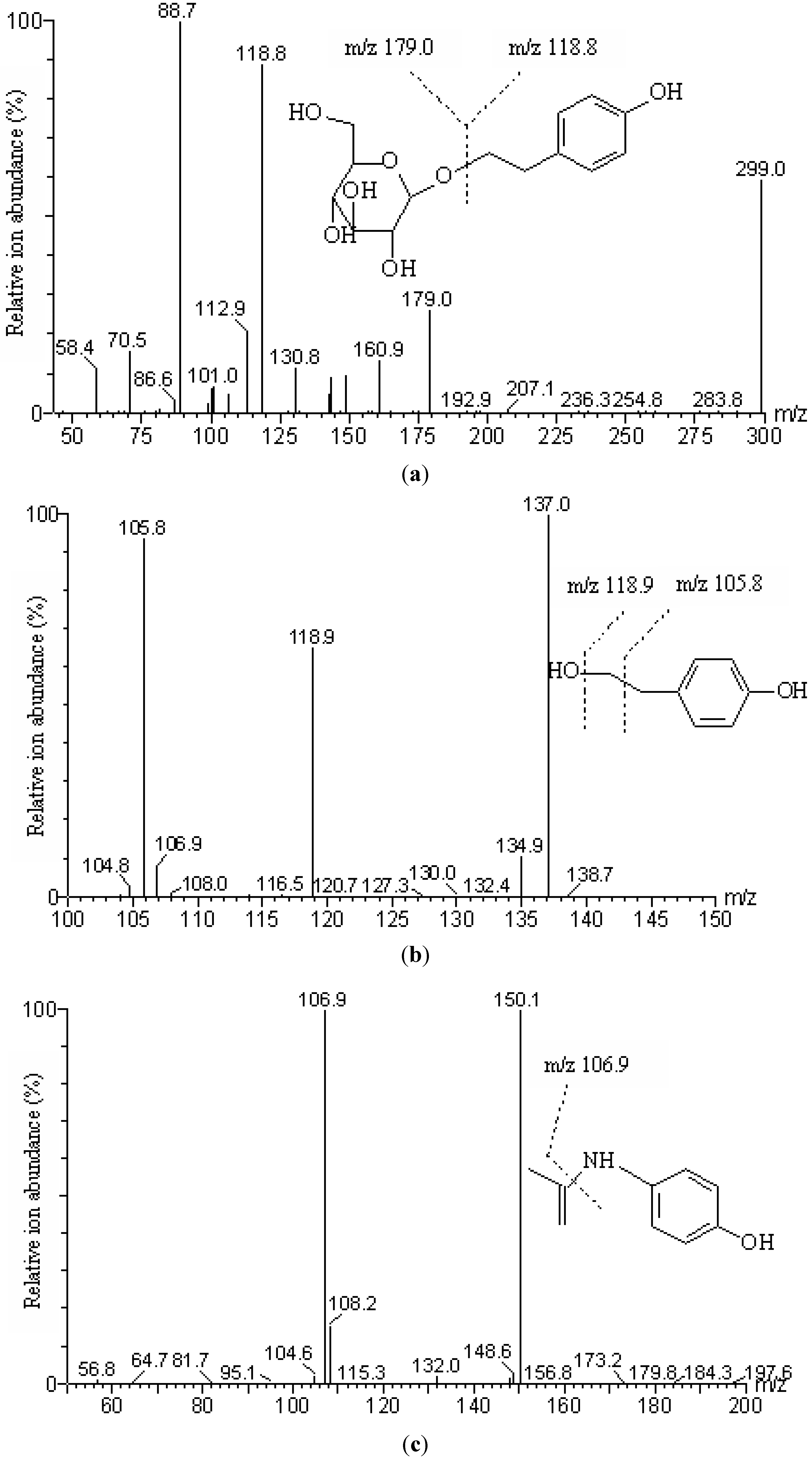
| Analyte | Precursor ion ( m/z) | Daughter ion ( m/z) | Dwell time (s) | Cone voltage (V) | Collision energy (eV) |
|---|---|---|---|---|---|
| Salidroside | 299.0 | 118.8 a, 179.0 | 0.2 | 28 | 14 |
| p-Tyrosol | 137.0 | 105.8,118.9 a | 0.2 | 35 | 16 |
| Paracetamol (IS) | 150.1 | 106.9 a | 0.2 | 35 | 20 |
2.2. Method Validation
2.2.1. Selectivity and Specificity
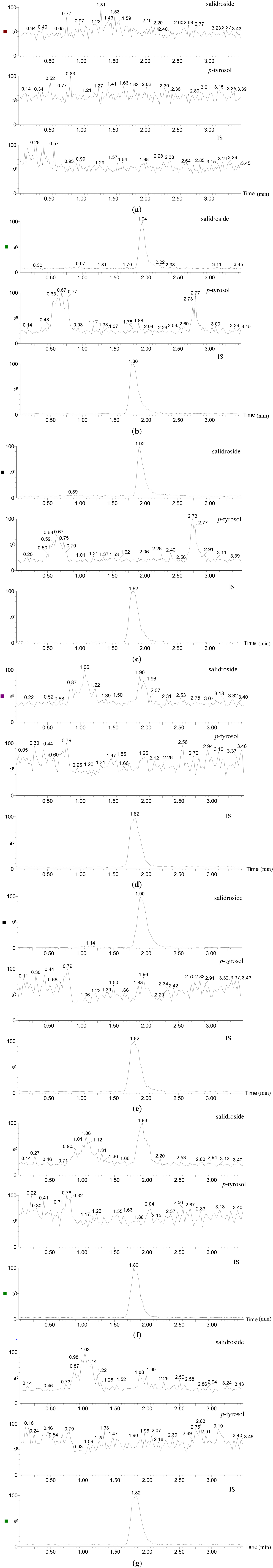
2.2.2. Calibration Curve, Linearity, LLOQ and LOD
2.2.3. Accuracy and Precision
| Analyte | Concentration (ng/mL) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | Precision (%) | Accuracy (%) | Mean ± SD (ng/mL) | Precision (%) | Accuracy (%) | ||
| salidroside | 50 | 49.84 ± 4.14 | 8.30 | 99.67 | 52.08 ± 1.82 | 3.49 | 104.17 |
| 500 | 500.68 ± 35.75 | 7.14 | 100.14 | 498.83 ± 49.83 | 9.99 | 99.77 | |
| 2,000 | 1,996.92 ± 62.58 | 3.13 | 99.85 | 2,011.80 ± 63.96 | 3.18 | 100.59 | |
| p-tyrosol | 20 | 23.16 ± 2.36 | 10.20 | 114.82 | 22.86 ± 2.62 | 11.44 | 114.29 |
| 100 | 107.08 ± 9.28 | 8.67 | 107.08 | 109.84 ± 2.83 | 2.57 | 109.84 | |
| 200 | 208.03 ± 15.80 | 7.59 | 104.02 | 206.04 ± 14.98 | 7.27 | 103.02 | |
2.2.4. Recovery and Matrix Effects
| Analyte | Concentration(ng/mL) | Recovery | Matrix effects | ||
|---|---|---|---|---|---|
| Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | ||
| salidroside | 50 | 83.98 ± 0.22 | 0.26 | 113.04 ± 6.50 | 5.75 |
| 500 | 104.11 ± 13.29 | 12.76 | 83.91 ± 5.91 | 7.05 | |
| 2,000 | 99.30 ± 2.33 | 3.82 | 89.33 ± 1.76 | 1.97 | |
| p-tyrosol | 20 | 103.71 ± 3.17 | 3.05 | 96.07 ± 0.91 | 0.95 |
| 100 | 94.67 ± 11.94 | 12.61 | 80.39 ± 10.54 | 13.11 | |
| 200 | 98.79 ± 8.70 | 8.81 | 90.84 ± 11.39 | 12.53 | |
2.2.5. Stability
| Experimental condition | Added, C (ng·mL−1) | Found, C ± S.D. (ng·mL−1) | RSD (%) | Accuracy (%) |
|---|---|---|---|---|
| Standard solution2 h at RT | 50 | 52.04 ± 1.89 | 3.63 | 104.08 |
| 500 | 517.76 ± 32.17 | 6.21 | 103.55 | |
| 2,000 | 2,041.93 ± 12.53 | 0.61 | 102.10 | |
