Baicalin Induces Apoptosis in SW620 Human Colorectal Carcinoma Cells in Vitro and Suppresses Tumor Growth in Vivo
Abstract
:1. Introduction
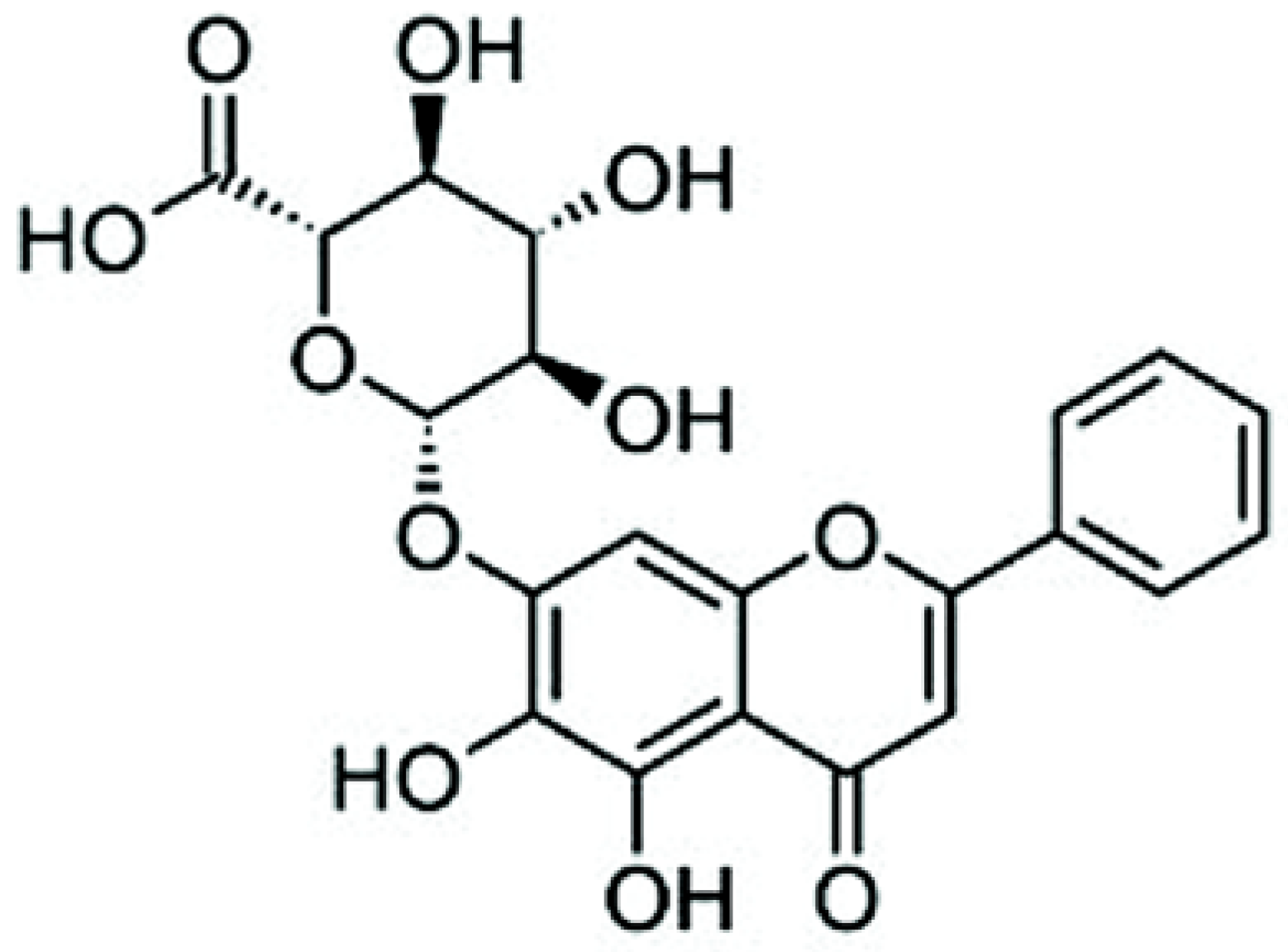
2. Results
2.1. Apoptotic Activity on SW620 Cells

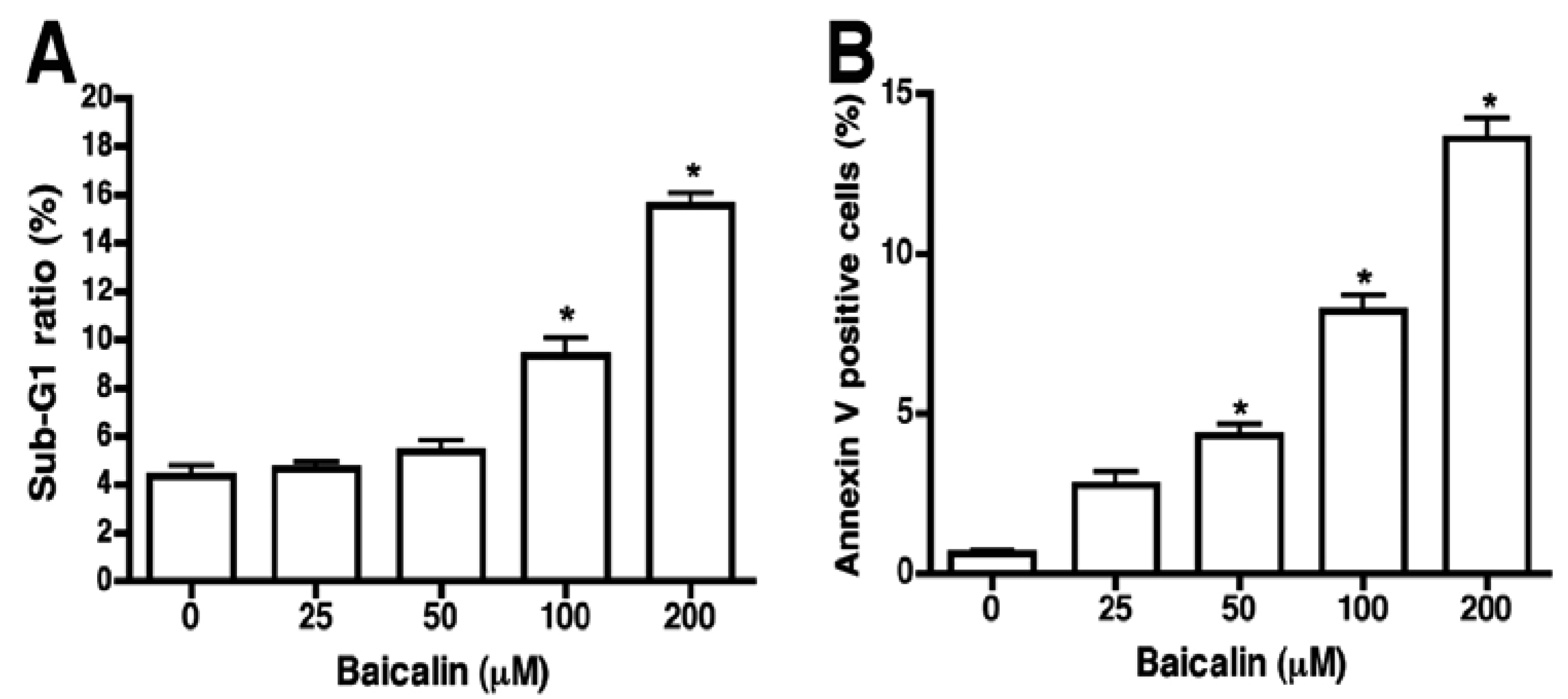
2.2. Flow Cytometric Assessment of Baicalin-Induced Apoptosis
2.3. Caspase Activation

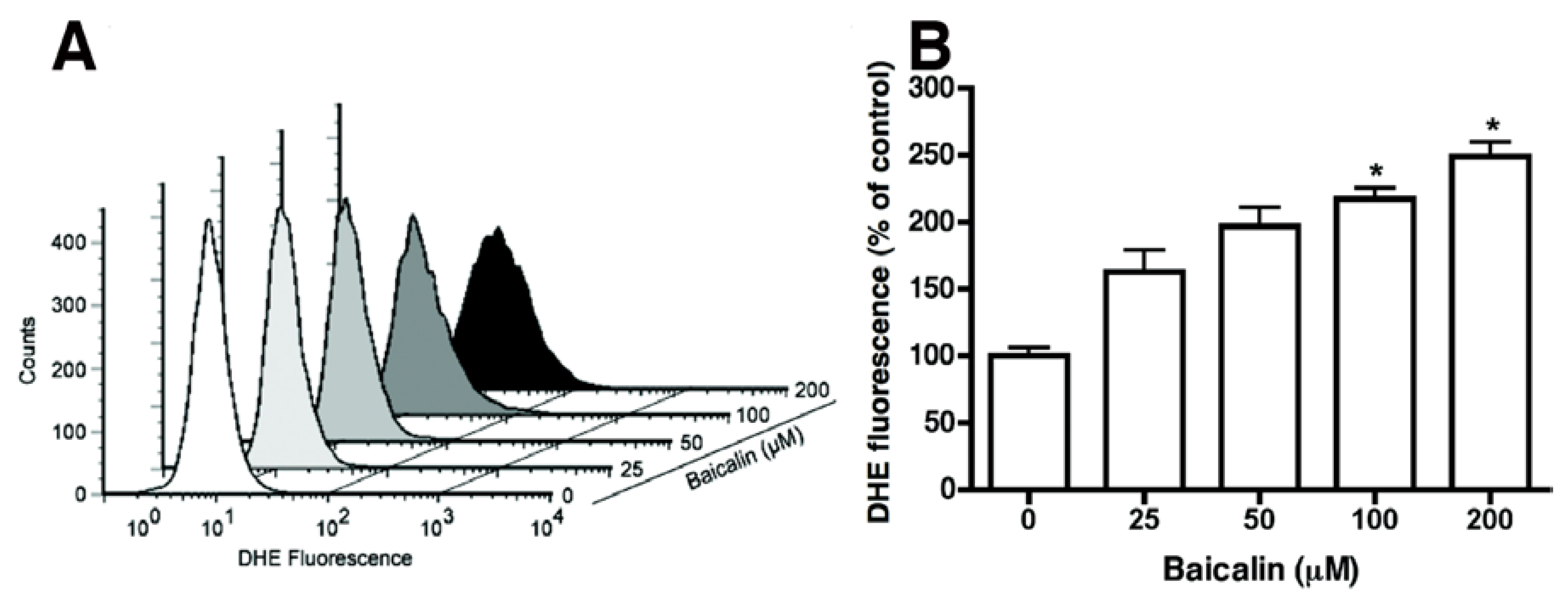
2.4. Baicalin-Induced Generation of Intracellular ROS and Caspase-Dependent Apoptosis
2.5. ROS Neutralization Conferred Resistance to Baicalin-Induced Apoptosis
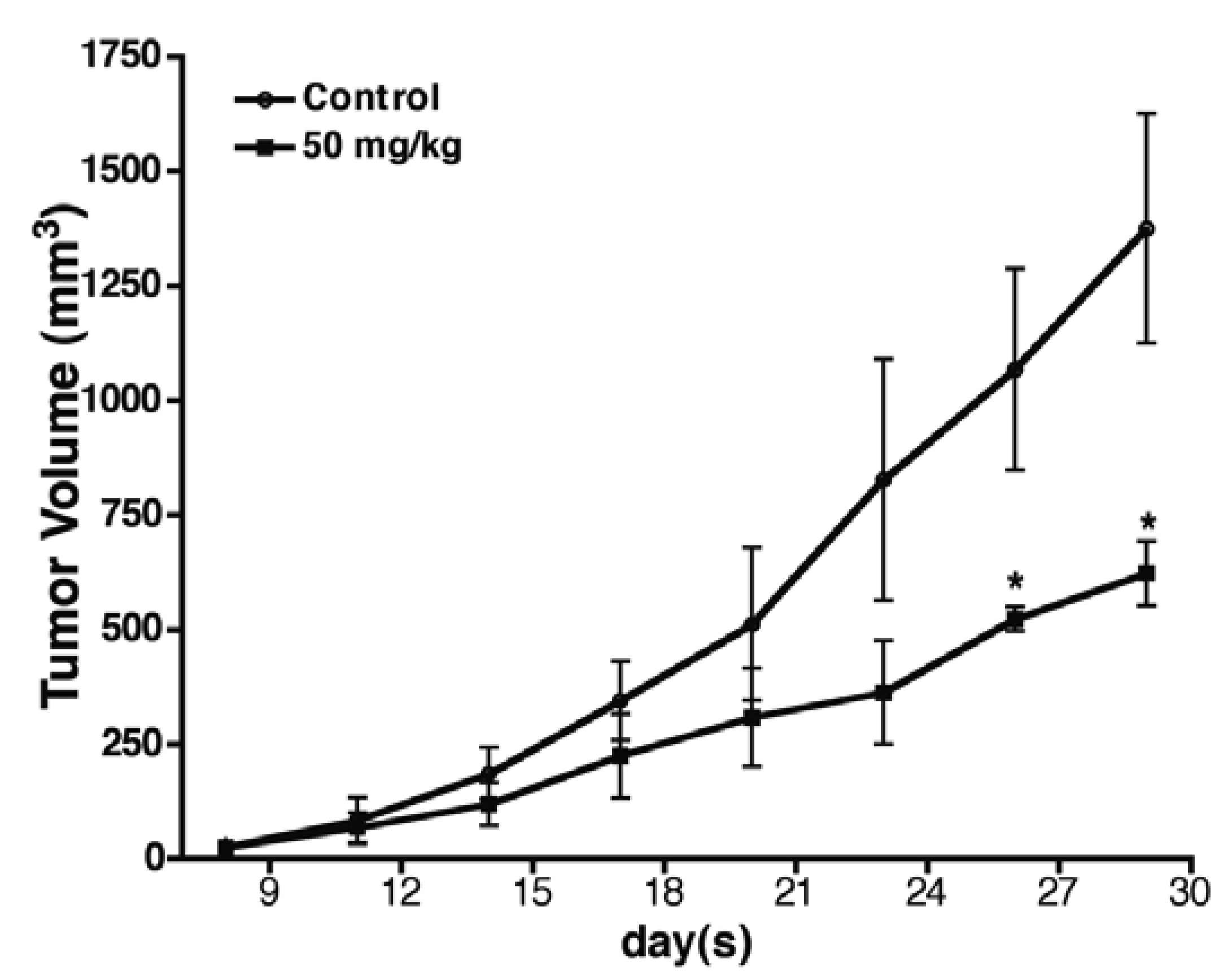
2.6. Baicalin Antitumor Activity in SW620 Human Colon Adenocarcinoma Cells in Vivo

3. Discussion
4. Experimental
4.1. Cell Culture
4.2. Reagents
4.3. Assessment of Cell Viability and Growth
4.4. DAPI Staining
4.5. Flow Cytometric Analysis
4.6. Caspase Activity Assay
4.7. Measurement of Reactive Oxygen Species
4.8. Measurement of Tumor Volume in Nude Mice
4.9. Statistical Analysis
5. Conclusions
Acknowledgment
References and Notes
- Schetter, A.J.; Harris, C.C. Alterations of microRNAs contribute to colon carcinogenesis. Semin. Oncol. 2011, 38, 734–742. [Google Scholar]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar]
- Osarogiagbon, R.U.; Sachdev, J.C.; Khattak, A.G.; Kronish, L.E. Pattern of use of adjuvant chemotherapy for stage II colon cancer: A single-institution experience. Clin. Colorectal. Cancer 2009, 8, 94–99. [Google Scholar]
