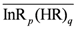Extraction Equilibrium of Indium(III) from Nitric Acid Solutions by Di(2-ethylhexyl)phosphoric Acid Dissolved in Kerosene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Equilibrium of Indium

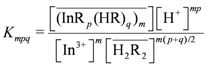

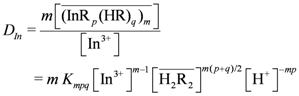
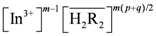 remained almost unchanged when D2EHPA was present at a low distribution ratio and its concentration in the organic phase varied insignificantly. Therefore, mp can be determined from the slope by plotting log DIn vs. pH. Figure 1 showed the impact of the pH on the distribution ratio when extracting indium(III) with various concentrations of D2EHPA from the nitric acid aqueous solutions.
remained almost unchanged when D2EHPA was present at a low distribution ratio and its concentration in the organic phase varied insignificantly. Therefore, mp can be determined from the slope by plotting log DIn vs. pH. Figure 1 showed the impact of the pH on the distribution ratio when extracting indium(III) with various concentrations of D2EHPA from the nitric acid aqueous solutions. 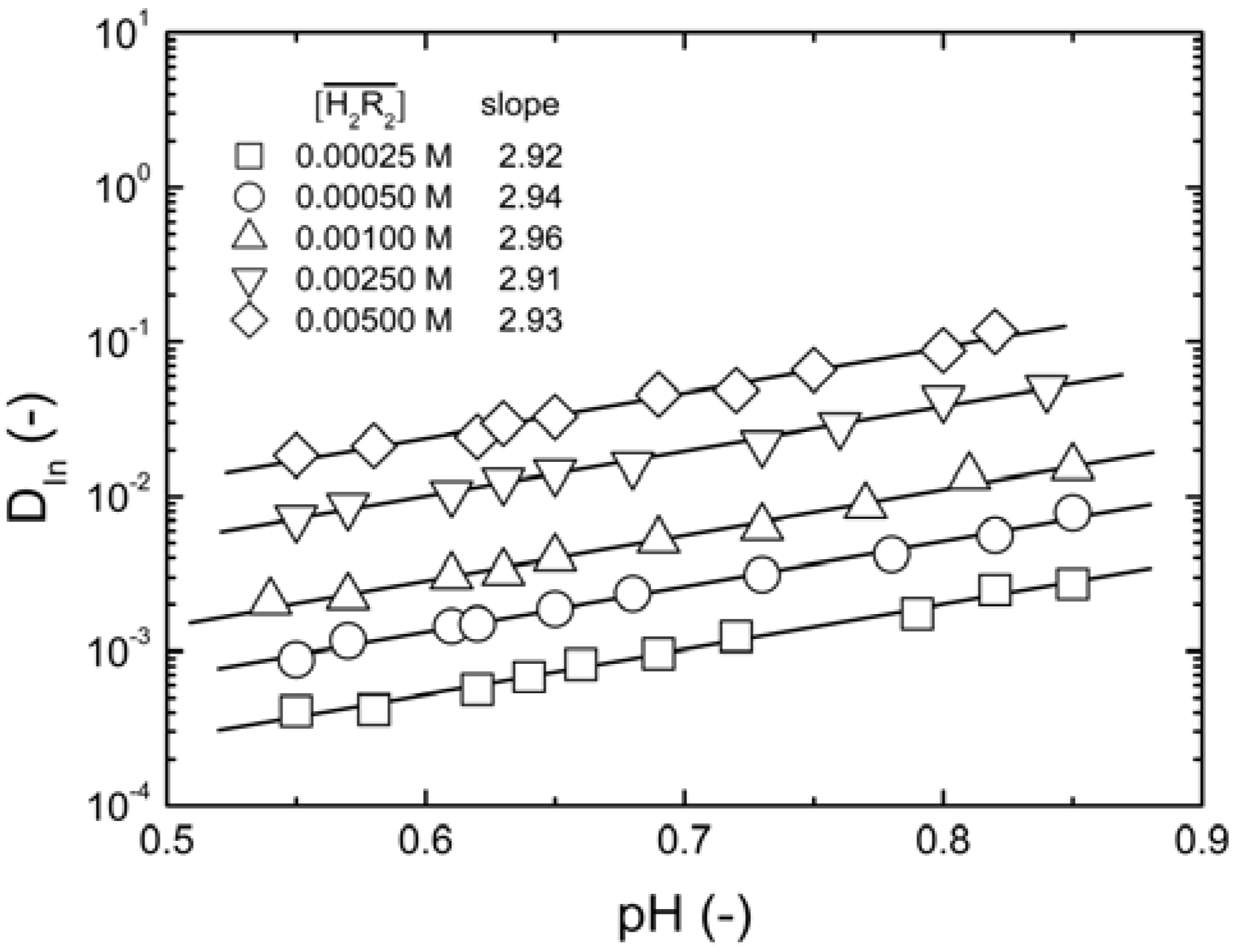

 vs.