| Standard solution30 days at 4 °C | 50 | 49.81 ± 4.78 | 0.59 | 99.62 |
| 500 | 498.05 ± 40.72 | 8.18 | 99.61 | |
| 2,000 | 1,989.01 ± 69.32 | 3.48 | 99.45 | |
| QC samplesAutosampler24 h at RT | 50 | 48.83 ± 5.37 | 11.00 | 97.67 |
| 500 | 519.87 ± 29.48 | 5.67 | 103.97 | |
| 2,000 | 1,997.68 ± 67.16 | 3.36 | 99.88 | |
| QC samples30 days storageat −20 °C | 50 | 51.55±1.83 | 3.56 | 103.09 |
| 500 | 501.92 ± 41.15 | 8.20 | 100.38 | |
| 2,000 | 2,015.99 ± 52.89 | 2.62 | 100.80 | |
| QC samples3 freeze-thaw cycles | 50 | 49.78 ± 4.78 | 9.60 | 99.56 |
| 500 | 512.25 ± 28.49 | 5.56 | 102.45 | |
| 2,000 | 2,011.61 ± 61.51 | 3.06 | 100.58 |
| Experimental condition | Added, C (ng·mL−1) | Found, C ± S.D. (ng·mL−1) | RSD (%) | Accuracy (%) |
|---|---|---|---|---|
| Standard solution2 h at RT | 20 | 21.53 ± 1.09 | 5.06 | 107.66 |
| 100 | 110.95 ± 4.06 | 3.66 | 110.95 | |
| 200 | 218.65 ± 8.27 | 3.78 | 109.32 | |
| Standard solution30 days at 4 °C | 20 | 23.55 ± 2.54 | 10.79 | 117.74 |
| 100 | 105. 20 ± 9.55 | 9.08 | 105.20 | |
| 200 | 203.25 ± 13.44 | 6.61 | 101.63 | |
| QC samplesAutosampler24 h at RT | 20 | 22.55 ± 2.73 | 12.09 | 112.73 |
| 100 | 105.86 ± 12.69 | 11.99 | 105.86 | |
| 200 | 213.41 ± 16.64 | 7.80 | 106.71 | |
| QC samples30 days storageat −20 °C | 20 | 22.55±2.22 | 9.86 | 112.76 |
| 100 | 111.03 ± 3.32 | 2.99 | 111.03 | |
| 200 | 211.31 ± 16.15 | 7.64 | 105.66 | |
| QC samples3 freeze-thaw cycles | 20 | 22.55 ± 2.23 | 9.87 | 112.76 |
| 100 | 106.04 ± 10.37 | 9.78 | 106.36 | |
| 200 | 212.71± 13.66 | 6.42 | 106.36 |
2.3. Application to Pharmacokinetic Studies
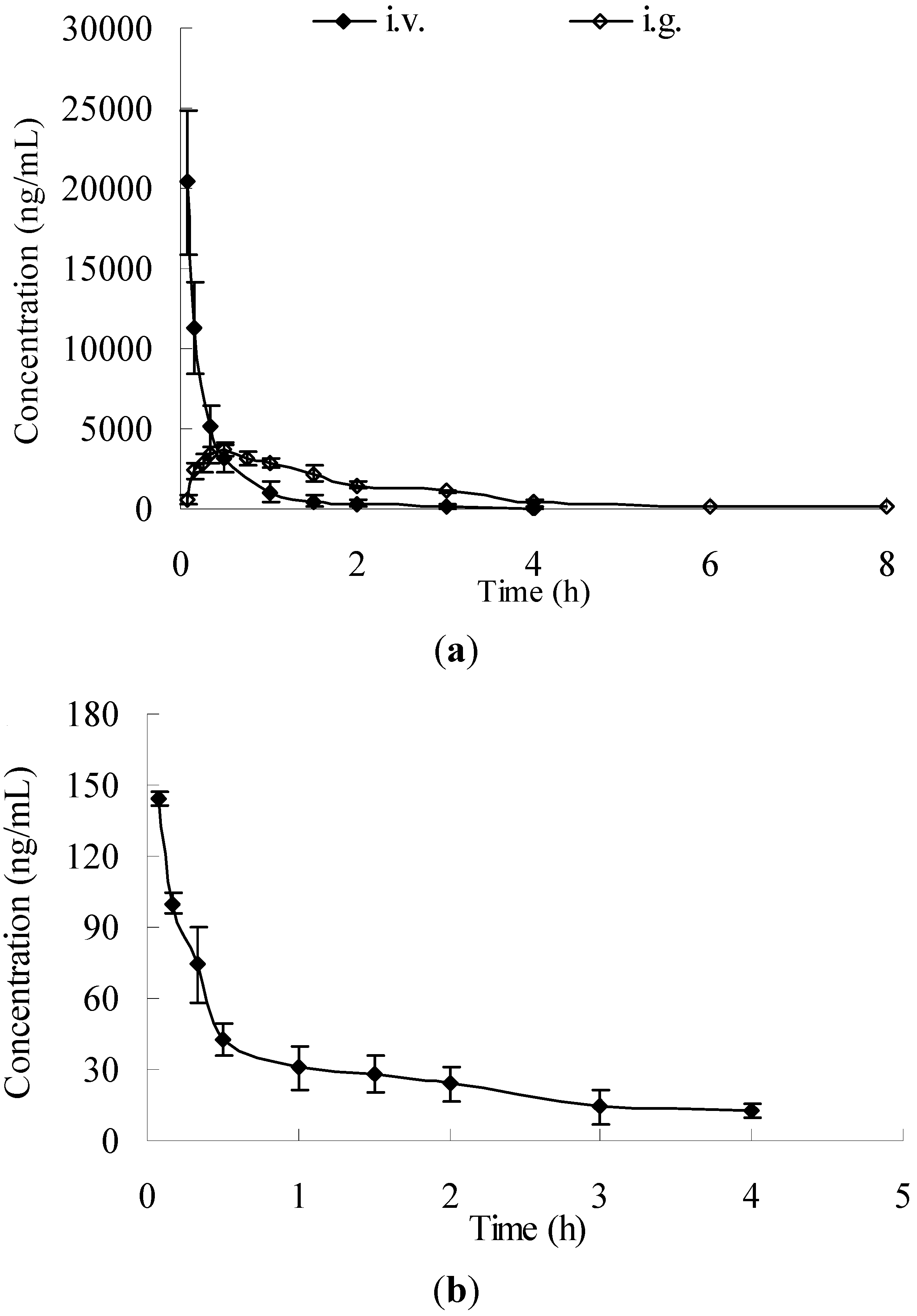
| Parameters | I.v. administration (50 mg/kg) | I.g. administration (100 mg/kg) | |
|---|---|---|---|
| Salidroside (mean ± SD) | p-Tyrosol(mean ± SD) | Salidroside (mean ± SD) | |
| Cmax (ng/mL) | - | - | 3,716.73 ± 860.13 |
| Tmax (h) | - | - | 0.30 ± 0.10 |
| T1/2 (h) | 0.70 ± 0.21 | 1.64 ± 0.30 | 1.32 ± 0.22 |