- Figueredo, A.; Coombes, M.E.; Mukherjee, S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst. Rev. 2008, 52, CD005390. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Bonham, M.; Posakony, J.; Coleman, I.; Montgomery, B.; Simon, J.; Nelson, P.S. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin. Cancer Res. 2005, 11, 3905–3914. [Google Scholar]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar]
- Shieh, D.E.; Cheng, H.Y.; Yen, M.H.; Chiang, L.C.; Lin, C.C. Baicalin-induced apoptosis is mediated by Bcl-2-dependent, but not p53-dependent, pathway in human leukemia cell lines. Am. J. Chin. Med. 2006, 34, 245–261. [Google Scholar] [CrossRef]
- Chang, W.H.; Chen, C.H.; Lu, F.J. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002, 68, 128–132. [Google Scholar]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta 2011, 1807, 735–745. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar]
- Denault, J.B.; Salvesen, G.S. Caspases: Keys in the ignition of cell death. Chem. Rev. 2002, 102, 4489–4500. [Google Scholar]
- Crow, J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: Implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1997, 1, 145–157. [Google Scholar] [CrossRef]
- Rothe, G.; Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J. Leukoc. Biol. 1990, 47, 440–448. [Google Scholar]
- Schepelmann, S.; Ogilvie, L.M.; Hedley, D.; Friedlos, F.; Martin, J.; Scanlon, I.; Chen, P.; Marais, R.; Springer, C.J. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res. 2007, 67, 4949–4955. [Google Scholar]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar]
