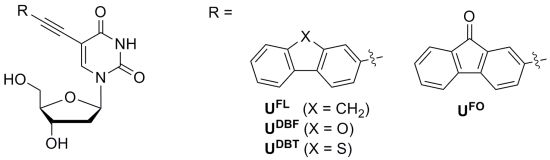
Synthesis and Photophysical Study of 2′-Deoxyuridines Labeled with Fluorene Derivatives
Abstract
:1. Introduction
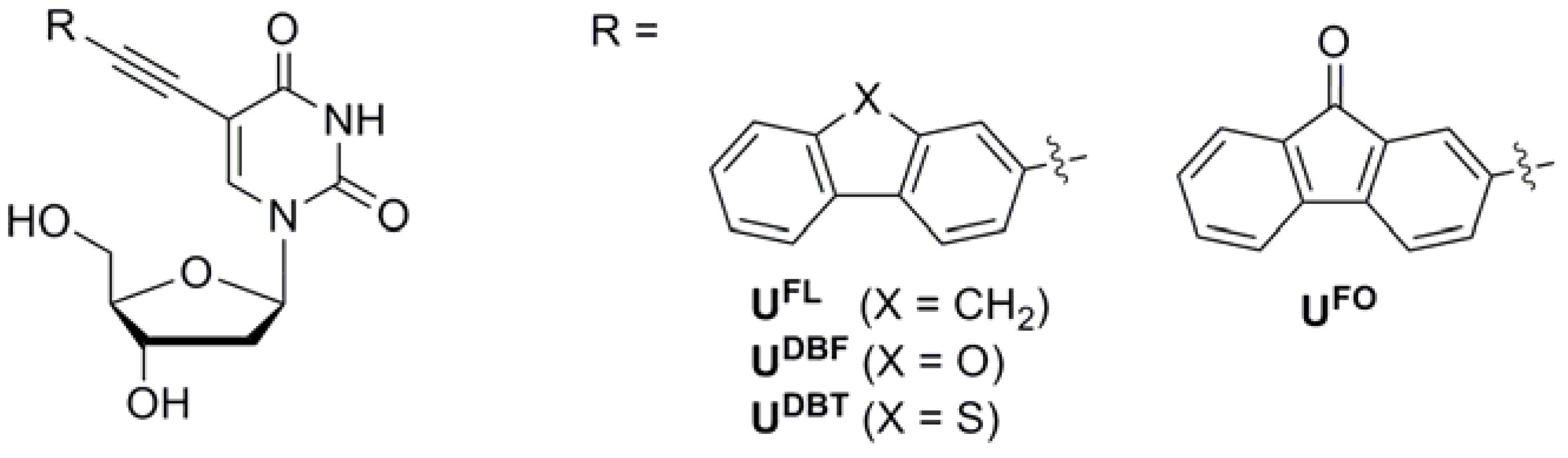
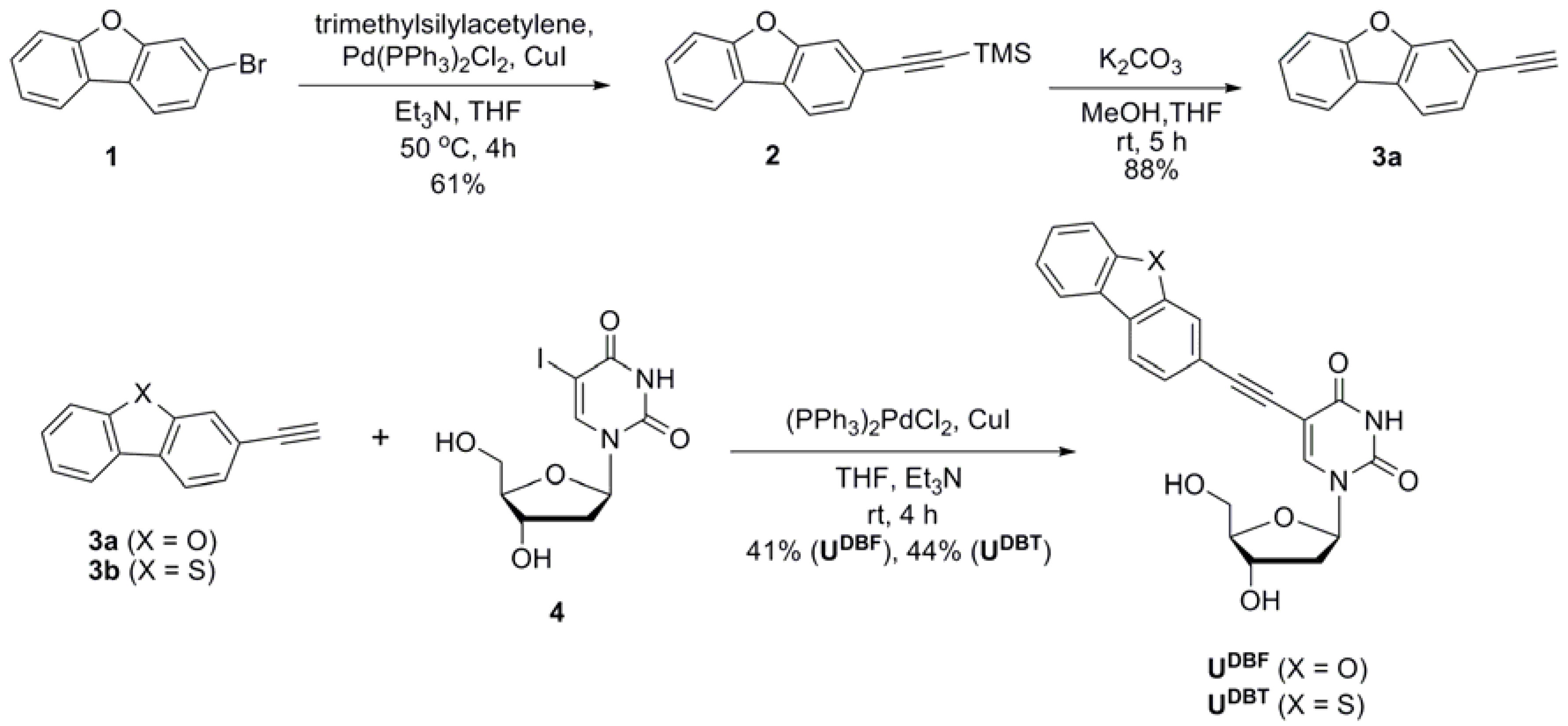
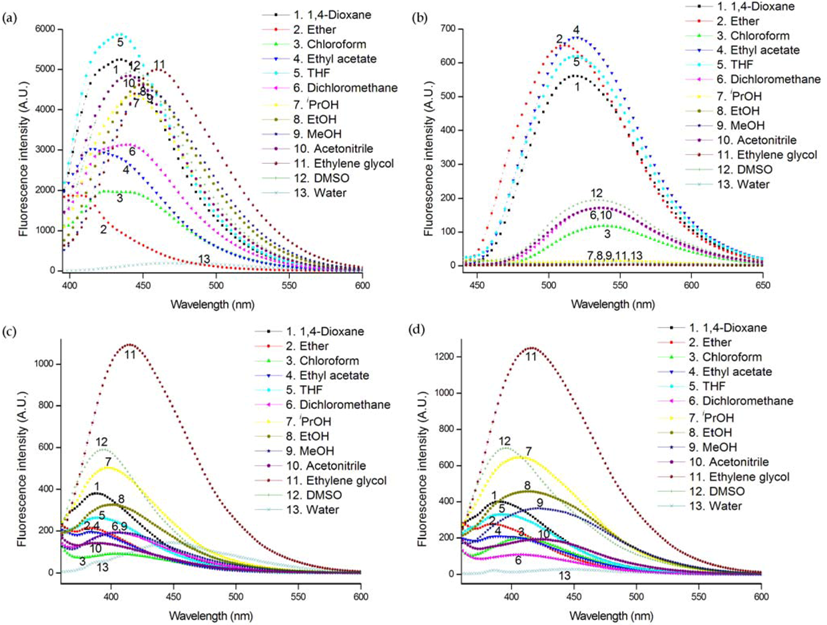
2. Results and Discussion
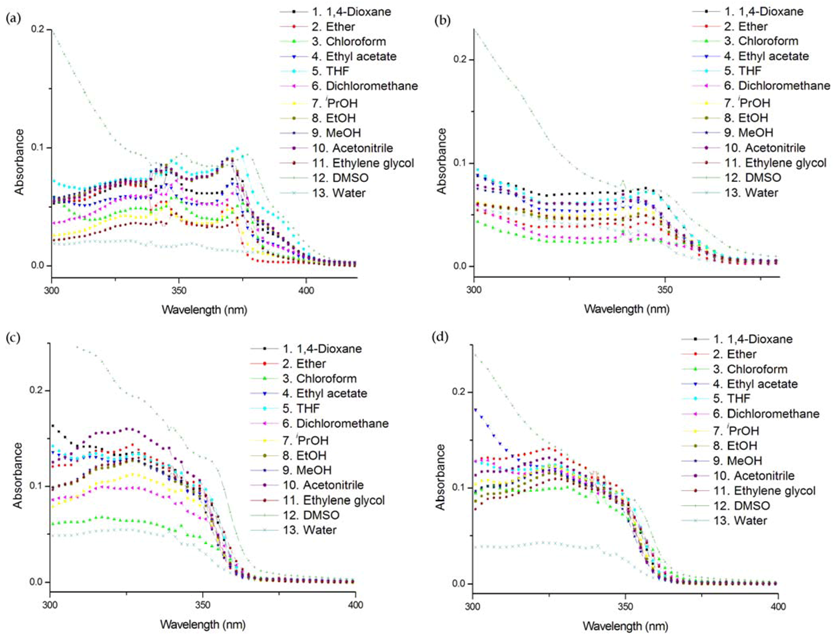
| Solvent | Compound | ET(30)11 | λmax (nm) a | ε (M−1 cm−1) | λem (nm) b | ΦF c | Brightness d |
|---|---|---|---|---|---|---|---|
| 1,4-Dioxane | UFL | 36 | 373 | 25,000 | 434 | 0.33 | 8,250 |
| Ether | 34.5 | 370 | 27,200 | 409 | 0.055 | 1,500 | |
| Chloroform | 39.1 | 375 | 20,100 | 420 | 0.18 | 3,620 | |
| Ethyl acetate | 38.1 | 371 | 19,900 | 414 | 0.14 | 2,790 | |
| THF | 37.4 | 373 | 29,700 | 434 | 0.31 | 9,200 | |
| Dichloromethane | 40.7 | 374 | 22,200 | 439 | 0.23 | 5,100 | |