| AUC0–4h (h·ng/mL) | 7,060.72 ± 1337.51 | 122.77 ± 25.90 | 7,552.92 ± 549.02 |
| AUC0–∞ (h·ng/mL) | 7,135.79 ± 1346.40 | 146.83 ± 32.49 | 7,724.52 ± 446.62 |
| MRT (h) | 0.41 ± 0.13 | 1.84 ± 0.32 | 2.07 ± 0.51 |
| Cl (L/h) | 1.78 ± 0.36 | 54.97 ± 18.56 | 2.54 ± 0.15 |
| Vss (L) | 0.98 ± 0.33 | 0.160 ± 0.05 | 4.46 ± 1.19 |
| F (%) | 51.97 ± 2.67 | ||
3. Experimental
3.1. Chemicals and Reagents
3.2. Chromatographic and Mass Spectrometric Conditions
3.3. Preparation of Stock Solutions, Calibration Standard (CS) and Quality Control (QC) Samples
3.4. Sample Processing
3.5. Method Validation
3.6. Stability
3.7. Pharmacokinetic and Bioavailability Studies
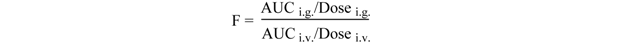
4. Conclusions
Acknowledgements
References and Notes
- Setchell, K.D.R.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar]
- Ioku, K.; Pongpiriyadacha, Y.; Konishi, Y.; Takei, Y.; Nakatani, N.; Terao, J. β-Glucosidase activity in the rat small intestine toward quercetin monoglucosides. Biosci. Biotechnol. Biochem. 1998, 62, 1428–1431. [Google Scholar] [CrossRef]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.A.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar]
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar]
- Díaz Lanza, A.M.; Abad Martínez, M.J.; Fernández Matellano, L.; Recuero Carretero, C.; Villaescusa Castillo, L.; Silván Sen, A.M.; Bermejo Benito, P. Lignan and phenylpropanoid glycosides from Phillyrea latifolia and their in vitro anti-inflammatory activity. Planta Med. 2001, 67, 219–223. [Google Scholar]
- Zhang, J.P.; Liu, A.H.; Hou, R.R.; Zhang, J.; Jia, X.; Jiang, W.F.; Chen, J.Z. Salidroside protects cardiomyocyte against hypoxia-induced death: A HIF-1α-activated and VEGF-mediated pathway. Eur. J. Pharmacol. 2009, 607, 6–14. [Google Scholar]
- Zhang, L.; Yu, H.X.; Sun, Y.; Lin, X.F.; Chen, B.; Tan, C.; Cao, G.X.; Wang, Z.W. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar]