- Johnson, J.J. Carnosol: A promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011, 305, 1–7. [Google Scholar]
- Khuri, F.R.; Nemunaitis, J.; Ganly, I.; Arseneau, J.; Tannock, I.F.; Romel, L.; Gore, M.; Ironside, J.; MacDougall, R.H.; Heise, C.; et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000, 6, 879–885. [Google Scholar]
- Manson, M.M. Cancer prevention—The potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS One 2011, 6, e27441. [Google Scholar]
- Banerjee, C.; Goswami, R.; Datta, S.; Rajagopal, R.; Mazumder, S. Arsenic-induced alteration in intracellular calcium homeostasis induces head kidney macrophage apoptosis involving the activation of calpain-2 and ERK in Clarias batrachus. Toxicol. Appl. Pharmacol. 2011, 256, 44–51. [Google Scholar]
- Youmba, S.B.; Belmonte, L.; Galas, L.; Boukhettala, N.; Bole-Feysot, C.; Dechelotte, P.; Coeffier, M. Methotrexate modulates tight junctions through NF-kappaB, MEK and JNK pathways. J. Pediatr. Gastroenterol. Nutr. 2011, 54, 463–470. [Google Scholar]
- Kim, J.K.; Park, G.M. Indirubin-3-monoxime exhibits anti-inflammatory properties by down-regulating NF-kappaB and JNK signaling pathways in lipopolysaccharide-treated RAW264.7 cells. Inflamm. Res. 2011, 61, 319–325. [Google Scholar]