vs. 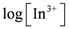 . As shown in Figure 2, the slope of the straight lines was 1, that was m = 1. Therefore, p = 3, and Equations (2) and (4) become:
. As shown in Figure 2, the slope of the straight lines was 1, that was m = 1. Therefore, p = 3, and Equations (2) and (4) become:
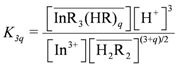

 vs.
vs.  at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. [In3+]t 0.020 to 0.15 kmol/m3.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. [In3+]t 0.020 to 0.15 kmol/m3.
 vs.
vs.  at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. [In3+]t 0.020 to 0.15 kmol/m3.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. [In3+]t 0.020 to 0.15 kmol/m3. 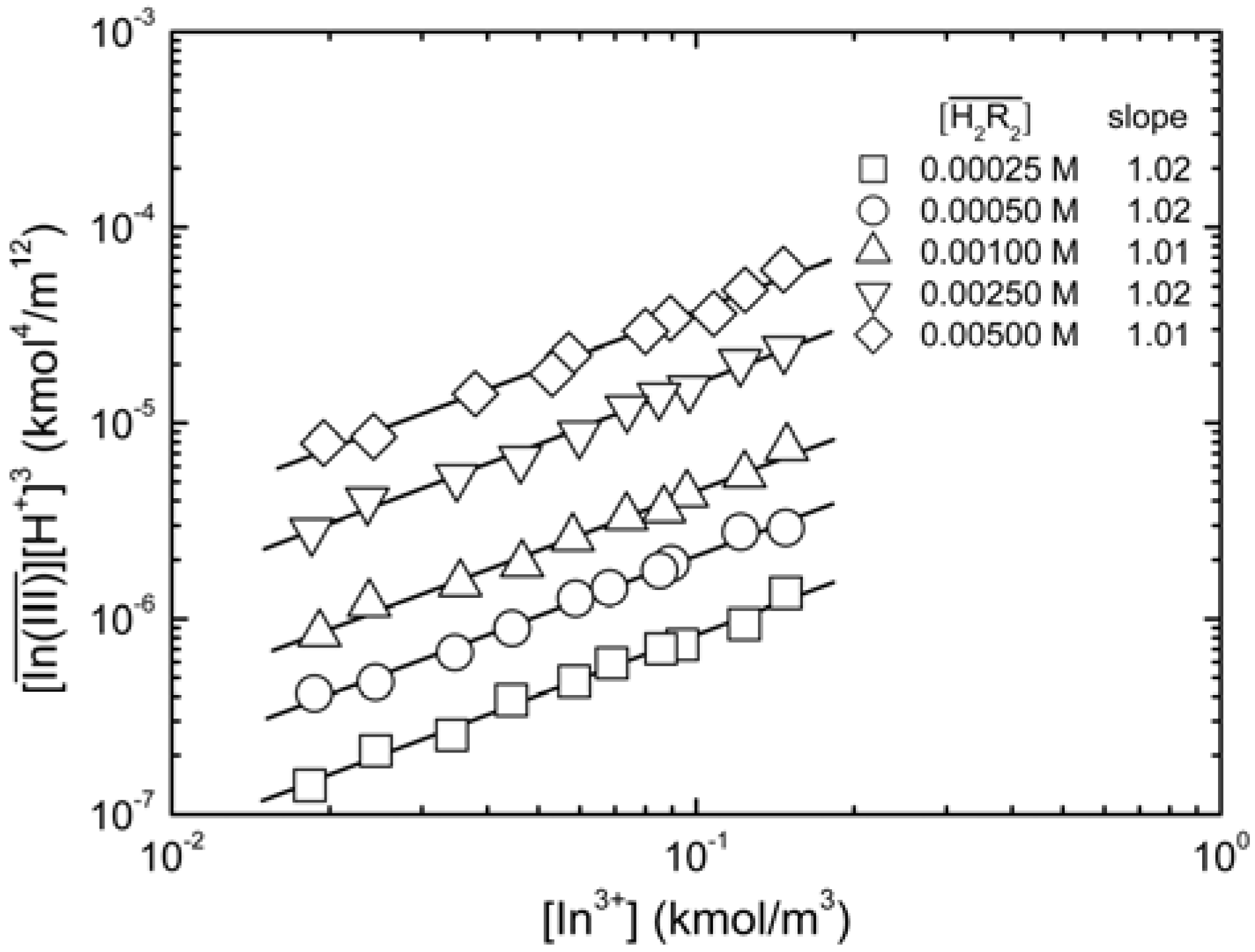
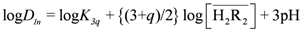
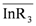 ; therefore, Equation (3) can be expressed as:
; therefore, Equation (3) can be expressed as:
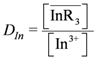
 vs.
vs.  at equilibrium with various D2EHPA concentrations in kerosene at 25 °C.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. 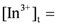 0.020 to 0.15 kmol/m3, slope = 1.62.
0.020 to 0.15 kmol/m3, slope = 1.62.
 vs.
