Effects of Botulinum Toxin Type A on Collagen Deposition in Hypertrophic Scars
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
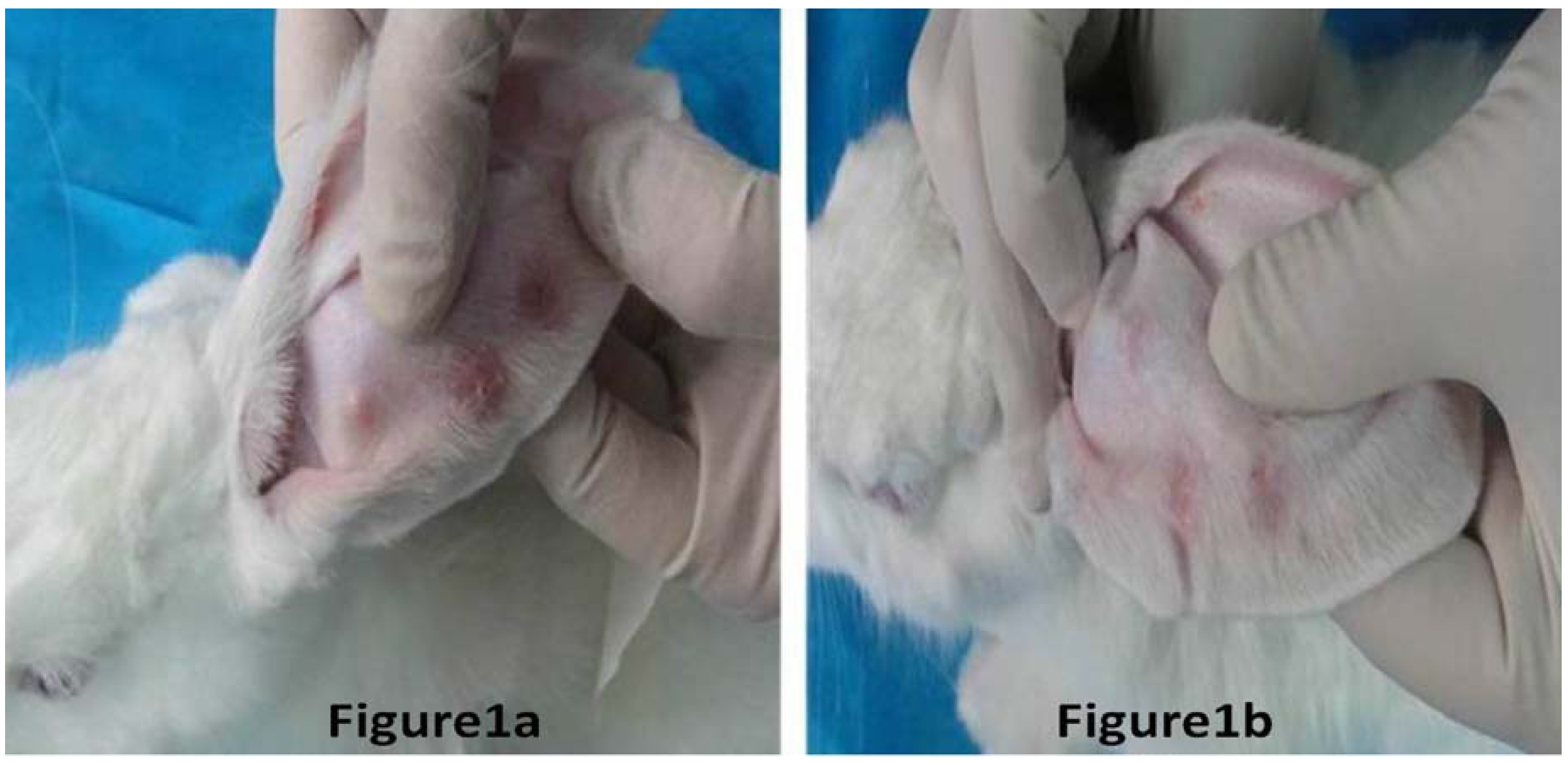

| Group difference | No of rabbits | |||||||
|---|---|---|---|---|---|---|---|---|
| Scar thicknessWithout BTXA (mm) | 1.92 ± 0.05 | 1.74 ± 0.03 | 1.57 ± 0.09 | 1.68 ± 0.02 | 1.37 ± 0.11 | 1.72 ± 0.8 | 1.64 ± 0.12 | 1.83 ± 0.09 |
| Scar thicknessWith BTXA (mm) | 0.90 ± 0.03 | 0.95 ± 0.18 | 1.16 ± 0.06 | 1.03 ± 0.11 | 1.08 ± 0.05 | 0.72 ± 0.09 | 0.69 ± 0.07 | 0.94 ± 0.02 |
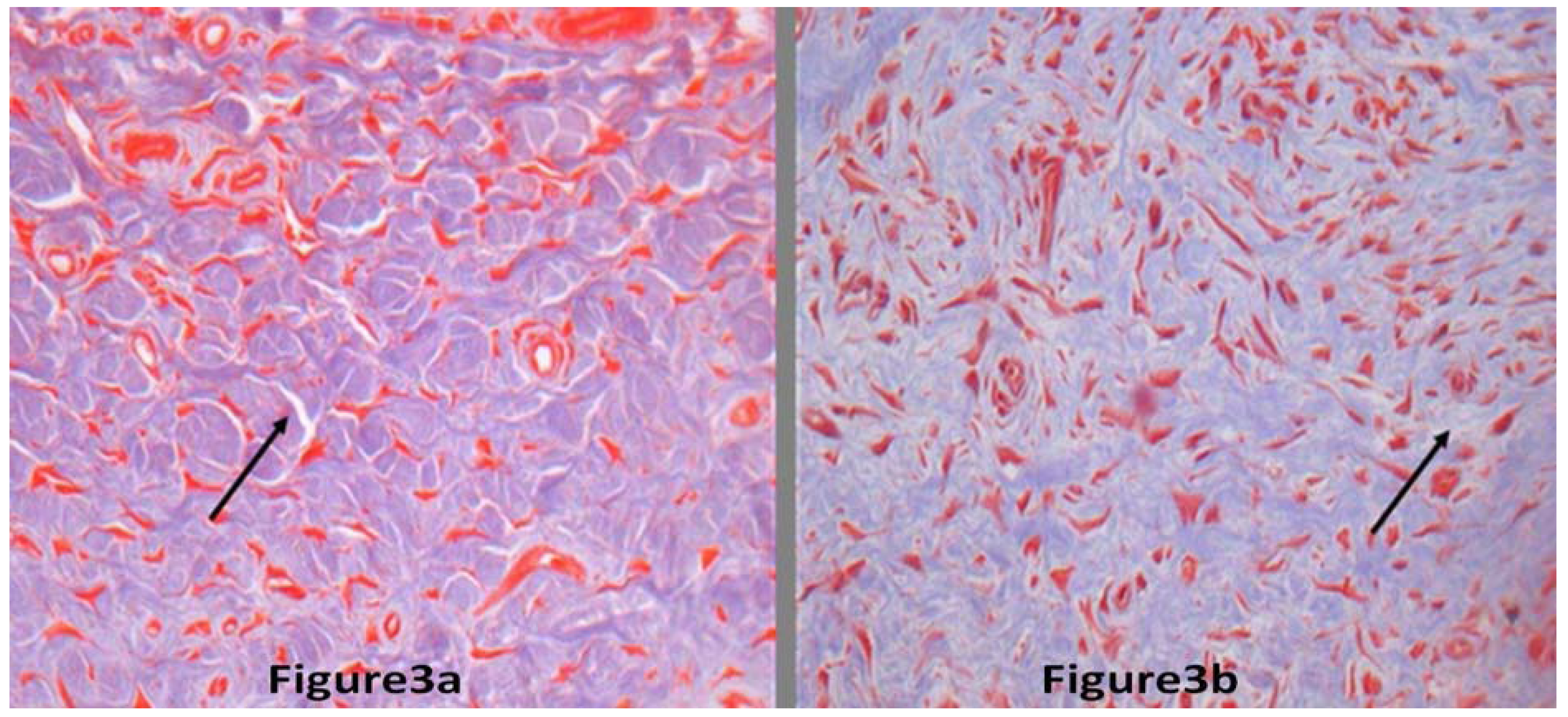
2.2. Discussion
3. Experimental
3.1. Hypertrophic Scar Model
3.2. Scar Treatment and Measurement
3.3. Statistical Analysis
4. Conclusions
Conflicts of Interest
Acknowledgements
References and Notes
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N. Keloid or hypertrophic scar: the controversy: Review of the literature. Ann. Plas. Surg. 2005, 54, 676–680. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, F.; Cui, Z. Treatment of hypertrophic scars with intra lesional botulinum toxin type A injections: A preliminary report. Aesthet. Plast. Surg. 2009, 33, 409–412. [Google Scholar] [CrossRef]
- Gassner, H.G.; Brissett, A.E.; Otley, C.C.; Boahene, D.K.; Boggust, A.J.; Weaver, A.L.; Sherris, D.A. Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo Clin. Proc. 2006, 81, 1023–1028. [Google Scholar] [CrossRef]
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261. [Google Scholar]