| iPrOH | 48.4 | 370 | 20,900 | 444 | 0.50 | 10,500 | |
| EtOH | 51.9 | 370 | 24,400 | 450 | 0.25 | 6,100 | |
| MeOH | 55.4 | 369 | 25,100 | 453 | 0.26 | 6,530 | |
| Acetonitrile | 45.6 | 370 | 26,200 | 440 | 0.28 | 7,340 | |
| Ethylene glycol | 56.3 | 374 | 12,900 | 460 | 0.27 | 3,480 | |
| DMSO | 45.1 | 377 | 27,100 | 443 | 0.18 | 4,880 | |
| Water | 63.1 | 384 | 4,930 | 467 | 0.062 | 305 | |
| 1,4-Dioxane | UFO | 36 | 344 | 20,800 | 518 | 0.056 | 1,160 |
| Ether | 34.5 | 345 | 21,200 | 511 | 0.080 | 1,700 | |
| Chloroform | 39.1 | 343 | 14,300 | 538 | 0.029 | 415 | |
| Ethyl acetate | 38.1 | 343 | 19,400 | 519 | 0.090 | 1,750 | |
| THF | 37.4 | 346 | 21,900 | 519 | 0.064 | 1,400 | |
| Dichloromethane | 40.7 | 342 | 15,800 | 535 | 0.040 | 632 | |