vs.  at equilibrium with various D2EHPA concentrations in kerosene at 25 °C.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. 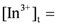 0.020 to 0.15 kmol/m3, slope = 1.62.
0.020 to 0.15 kmol/m3, slope = 1.62.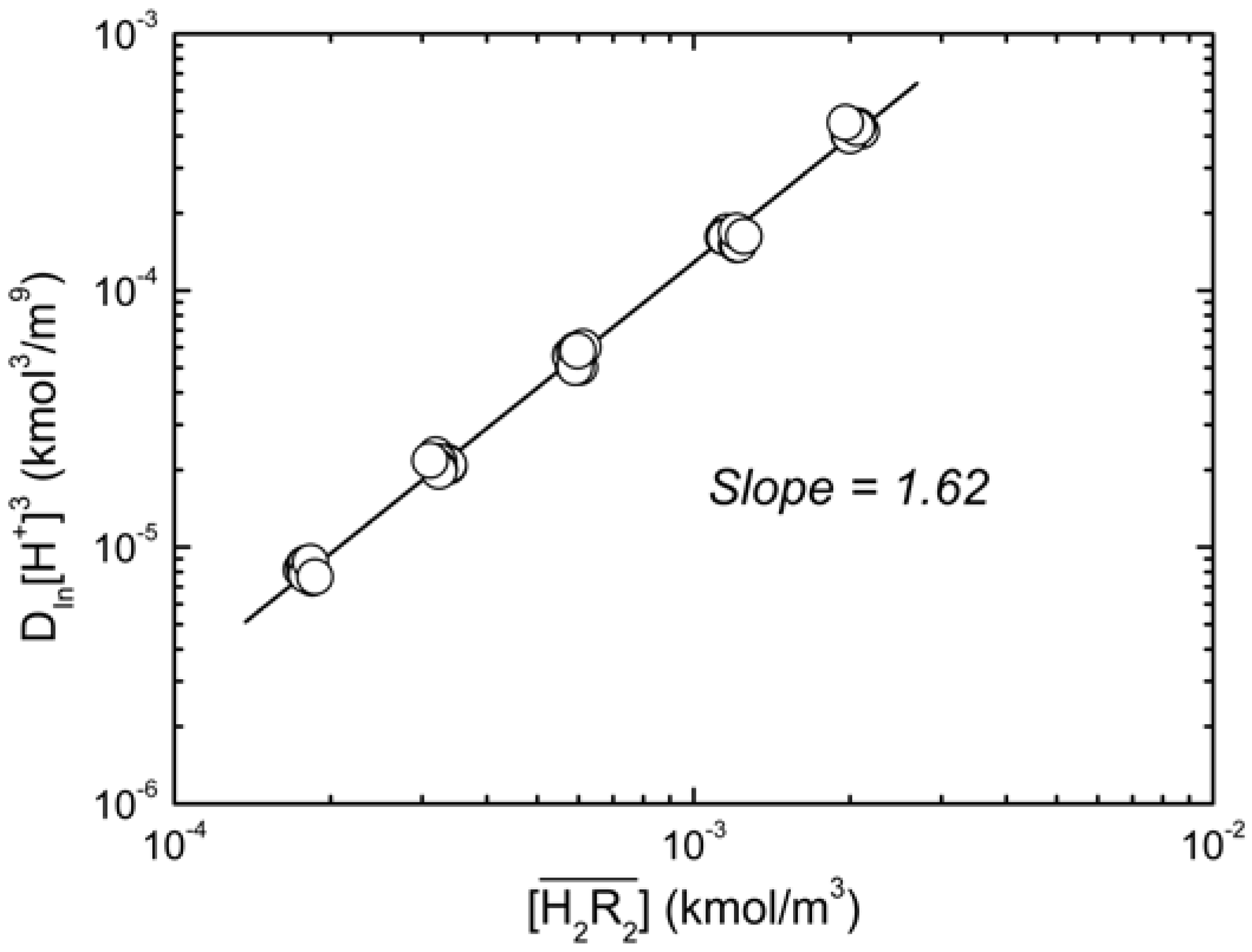

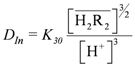
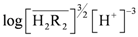 . When the slope is 1, the intercept was log K30. As shown in Figure 4, the intercept for the straight line is 0.55, that was K30 = 3.55 (kmol/m3)3/2.
. When the slope is 1, the intercept was log K30. As shown in Figure 4, the intercept for the straight line is 0.55, that was K30 = 3.55 (kmol/m3)3/2. at equilibrium with various D2EHPA concentrations in kerosene at 25 °C.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. 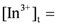 0.020 to 0.15 kmol/m3.
0.020 to 0.15 kmol/m3.
 at equilibrium with various D2EHPA concentrations in kerosene at 25 °C.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. 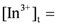 0.020 to 0.15 kmol/m3.
0.020 to 0.15 kmol/m3.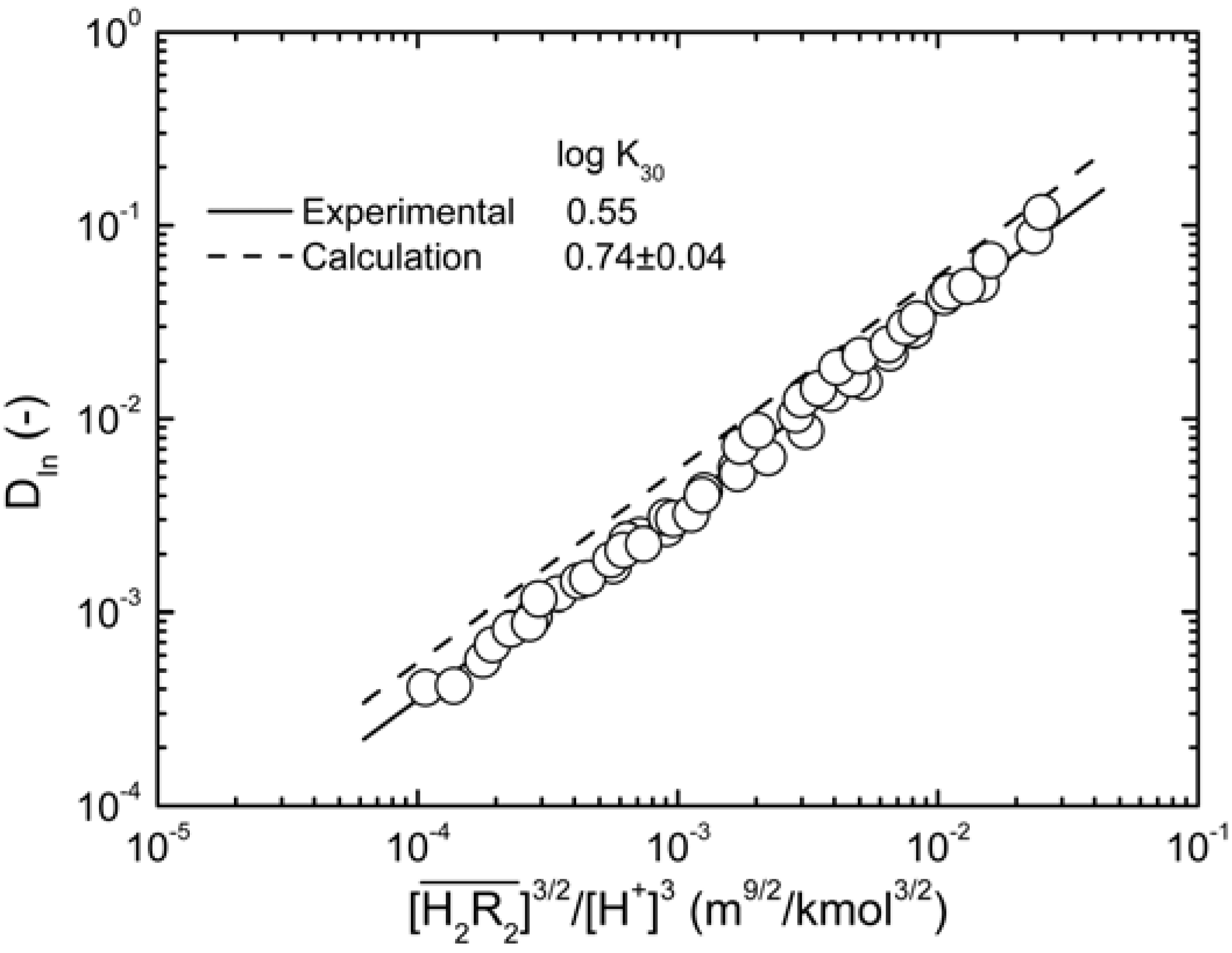
2.2. Reconfirmation of Extraction Equilibrium Formation by Computer Analysis

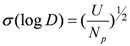
