Phytochemical and Antioxidant-Related Investigations on Bark of Abies spectabilis (D. Don) Spach. from Nepal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity and Iron (II) Chelating Capacity
| Extract | DPPH IC50 μg/mL | GAE μg/g |
|---|---|---|
| Methanol | 4.13 ± 0.02 | 3.9 ± 0.1 |
| Chloroform | >100 | n.d. |
| Rutin | 4.80 ± 0.02 | n.d. |
| Ascorbic acid | 3.50 ± 0.01 | n.d. |
2.2. Phytochemical Composition of Methanol Extract
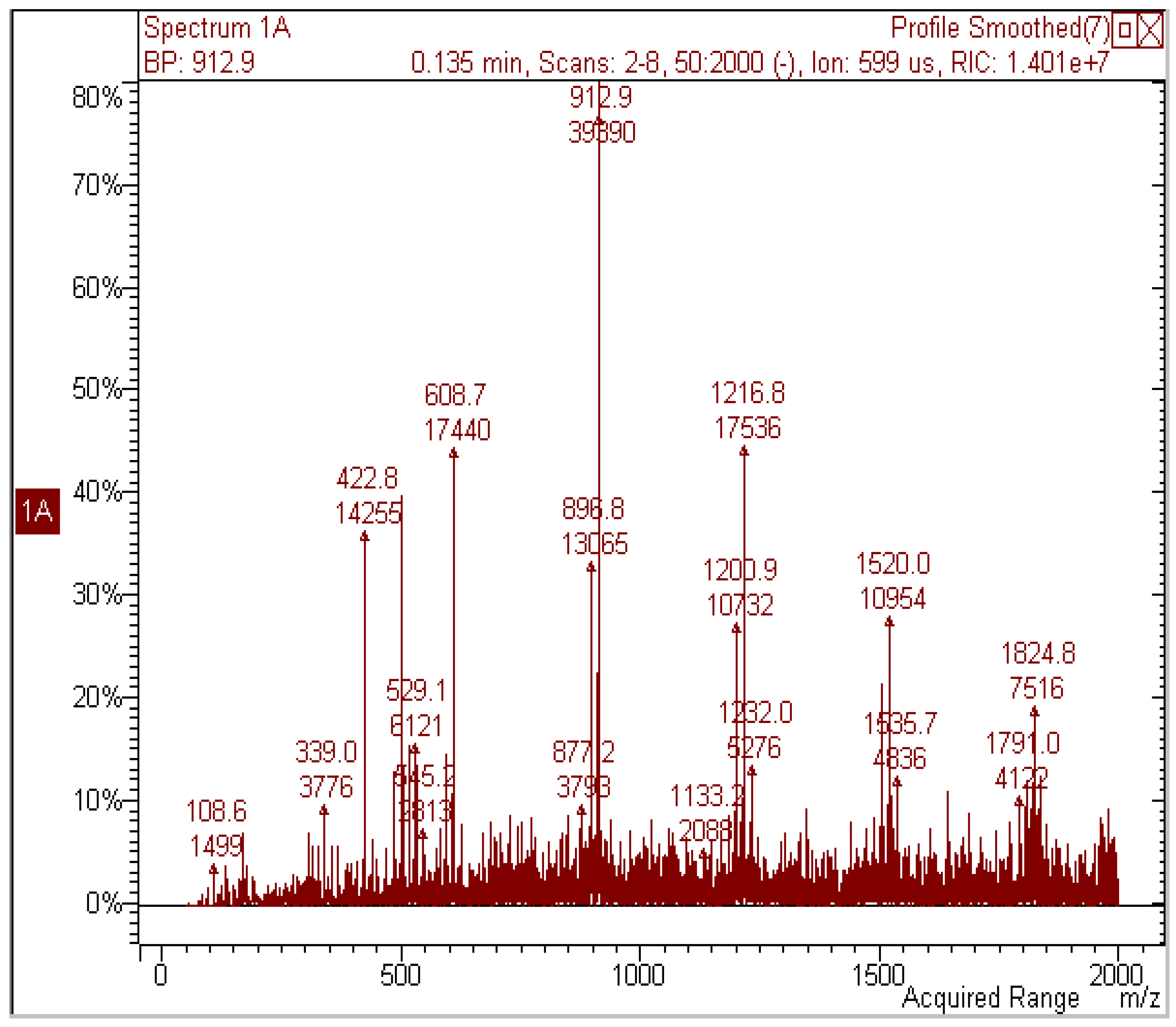
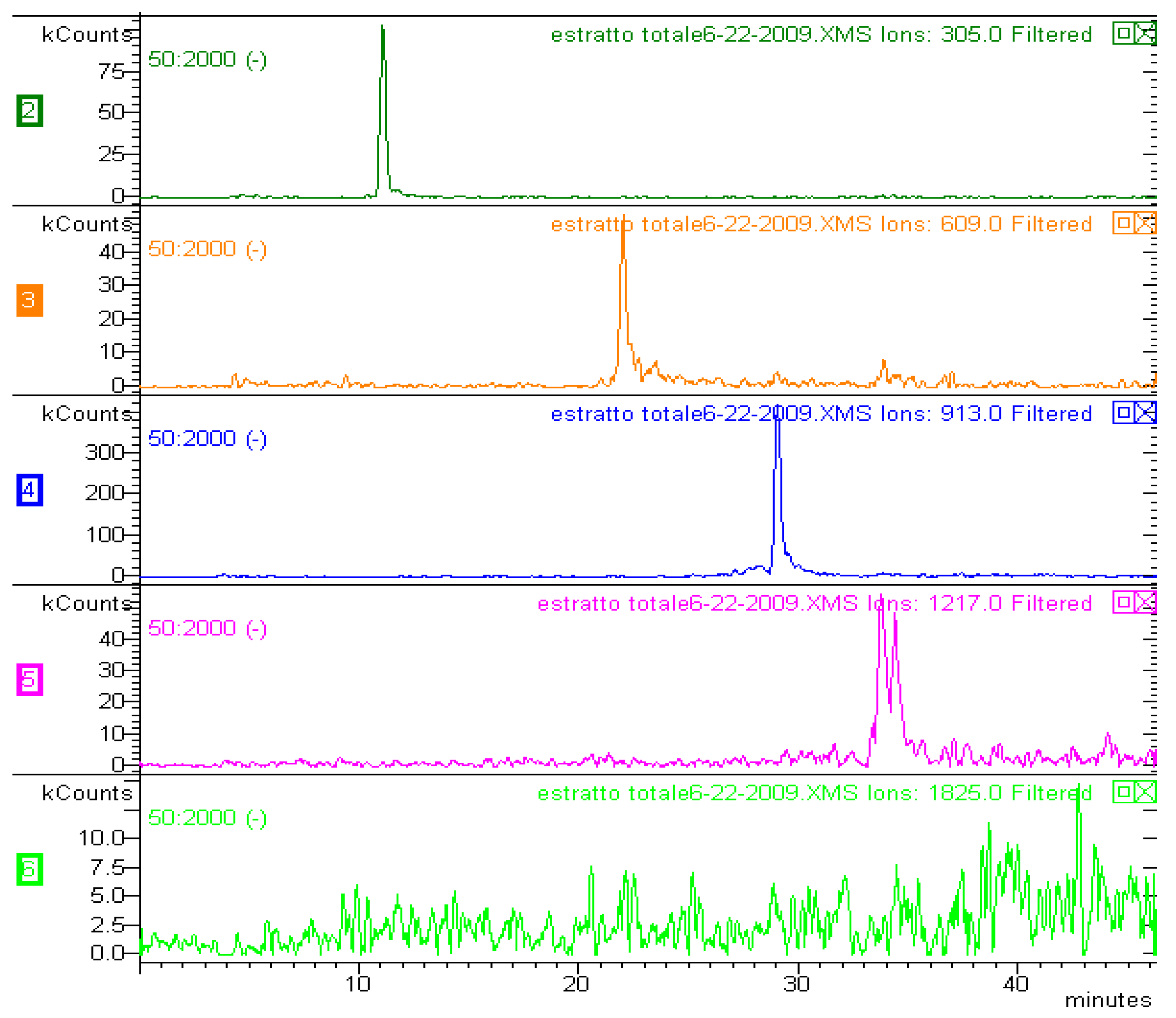