- Karsenty, G.; Rocha, J.; Chevalier, S.; Scarlata, E.; Andrieu, C.; Zouanat, F.Z.; Rocchi, P.; Giusiano, S.; Elzayat, E.A.; Corcos, J. Botulinum toxin type A inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo. Prostate 2009, 69, 1143–1150. [Google Scholar] [CrossRef]
- Rohrbach, S.; Olthoff, A.; Laskawi, R.; Giefer, B.; Götz, W. Botulinum toxin type A induces apoptosis in nasal glands of guinea pigs. Ann. Oto. Rhinol. Laryn. 2001, 110, 1045–1050. [Google Scholar]
- Reid, R.R.; Roy, N.; Mogford, J.E.; Zimmerman, H.; Lee, C.; Mustoe, T.A. Reduction of hypertrophic scar via retroviral delivery of adominant negative TGF-beta receptor II. J. Plast. Reconstr. Aes. 2007, 60, 64–72. [Google Scholar] [CrossRef]
- Lu, L.; Saulis, A.S.; Liu, W.R.; Roy, N.K.; Chao, J.D.; Ledbetter, S.; Mustoe, T.A. The Temporal Effects of Anti-TGF-β1, 2, and 3 Monoclonal Antibody on Wound Healing and Hypertrophic Scar Formation. J. Am. Coll. Surg. 2005, 201, 391–397. [Google Scholar]
- Xiao, Z.; Zhang, F.; Lin, W.; Zhang, M.; Liu, Y. Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: A preliminary report. Aesthet. Plast. Surg. 2010, 34, 424–427. [Google Scholar] [CrossRef]
- Kloeters, O.; Tandara, A.A.; Mustoe, T.A. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007, 15, S40–S45. [Google Scholar] [CrossRef]
- Ahmed, K.; Oas, K.H.; Mack, K.J.; Garza, I. Experience with botulinum toxin type A in medically intractable pediatric chronic daily headache. Pediatr. Neurol. 2010, 43, 316–319. [Google Scholar] [CrossRef]
- Scheffer, A.R.T.; Erasmus, C.; van Hulst, K.; van Limbeek, J.; Jongerius, P.H.; van den Hoogen, F.J.A. Efficacy and duration of botulinum toxin treatment for drooling in 131 children. Arch. Otolaryngol. 2010, 136, 873–877. [Google Scholar] [CrossRef]