| iPrOH | 48.4 | 344 | 16,500 | 552 | 0.0034 | 56.1 | |
| EtOH | 51.9 | 343 | 16,300 | 554 | 0.00097 | 15.8 | |
| MeOH | 55.4 | 342 | 17,900 | 558 | 0.0014 | 25.1 | |
| Acetonitrile | 45.6 | 342 | 18,400 | 537 | 0.0219 | 403 | |
| Ethylene glycol | 56.3 | 345 | 14,000 | 556 | 0.00076 | 10.6 | |
| DMSO | 45.1 | 347 | 21,500 | 535 | 0.018 | 387 | |
| Water | 63.1 | 341 | 13,200 | 552 | 0.0038 | 50.2 | |
| 1,4-Dioxane | UDBF | 36 | 328 | 23,300 | 388 | 0.049 | 1,140 |
| Ether | 34.5 | 327 | 27,300 | 383 | 0.029 | 792 | |
| Chloroform | 39.1 | 317 | 17,100 | 404 | 0.086 | 1,470 | |
| Ethyl acetate | 38.1 | 327 | 24,500 | 383 | 0.027 | 662 | |
| THF | 37.4 | 329 | 24,700 | 388 | 0.035 | 865 | |
| Dichloromethane | 40.7 | 328 | 21,400 | 405 | 0.044 | 942 | |
| iPrOH | 48.4 | 328 | 22,500 | 397 | 0.086 | 1,940 | |