| [M−H]− m/z | MS2 | MS3 (parent ion) | Proposed structure |
|---|---|---|---|
| 593 | 467; 441; 423; 305 | (467) 305; (441) 423 | G-C |
| 609 | 591; 483; 441; 423; 305 | (483) 305; (441) 423 | G-G |
| 897 | 771; 729 ; 711 | (771) 593; (729) 711 | G-C-G |
| 913 | 787; 745; 609 | (787) 609, 483; (745) 727 | G-G-G |
| 1217 | 1091; 1049; 305 | (1091) 913; (1049) 1031 | G-G-G-G |
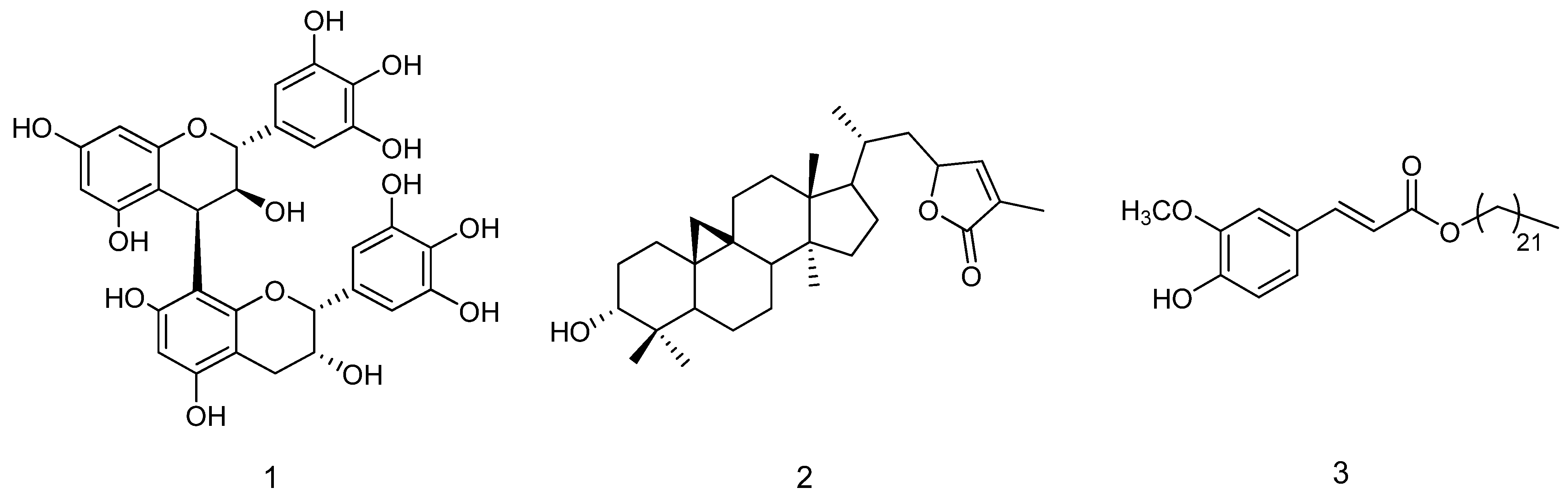
2.3. Phytochemical Investigation of Chloroform Extract
3. Experimental
3.1. Chemicals
3.2. General Experimental Procedures
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Antioxidant Activity
3.5.1. DPPH Assay
3.5.2. BR (Briggs-Rauscher Oscillating Reaction) Method

3.5.3. TEAC (Trolox Equivalent Antioxidant Capacity) Assay
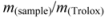
3.6. Determination of Total Phenols (Total Reducing Power Quantification)
3.7. Iron (II) Chelating Capacity
4. Conclusions
Supplementary Materials
Acknowledgements
- Sample Availability: Samples of the compounds and methanol extract are available from the authors.