- Zhu, Y.; Shi, Y.P.; Wu, D.; Ji, Y.J.; Wang, X.; Chen, H.L.; Wu, S.S.; Huang, D.J.; Jiang, W. Salidroside protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway. DNA Cell Biol. 2011, 30, 809–819. [Google Scholar]
- Hu, X.; Lin, S.; Yu, D.; Qiu, S.; Zhang, X.; Mei, R. A preliminary study: The anti-proliferation effect of salidroside on different human cancer cell lines. Cell Biol. Toxicol. 2010, 26, 499–507. [Google Scholar]
- Nan, J.X.; Jiang, Y.Z.; Park, E.J.; Ko, G.; Kim, Y.C.; Sohn, D.H. Protective effect of Rhodiola sachalinensis extract on carbon tetrachloride-induced liver injury in rats. J. Ethnopharmacol. 2003, 84, 143–148. [Google Scholar] [CrossRef]
- Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar]
- Chang, Y.W.; Yao, H.T.; Hsieh, S.H.; Lu, T.J.; Yeh, T.K. Quantitative determination of salidroside in rat plasma by on-line solid-phase extraction integrated with high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 857, 164–169. [Google Scholar]
- di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; de Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar]
- de la Puerta, R.; Ruiz Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar]
- Zhang, J.; Chen, X.H.; Wang, P.; Huo, L.; Shen, Z.D.; Guo, X.R.; Cheng, W.M.; Bi, K.S. LC-MS determination and pharmacokinetic study of salidroside in rat plasma after oral administration of traditional Chinese medicinal preparation Rhodiola crenulata extract. Chromatographia 2008, 67, 695–700. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.R.; Zhang, X.D.; Lu, G.C. Development of an HPLC method for the determination of salidroside in beagle dog plasma after administration of salidroside injection: Application to a pharmacokinetics study. J. Sep. Sci. 2007, 30, 3218–3222. [Google Scholar]
- Yu, S.; Liu, L.; Wen, T.; Liu, Y.C.; Wang, D.L.; He, Y.X.; Liang, Y.; Liu, X.D.; Xie, L.; Wang, G.J.; et al. Development and validation of a liquid chromatographic/electrospray ionization mass spectrometric method for the determination of salidroside in rat plasma: Application to the pharmacokinetics study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 861, 10–15. [Google Scholar] [CrossRef]