| EtOH | 51.9 | 327 | 24,300 | 401 | 0.074 | 1,800 | |
| MeOH | 55.4 | 326 | 23,000 | 406 | 0.047 | 1,080 | |
| Acetonitrile | 45.6 | 326 | 24,000 | 386 | 0.026 | 624 | |
| Ethylene glycol | 56.3 | 329 | 21,300 | 415 | 0.23 | 4,900 | |
| DMSO | 45.1 | nd e | nd e | 394 | 0.084 | nd e | |
| Water | 63.1 | 325 | 11,700 | 449 | 0.047 | 550 | |
| 1,4-Dioxane | UDBT | 36 | 327 | 26,500 | 390 | 0.047 | 1,250 |
| Ether | 34.5 | 325 | 28,900 | 358 | 0.020 | 578 | |
| Chloroform | 39.1 | 329 | 20,500 | 407 | 0.050 | 1,030 | |
| 1,4-Dioxane | UDBT | 36 | 327 | 26,500 | 390 | 0.047 | 1,250 |
| Ethyl acetate | 38.1 | 325 | 24,200 | 389 | 0.024 | 581 | |
| THF | 37.4 | 326 | 24,900 | 391 | 0.033 | 822 | |
| Dichloromethane | 40.7 | 327 | 24,600 | 408 | 0.029 | 713 | |
| iPrOH | 48.4 | 325 | 24,300 | 407 | 0.064 | 1,560 | |