References and Notes
- Wada, S.-I.; Hitomi, T.; Tanaka, R. Phenolic Compounds Isolated from the Bark of Abies Sachalinensis. Helv. Chim. Acta 2009, 92, 1610–1620. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsuo, Y.; Yamada, Y.; Kouno, I. Structure of Polymeric Polyphenols of Cinnamon Bark Deduced from Condensation Products of Cinnamaldehyde with Catechin and Procyanidins. J. Agric. Food Chem. 2008, 56, 5864–5870. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Turchetti, B.; Mulinacci, N.; Vincieri, F.F.; Buzzini, P. Analysis of Condensed and Hydrolysable Tannins from Commercial Plant Extracts. J. Pharm. Biomed. Anal. 2006, 41, 415–420. [Google Scholar] [CrossRef]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Study on Chemical Composition, Antioxidant and Anti-Inflammatory Activities of Hot Water Extract from Picea Mariana Bark and its Proanthocyanidin-Rich Fractions. Food Chem. 2009, 113, 897–902. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Errichi, B.; Di Renzo, A.; Grossi, M.G.; Ricci, A.; Dugall, M.; Cornelli, U.; Cacchio, M.; Rohdewald, P. Pycnogenol® Treatment of Acute Hemorrhoidal Episodes. Phytother. Res. 2010, 24, 438–444. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; et al. Treatment of Osteoarthritis with Pycnogenol®. the SVOS (San Valentino Osteo-Arthrosis Study). Evaluation of Signs, Symptoms, Physical Performance and Vascular Aspects. Phytother. Res. 2008, 22, 518–523. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Rohdewald, P.; Pellegrini, L.; Ledda, A.; Vinciguerra, G.; Ricci, A.; Gizzi, G.; Ippolito, E.; Fano, F.; et al. Improvement of Diabetic Microangiopathy with Pycnogenol®: A Prospective, Controlled Study. Angiology 2006, 57, 431–436. [Google Scholar]
- Rohdewald, P.; Beil, W. In Vitro Inhibition of Helicobacter Pylori Growth and Adherence to Gastric Mucosal Cells by Pycnogenol®. Phytother. Res. 2008, 22, 685–688. [Google Scholar] [CrossRef]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of Cardiovascular Risk Factors in Subjects with Type 2 Diabetes by Pycnogenol Supplementation. Nutr. Res. 2008, 28, 315–320. [Google Scholar] [CrossRef]
- Zibadi, S.; Yu, Q.; Rohdewald, P.J.; Larson, D.F.; Watson, R.R. Impact of Pycnogenol® on Cardiac Extracellular Matrix Remodeling Induced by L-NAME Administration to Old Mice. Cardiovasc. Toxicol. 2007, 7, 10–18. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Ganzera, M.; Akgun, I.; Sevimli, C.; Korkmaza, K.S.; Bedira, E. Determination of Polyphenolic Constituents and Biological Activities of Bark Extracts from Different Pinus Species. J. Sci. Food Agric. 2009, 89, 1339–1345. [Google Scholar] [CrossRef]
- D’Andrea, G. Pycnogenol: A Blend of Procyanidins with Multifaceted Therapeutic Applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef]
- Chaudhary, R.P. Biodiversity in Nepal: Status and Conservation; S. Devi: Saharanpur (U.P.) India and Tecpress Books: Bangkok, Thailand, 1998. [Google Scholar]
- Dall’Acqua, S.; Shrestha, B.B.; Comai, S.; Innocenti, G.; Gewali, M.B.; Jha, P.K. Two Phenolic Glycosides from Curculigo Orchioides Gaertn. Fitoterapia 2009, 80, 279–282. [Google Scholar] [CrossRef]
- Comai, S.; Dall’Acqua, S.; Grillo, A.; Castagliuolo, I.; Gurung, K.; Innocenti, G. Essential Oil of Lindera Neesiana Fruit: Chemical Analysis and its Potential Use in Topical Applications. Fitoterapia 2010, 81, 11–16. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Shrestha, B.B.; Gewali, M.B.; Jha, P.K.; Carrara, M.; Innocenti, G. Diterpenoid Alkaloids and Phenol Glycosides from Aconitum Naviculare (Brühl) Stapf. Nat. Prod. Commun. 2008, 3, 1985–1989. [Google Scholar]
- Shrestha, B.B.; Dall’Acqua, S.; Gewali, M.B.; Jha, P.K.; Innocenti, G. New Flavonoid Glycosides from Aconitum Naviculare (Brühl) Stapf, a Medicinal Herb from the Trans-Himalayan Region of Nepal. Carbohydr. Res. 2006, 341, 2161–2165. [Google Scholar] [CrossRef]
- Innocenti, G.; Dall’Acqua, S.; Scialino, G.; Banfi, E.; Sosa, S.; Gurung, K.; Barbera, M.; Carrara, M. Chemical Composition and Biological Properties of Rhododendron Anthopogon Essential Oil. Molecules 2010, 15, 2326–2338. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in Vitro Antioxidant Properties of some Traditional Sardinian Medicinal Plants: Investigation of the High Antioxidant Capacity of Rubus Ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and their Antioxidant Activity in Vegetables—Evaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry Behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar]