- Sherris, D.A.; Gassner, H.G. Botulinum toxin to minimize facial scarring. Facial Plast. Surg. 2002, 18, 35–39. [Google Scholar] [CrossRef]
- Xiao, Z.; Miaobo, Z. Botulinum toxin type A affects cell cycle distribution of fibroblasts derived from hypertrophic scar. J. Plast. Reconstr. Aes. 2008, 61, 1128–1129. [Google Scholar] [CrossRef]
- Xiao, Z.; Miaobo, Z. Potential therapeutical effects of botulinum toxin type A in keloid management. Med. Hypotheses 2008, 71, 623–624. [Google Scholar] [CrossRef]
- Lee, J.P.; Jalili, R.B.; Tredget, E.E.; Demare, J.A.; Ghahary, A. Antifibrogenic Effects of Liposome-Encapsulated IFN-α2b Cream on Skin Wounds in a Fibrotic Rabbit Ear Model. J. Interf. Cytok. Res. 2005, 25, 627–631. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiao, Z.; Qu, G. Effects of Botulinum Toxin Type A on Collagen Deposition in Hypertrophic Scars. Molecules 2012, 17, 2169-2177. https://doi.org/10.3390/molecules17022169
Xiao Z, Qu G. Effects of Botulinum Toxin Type A on Collagen Deposition in Hypertrophic Scars. Molecules. 2012; 17(2):2169-2177. https://doi.org/10.3390/molecules17022169
Chicago/Turabian StyleXiao, Zhibo, and Guofan Qu. 2012. "Effects of Botulinum Toxin Type A on Collagen Deposition in Hypertrophic Scars" Molecules 17, no. 2: 2169-2177. https://doi.org/10.3390/molecules17022169
APA StyleXiao, Z., & Qu, G. (2012). Effects of Botulinum Toxin Type A on Collagen Deposition in Hypertrophic Scars. Molecules, 17(2), 2169-2177. https://doi.org/10.3390/molecules17022169