- Qi, S.; Xin, Y.; Guo, Y.; Diao, Y.; Kou, X.; Luo, L.; Yin, Z. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-kappaB signaling pathways. Int. Immunopharmacol. 2012, 12, 278–287. [Google Scholar]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and beyond: Cytometry in studies of programmed cell death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar]
- Hoffman, B.; Liebermann, D.A. The proto-oncogene c-myc and apoptosis. Oncogene 1998, 17, 3351–3357. [Google Scholar]
- Johansson, M.; Persson, J.L. Cancer therapy: Targeting cell cycle regulators. Anti-Cancer Agents Med. Chem. 2008, 8, 723–731. [Google Scholar]
- Bobe, G.; Sansbury, L.B.; Albert, P.S.; Cross, A.J.; Kahle, L.; Ashby, J.; Slattery, M.L.; Caan, B.; Paskett, E.; Iber, F.; et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1344–1353. [Google Scholar]
- Magee, P.J.; Rowland, I.R. Phyto-oestrogens, their mechanism of action: Current evidence for a role in breast and prostate cancer. Br. J. Nutr. 2004, 91, 513–531. [Google Scholar]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003, 21, 744–757. [Google Scholar]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008, 44, 1833–1845. [Google Scholar]
- Lee, Y.K.; Park, O.J. Soybean isoflavone genistein regulates apoptosis through NF-kappaB dependent and independent pathways. Exp. Toxicol. Pathol. 2011, in press.. [Google Scholar]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar]
- Sergeev, I.N. Genistein induces Ca2+ -mediated, calpain/caspase-12-dependent apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2004, 321, 462–467. [Google Scholar]