- Kehrer, J.P. The Haber-Weiss Reaction and Mechanisms of Toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Lantto, T.A.; Dorman, H.J.D.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R.; Raasmaja, A. Chemical Composition, Antioxidative Activity and Cell Viability Effects of a Siberian Pine (Pinus Sibirica Du Tour) Extract. Food Chem. 2009, 112, 936–943. [Google Scholar] [CrossRef]
- Pan, H.; Lundgren, L.N. Phenolics from Inner Bark of Pinus Sylvestris. Phytochemistry 1996, 42, 1185–1189. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Molan, A.L.; Woodfield, D.R.; McNabb, W.C. The Phenols and Prodelphinidins of White Clover Flowers. Phytochemistry 2000, 54, 539–548. [Google Scholar] [CrossRef]
- Hümmer, W.; Schreier, P. Analysis of Proanthocyanidins. Mol. Nutr. Food Res. 2008, 52, 1381–1398. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Zhang, Z.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Liquid Chromatographic/electrospray Ionization Mass Spectrometric Studies of Proanthocyanidins in Foods. J. Mass Spectrom. 2003, 38, 1272–1280. [Google Scholar] [CrossRef]
- Kelm, M.A.; Johnson, J.C.; Robbins, R.J.; Hammerstone, J.F.; Schmitz, H.H. High-Performance Liquid Chromatography Separation and Purification of Cacao (Theobroma Cacao L.) Procyanidins According to Degree of Polymerization using a Diol Stationary Phase. J. Agric. Food Chem. 2006, 54, 1571–1576. [Google Scholar]
- Del Río, J.C.; Gutiérrez, A.; Martínez, Á.T. Identifying Acetylated Lignin Units in Non-Wood Fibers using Pyrolysis-Gas Chromatography/mass Spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1181–1185. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Demonstration of Long-Chain n-Alkyl Caffeates and Δ7-Steryl Glucosides in the Bark of Acacia Species by Gas Chromatography-Mass Spectrometry. Phytochem. Anal. 2007, 18, 151–156. [Google Scholar] [CrossRef]
- Kutney, J.P.; Grierson, D.S.; Knowles, G.D.; Westcott, N.D.; Rogers, I.H. Studies on Constituents of Abies Grandis. the Structures and Absolute Stereochemistry of Cyclograndisolide and Epicyclograndisolide, Novel Cyclopropane Triterpene Lactones. Tetrahedron 1973, 29, 13–20. [Google Scholar]
- Cervellati, R.; Renzulli, C.; Guerra, M.C.; Speroni, E. Evaluation of Antioxidant Activity of some Natural Polyphenolic Compounds using the Briggs-Rauscher Reaction Method. J. Agric. Food Chem. 2002, 50, 7504–7509. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Carter, P. Spectrophotometric Determination of Serum Iron at the Submicrogram Level with a New Reagent (Ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar]
- Speroni, E.; Cervellati, R.; Dall’Acqua, S.; Guerra, M.C.; Greco, E.; Govoni, P.; Innocenti, G. Gastroprotective effect and antioxidant properties of different Laurus nobilis L. leaf extracts. J. Med. Food 2011, 14, 499–504. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dall’Acqua, S.; Minesso, P.; Shresta, B.B.; Comai, S.; Jha, P.K.; Gewali, M.B.; Greco, E.; Cervellati, R.; Innocenti, G. Phytochemical and Antioxidant-Related Investigations on Bark of Abies spectabilis (D. Don) Spach. from Nepal. Molecules 2012, 17, 1686-1697. https://doi.org/10.3390/molecules17021686
Dall’Acqua S, Minesso P, Shresta BB, Comai S, Jha PK, Gewali MB, Greco E, Cervellati R, Innocenti G. Phytochemical and Antioxidant-Related Investigations on Bark of Abies spectabilis (D. Don) Spach. from Nepal. Molecules. 2012; 17(2):1686-1697. https://doi.org/10.3390/molecules17021686
Chicago/Turabian StyleDall’Acqua, Stefano, Paola Minesso, Bharat Babu Shresta, Stefano Comai, Pramod Kumar Jha, Mohan Bikram Gewali, Emanuela Greco, Rinaldo Cervellati, and Gabbriella Innocenti. 2012. "Phytochemical and Antioxidant-Related Investigations on Bark of Abies spectabilis (D. Don) Spach. from Nepal" Molecules 17, no. 2: 1686-1697. https://doi.org/10.3390/molecules17021686
APA StyleDall’Acqua, S., Minesso, P., Shresta, B. B., Comai, S., Jha, P. K., Gewali, M. B., Greco, E., Cervellati, R., & Innocenti, G. (2012). Phytochemical and Antioxidant-Related Investigations on Bark of Abies spectabilis (D. Don) Spach. from Nepal. Molecules, 17(2), 1686-1697. https://doi.org/10.3390/molecules17021686