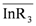 . This result was consistent with that obtained from graphical treatment, where the calculated equilibrium constant was log K30 = 0.74 ± 0.04 (the error provided corresponds to 3s(log K)). Subsequently, the distribution ratio was calculated as shown in Figure 4, and compared with the experimental value. The results demonstrate that the calculated and the experimental values were very close.
. This result was consistent with that obtained from graphical treatment, where the calculated equilibrium constant was log K30 = 0.74 ± 0.04 (the error provided corresponds to 3s(log K)). Subsequently, the distribution ratio was calculated as shown in Figure 4, and compared with the experimental value. The results demonstrate that the calculated and the experimental values were very close. is:
is:
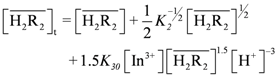
| Reaction | Constant |
|---|---|
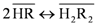 | 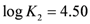 |
 |  |
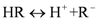 | 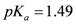 |
2.3. Complexation of Indium in Nitrate Solutions

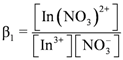
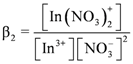
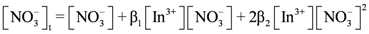
 concentration increased as the total concentration of D2EHPA increased; while the concentrations of In3+, In(NO3)2+, In(NO3)2+ decreased. Figure 6a,b show that, under the same indium ions concentrations, the higher pH of aqueous phase, the better extraction efficiency of D2EHPA for indium(III). In other words, it is easier to form
concentration increased as the total concentration of D2EHPA increased; while the concentrations of In3+, In(NO3)2+, In(NO3)2+ decreased. Figure 6a,b show that, under the same indium ions concentrations, the higher pH of aqueous phase, the better extraction efficiency of D2EHPA for indium(III). In other words, it is easier to form  in the organic phase. Figure 6b,c indicated the extraction amount of indium by D2EHPA can be increased by raising the indium ions concentration in the aqueous phase at the same pH. However, only at higher D2EHPA concentration, indium(III) can be extracted totally from the aqueous phase. Table 2 displays the different equilibrium constants used in this study and discussed in literatures.
in the organic phase. Figure 6b,c indicated the extraction amount of indium by D2EHPA can be increased by raising the indium ions concentration in the aqueous phase at the same pH. However, only at higher D2EHPA concentration, indium(III) can be extracted totally from the aqueous phase. Table 2 displays the different equilibrium constants used in this study and discussed in literatures.