- van Erk, M.J.; Roepman, P.; van der Lende, T.R.; Stierum, R.H.; Aarts, J.M.; van Bladeren, P.J.; van Ommen, B. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. Eur. J. Nutr. 2005, 44, 143–156. [Google Scholar] [CrossRef]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012. [Google Scholar]
- Sample Availability: Samples of the compounds are availablefrom the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, W.-C.; Kuo, T.-H.; Tzeng, Y.-S.; Tsai, Y.-C. Baicalin Induces Apoptosis in SW620 Human Colorectal Carcinoma Cells in Vitro and Suppresses Tumor Growth in Vivo. Molecules 2012, 17, 3844-3857. https://doi.org/10.3390/molecules17043844
Chen W-C, Kuo T-H, Tzeng Y-S, Tsai Y-C. Baicalin Induces Apoptosis in SW620 Human Colorectal Carcinoma Cells in Vitro and Suppresses Tumor Growth in Vivo. Molecules. 2012; 17(4):3844-3857. https://doi.org/10.3390/molecules17043844
Chicago/Turabian StyleChen, Wen-Cheng, Tsu-Hsiang Kuo, Yi-Shiuan Tzeng, and Ying-Chieh Tsai. 2012. "Baicalin Induces Apoptosis in SW620 Human Colorectal Carcinoma Cells in Vitro and Suppresses Tumor Growth in Vivo" Molecules 17, no. 4: 3844-3857. https://doi.org/10.3390/molecules17043844
APA StyleChen, W.-C., Kuo, T.-H., Tzeng, Y.-S., & Tsai, Y.-C. (2012). Baicalin Induces Apoptosis in SW620 Human Colorectal Carcinoma Cells in Vitro and Suppresses Tumor Growth in Vivo. Molecules, 17(4), 3844-3857. https://doi.org/10.3390/molecules17043844