| EtOH | 51.9 | 326 | 22,900 | 411 | 0.061 | 1,400 | |
| MeOH | 55.4 | 325 | 24,400 | 421 | 0.045 | 1,100 | |
| Acetonitrile | 45.6 | 325 | 24,800 | 423 | 0.020 | 496 | |
| Ethylene glycol | 56.3 | 328 | 21,900 | 417 | 0.11 | 2,410 | |
| DMSO | 45.1 | nd e | nd e | 395 | 0.082 | nd e | |
| Water | 63.1 | 324 | 8,600 | 451 | 0.0079 | 67.9 |
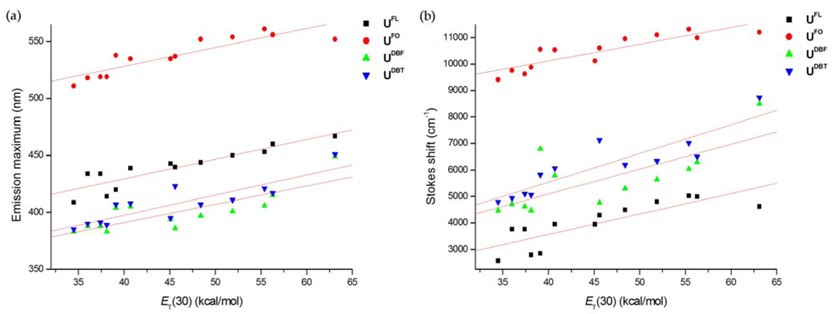
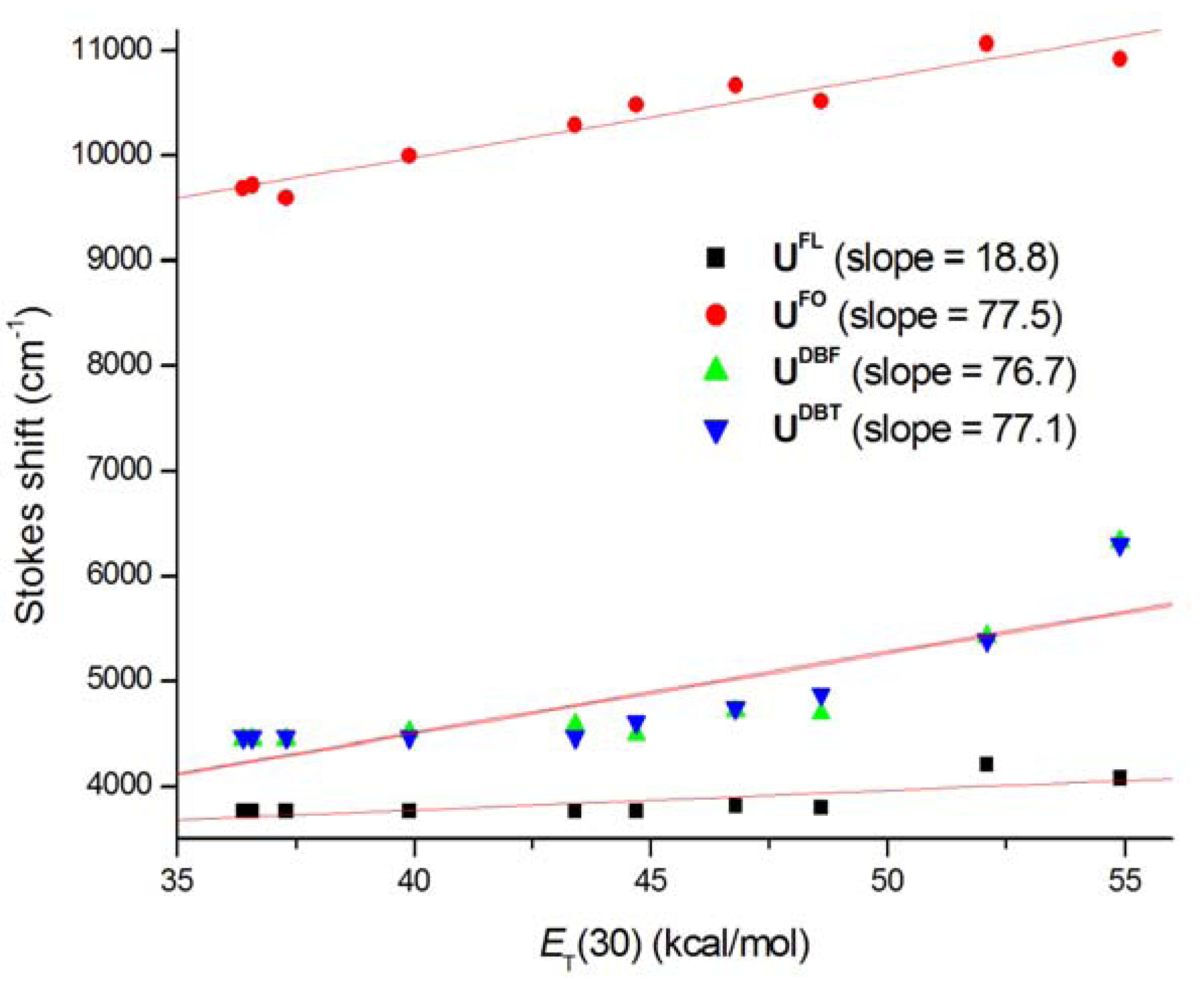
3. Experimental
3.1. General
3.2. Materials
3.3. Preparation of 3-[2-(Trimethylsilyl)ethynyl]dibenzofuran (2)
3.4. Preparation of 3-Ethynyldibenzofuran (3a)
3.5. General Procedure for Nucleoside Synthesis
3.6. UV and Fluorescence Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
- Sample Availability: Samples of the compounds used in this study are available from the authors.