2.4. Recovery Efficiency of Indium
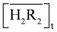 was plotted in Figure 7. As the D2EHPA concentration increased, the recovery efficiency of indium(III) increased accordingly; when
was plotted in Figure 7. As the D2EHPA concentration increased, the recovery efficiency of indium(III) increased accordingly; when 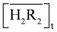 = 0.10 kmol/m3, the recovery efficiency could be as high as 99.09%.
= 0.10 kmol/m3, the recovery efficiency could be as high as 99.09%.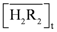 at equilibrium with various D2EHPA concentrations in kerosene at 25 °C.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. 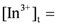 0.025 kmol/m3, pH = 0.14.
0.025 kmol/m3, pH = 0.14.
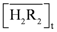 at equilibrium with various D2EHPA concentrations in kerosene at 25 °C.
at equilibrium with various D2EHPA concentrations in kerosene at 25 °C. 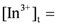 0.025 kmol/m3, pH = 0.14.
0.025 kmol/m3, pH = 0.14.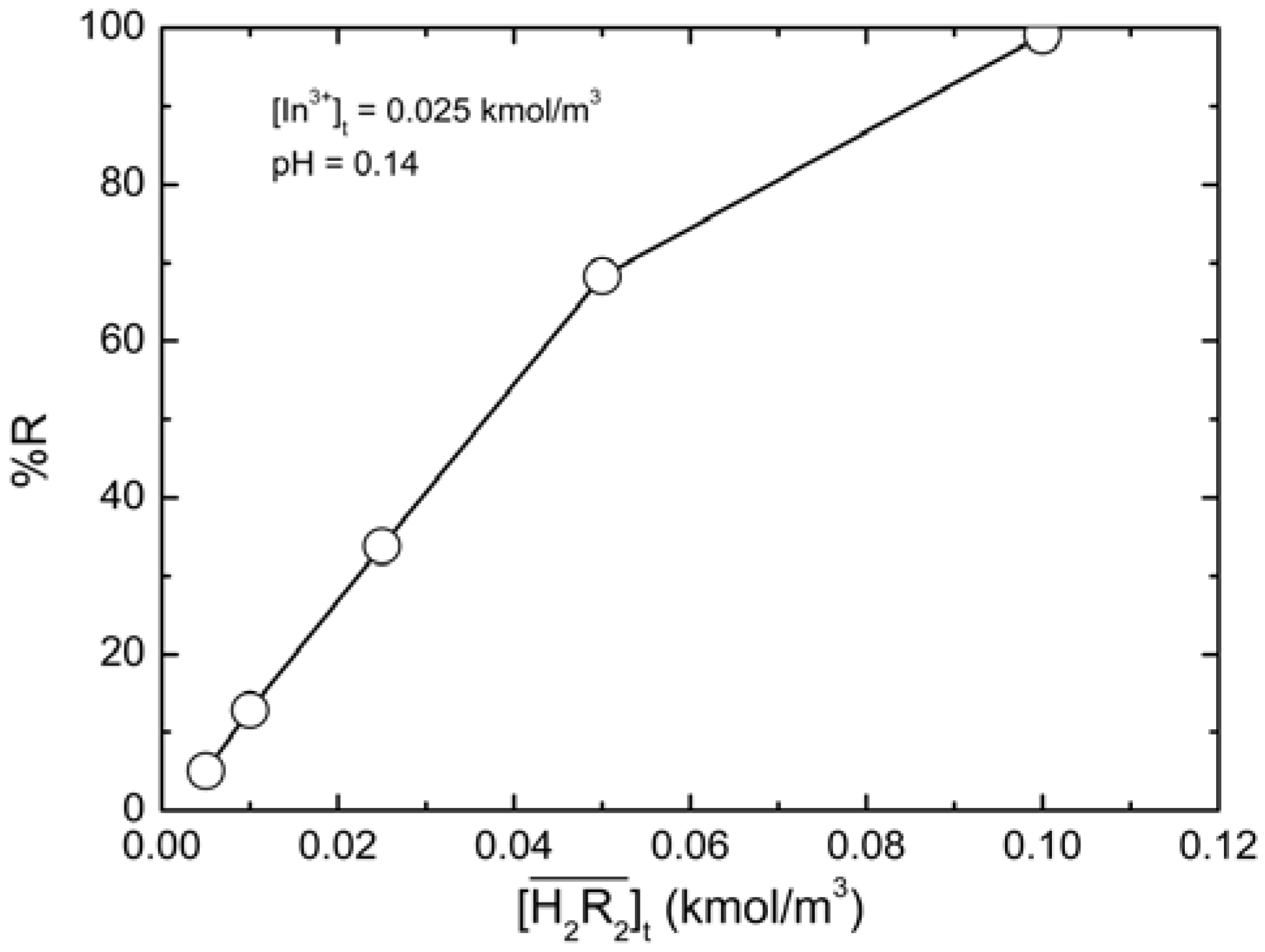
3. Experimental
3.1. Reagents and Solutions
3.2. Procedure
4. Conclusions
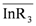 , in which the equilibrium constants of experimental and calculated values were log K30 = 0.55 and 0.74 ± 0.04, respectively. No significant effects on the extraction distribution ratio of indium with increased nitrate ions concentration were found. The
, in which the equilibrium constants of experimental and calculated values were log K30 = 0.55 and 0.74 ± 0.04, respectively. No significant effects on the extraction distribution ratio of indium with increased nitrate ions concentration were found. The  concentration in the organic phase increased as the total concentration of D2EHPA increased, while the concentrations of In3+, In(NO3)2+, In(NO3)2+ in the aqueous phase decreased. As for the metal recovery, when