- Chernysheva, G.A.; Smol’yakova, V.I.; Cherkashina, I.V.; Plotnikov, M.B.; Tolstikova, T.G.; Krysin, A.P.; Sorokina, I.V. The main pharmacokinetic parameters of p-tyrosol upon intravenous injection in rats. Eksp. Klin. Farmakol. 2005, 68, 43–44. [Google Scholar]
- Chernysheva, G.A.; Plotnikov, M.B.; Smol’yakova, V.I.; Cherkashina, I.V.; Tolstikova, T.G.; Krysin, A.P.; Sorokina, I.V. The main pharmacokinetic parameters of p-tyrosol upon intravenous injection in rats. II. Verification of the pharmacokinetics linearity and evaluation of the possible accumulation. Eksp. Klin. Farmakol. 2006, 69, 57–59. [Google Scholar]
- Li, Z.H.; Zhu, S.Y.; Du, G.H. Comparison of the pharmacokinetics of salidroside and salidroside in the extracts of Rhodiola rosea L. in rats. Asian J. Pharmacodyn. Pharmacokinet. 2006, 6, 224–226. [Google Scholar]
- Wang, Y.; Yu, T.; Yan, X.F. Determination of contents of salidroside and tyrosol in Rhodiola roots by HPLC. Chem. Ind. Forest Prod. 2006, 26, 51–54. [Google Scholar]
- Mao, Y.; Li, Y.; Yao, N. Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 45, 510–515. [Google Scholar] [CrossRef]
- Anderson, L.; Hunter, C.L. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 2006, 5, 573–588. [Google Scholar]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar]
- Day, A.J.; Williamson, G. Biomarkers for exposure to dietary flavonoids: A review of the current evidence for identification of quercetin glycosides in plasma. Br. J. Nutr. 2001, 86, S105–S110. [Google Scholar]
- Spencer, J.P. Metabolism of tea flavonoids in the gastrointestinal tract. J. Nutr. 2003, 133, 3255S–3261S. [Google Scholar]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar]
- Spencer, J.P.; Schroeter, H.; Rechner, A.R.; Rice-Evans, C. Bioavailability of flavan-3-ols and procyanidins: Gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid. Redox Signal. 2001, 3, 1023–1039. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Evans, C.R. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar]