References
- Ranasinghe, R.T.; Brown, T. Fluorescence based strategies for genetic analysis. Chem. Commun. 2005, 5487–5502. [Google Scholar] [CrossRef]
- Wilson, J.N.; Kool, E.T. Fluorescent DNA base replacements: Reporters and sensors for biological systems. Org. Biomol. Chem. 2006, 4, 4265–4274. [Google Scholar] [CrossRef]
- Venkatesan, N.; Seo, Y.J.; Bang, E.K.; Park, S.M.; Lee, Y.S.; Kim, B.H. Chemical modification of nucleic acids toward functional nucleic acid systems. Bull. Korean Chem. Soc. 2006, 28, 613–630. [Google Scholar]
- Venkatesan, N.; Seo, Y.J.; Kim, B.H. Quencher-free molecular beacons: A new strategy in fluorescence based nucleic acid analysis. Chem. Soc. Rev. 2008, 37, 648–663. [Google Scholar]
- Dodd, D.W.; Hudson, R.H.E. Intrinsically fluorescent base-discriminating nucleoside analogs. Mini-Rev. Org. Chem. 2009, 6, 378–391. [Google Scholar]
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Fluorescent analogs of biomolecular building blocks: Design, properties, and application. Chem. Rev. 2010, 110, 2579–2619. [Google Scholar]
- Dai, N.; Kool, E.T. Fluorescent DNA-based enzyme sensors. Chem. Soc. Rev. 2011, 40, 5756–5770. [Google Scholar] [CrossRef]
- Østergaard, M.E.; Hrdlicka, P.J. Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): Tools for fundamental research, diagnostics, and nanotechnolog. Chem. Soc. Rev. 2011, 40, 5771–5788. [Google Scholar] [CrossRef]
- Hwang, G.T.; Seo, Y.J.; Kim, B.H. A highly discriminating quencher-free molecular beacon for probing DNA. J. Am. Chem. Soc. 2004, 126, 6528–6529. [Google Scholar] [CrossRef]
- Ryu, J.H.; Seo, Y.J.; Hwang, G.T.; Lee, J.Y.; Kim, B.H. Triad base pairs containing fluorene unit for quencher-free SNP typing. Tetrahedron 2007, 63, 3538–3547. [Google Scholar]
- Ryu, J.H.; Heo, J.Y.; Bang, E.-K.; Hwang, G.T.; Kim, B.H. Quencher-free linear beacon systems containing 2-ethynylfluorenone-labeled 2′-deoxyuridine units. Tetrahedron 2012, 68, 72–78. [Google Scholar] [CrossRef]
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Polarity of major grooves explored by using an isosteric emissive nucleoside. ChemBioChem 2008, 9, 706–709. [Google Scholar] [CrossRef]
- Østergaard, M.E.; Guenther, D.C.; Kumar, P.; Baral, B.; Deobald, L.; Paszczynski, A.J.; Sharma, P.K.; Hrdlicka, P.J. Pyrene-functionalized triazole-linked 2′-deoxyuridines-probes for discrimination of single nucleotide polymorphisms (SNPs). Chem. Commun. 2010, 46, 4929–4931. [Google Scholar]
- Varghese, R.; Wagenknecht, H.-A. Non-covalent versus covalent control of self-assembly and chirality of Nile red-modified nucleoside and DNA. Chem. Eur. J. 2010, 16, 9040–9046. [Google Scholar] [CrossRef]
- Bag, S.S.; Kundu, R.; Matsumoto, K.; Saito, Y.; Saito, I. Singly and doubly labeled base-discriminating fluorescent oligonucleotide probes containing oxo-pyrene chromophore. Bioorg. Med. Chem. Lett. 2010, 20, 3227–3230. [Google Scholar]
- Pawar, M.G.; Srivatsan, S.G. Synthesis, photophysical characterization, and enzymatic incorporation of a microenvironment-sensitive fluorescent uridine analog. Org. Lett. 2011, 13, 1114–1117. [Google Scholar]
- Saito, Y.; Miyamoto, S.; Suzuki, A.; Matsumoto, K.; Ishihara, T.; Saito, I. Fluorescent nucleosides with “on-off”switching function, pH-responsive fluorescent uridine derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 2753–2756. [Google Scholar]
- Tanaka, M.; Oguma, K.; Saito, Y.; Saito, I. Enhancement of fluorescence quenching and exciplex formation in DNA major groove by double incorporation of modified fluorescent deoxyuridines. Bioorg. Med. Chem. Lett. 2012, 22, 4103–4106. [Google Scholar] [CrossRef]
- Barrois, S.; Beyer, C.; Wagenknecht, H.-A. Covalent modification of 2′-deoxyuridine with two different molecular switches. Synlett 2012, 23, 711–716. [Google Scholar] [CrossRef]
- Tanaka, M.; Oguma, K.; Saito, Y.; Saito, I. Drastic enhancement of excess electron-transfer efficiency through DNA by inserting consecutive 5-phenylethynyl-2'-deoxyuridines as a modulator. Chem. Commun. 2012, 48, 9394–9396. [Google Scholar]
- Parker, C.A. Photoluminescence of Solutions; Elsevier: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Saito, Y.; Koda, M.; Shinohara, Y.; Saito, I. Synthesis and photophysical properties of 8-arylbutadienyl 2′-deoxyguanosines. Tetrahedron Lett. 2011, 52, 491–494. [Google Scholar] [CrossRef]
- Saito, Y.; Shinohara, Y.; Ishioroshi, S.; Suzuki, A.; Tanaka, M.; Saito, I. Synthesis of environmentally sensitive 2′-deoxyguanosine containing solvatochromic pyrene dluorophore. Tetrahedron Lett. 2011, 52, 2359–2361. [Google Scholar]
- Panozzo, S.; Vial, J.-C.; Kervella, Y.; Stéphan, O. Fluorene-fluorenone copolymer: Stable and efficient yellow-emitting material for electroluminescent devices. J. Appl. Phys. 2002, 92, 3495–3502. [Google Scholar] [CrossRef]
- Józefowicz, M. Determination of reorganization energy of fluorenone and 4-hydroxyfluorenone in neat and binary solvent mixtures. Spectrochim. Acta A 2007, 67, 444–449. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Trunk, J.; Nakhimovsky, L.; Spanget-Larsen, J. Electronic transitions of fluorene, dibenzofuran, carbazole, and dibenzothiophene: From the onset of absorption to the ionization threshold. J. Mol. Spectrosc. 2010, 264, 19–25. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Hwang, G.T.; Son, H.S.; Ku, J.K.; Kim, B.H. Synthesis and photophysical studies of bis-enediynes as tunable fluorophores. J. Am. Chem. Soc. 2003, 125, 11241–11248. [Google Scholar]
- Sekine, C.; Ishitobi, M.; Iwakura, K.; Minai, M.; Fujisawa, K. Novel high birefringence dibenzothiophenylacetylene liquid crystals. Liq. Cryst. 2002, 29, 355–367. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed; Springer: New York, NY, USA, 2006; pp. 205–235. [Google Scholar]
- Eastman, J.W. Quantitative spectrofluorimetry—The fluorescence quantum yield of quinine sulfate. Photochem. Photobiol. 1967, 6, 55–72. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Sinkeldam, R.W.; Marcus, P.; Uchenik, D.; Tor, Y. Multisensing emissive pyrimidine. Chem. Phys. Chem. 2011, 12, 2260–2265. [Google Scholar] [CrossRef]
- Sinkeldam, R.W.; Tor, Y. To D or not to D? On estimating the microenvironment polarity of biomolecular cavities. Org. Biomol. Chem. 2007, 5, 2523–2528. [Google Scholar] [CrossRef]
- Cullinane, N.M.; Padfield, H.J.H. Investigations in the diphenylene oxide series. Part V. J. Chem. Soc. 1935, 1131–1134. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cho, H.Y.; Woo, S.K.; Hwang, G.T. Synthesis and Photophysical Study of 2′-Deoxyuridines Labeled with Fluorene Derivatives. Molecules 2012, 17, 12061-12071. https://doi.org/10.3390/molecules171012061
Cho HY, Woo SK, Hwang GT. Synthesis and Photophysical Study of 2′-Deoxyuridines Labeled with Fluorene Derivatives. Molecules. 2012; 17(10):12061-12071. https://doi.org/10.3390/molecules171012061
Chicago/Turabian StyleCho, Hyun Yi, Sang Keun Woo, and Gil Tae Hwang. 2012. "Synthesis and Photophysical Study of 2′-Deoxyuridines Labeled with Fluorene Derivatives" Molecules 17, no. 10: 12061-12071. https://doi.org/10.3390/molecules171012061
APA StyleCho, H. Y., Woo, S. K., & Hwang, G. T. (2012). Synthesis and Photophysical Study of 2′-Deoxyuridines Labeled with Fluorene Derivatives. Molecules, 17(10), 12061-12071. https://doi.org/10.3390/molecules171012061