- Chao, P.D.L.; Hsiu, S.L.; Hou, Y.C. Bioavailability, Metabolism, and Pharmacokinetics of Glycosides in Chinese Herbs. In Herbs: Challenges in Chemistry and Biology; Oxford University Press: Cary, NC, USA, 2006; pp. 212–223. [Google Scholar]
- Yang, X.J.; Zhou, G.S.; Zou, P.P.; Ning, Y.; Zan, K.; Tu, P.F.; Jiang, Y. A rapid and sensitive HPLC-MS/MS analysis and preliminary pharmacokinetic characterization of sibiricaxanthone F in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2513–2518. [Google Scholar]
- Chen, J.; Lin, H.; Hu, M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003, 304, 1228–1235. [Google Scholar]
- Samples Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, N.; Hu, Z.; Fan, X.; Zheng, J.; Zhang, D.; Xu, T.; Yu, T.; Wang, Y.; Li, H. Simultaneous Determination of Salidroside and Its Aglycone Metabolite p-Tyrosol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2012, 17, 4733-4754. https://doi.org/10.3390/molecules17044733
Guo N, Hu Z, Fan X, Zheng J, Zhang D, Xu T, Yu T, Wang Y, Li H. Simultaneous Determination of Salidroside and Its Aglycone Metabolite p-Tyrosol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Molecules. 2012; 17(4):4733-4754. https://doi.org/10.3390/molecules17044733
Chicago/Turabian StyleGuo, Na, Zhiwei Hu, Xiaoxu Fan, Jian Zheng, Dehui Zhang, Tao Xu, Tao Yu, Yang Wang, and Haiying Li. 2012. "Simultaneous Determination of Salidroside and Its Aglycone Metabolite p-Tyrosol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry" Molecules 17, no. 4: 4733-4754. https://doi.org/10.3390/molecules17044733
APA StyleGuo, N., Hu, Z., Fan, X., Zheng, J., Zhang, D., Xu, T., Yu, T., Wang, Y., & Li, H. (2012). Simultaneous Determination of Salidroside and Its Aglycone Metabolite p-Tyrosol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Molecules, 17(4), 4733-4754. https://doi.org/10.3390/molecules17044733