concentration in the organic phase increased as the total concentration of D2EHPA increased, while the concentrations of In3+, In(NO3)2+, In(NO3)2+ in the aqueous phase decreased. As for the metal recovery, when 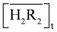 = 0.10 kmol/m3, the recovery efficiency of indium(III) could be as high as 99.09%, even when the pH of the solution was 0.14. D2EHPA had significant extraction efficiency for indium in the strong acidic solution.
= 0.10 kmol/m3, the recovery efficiency of indium(III) could be as high as 99.09%, even when the pH of the solution was 0.14. D2EHPA had significant extraction efficiency for indium in the strong acidic solution.References and Notes
- Tolcin, A.C. Mineral Commodity Summaries 2011; U.S. Geological Survey: Virginia, VA, USA, 2011; pp. 74–75. [Google Scholar]
- Huang, T.-C.; Tsai, T.-H. Extraction equilibrium of nickel(II) from sulphate solutions by di(2-ethylhexyl)phosphoric acid dissolved in kerosene. Ind. Eng. Chem. Res. 1989, 28, 1557–1562. [Google Scholar] [CrossRef]
- Huang, T.-C.; Tsai, T.-H. Extraction equilibrium of cobalt(II) from sulphate solutions by di(2-ethylhexyl)phosphoric acid dissolved in kerosene. Polyhedron 1990, 9, 1147–1153. [Google Scholar] [CrossRef]
- Guerriero, R.; Meregalli, L. Indium recovery from sulphuric solutions by supported liquid membranes. Hydrometallurgy 1988, 20, 109–120. [Google Scholar] [CrossRef]
- Gupta, B.; Mudhar, N.; Singh, I. Separations and recovery of indium and gallium using bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272). Sep. Purif. Technol. 2007, 57, 294–303. [Google Scholar] [CrossRef]
- Kondo, K.; Yamamoto, Y.; Matsumoto, M. Separation of indium(III) and gallium(III) by a supported liquid membrane containing diisostearylphosphoric acid as a carrier. J. Memb. Sci. 1997, 137, 9–15. [Google Scholar] [CrossRef]
- Lee, M.S.; Ahn, J.G.; Lee, E.C. Solvent extraction separation of indium and gallium from sulphate solutions using D2EHPA. Hydrometallurgy 2002, 63, 269–276. [Google Scholar] [CrossRef]
- Inoue, K.; Baba, Y.; Yoshizuka, K. Equilibria in the solvent extraction of indium (III) from nitric acid with acidic organophosphorus compounds. Hydrometallurgy 1988, 19, 393–399. [Google Scholar] [CrossRef]
- Naik, M.T.; Dhadke, P.M. Solvent extraction of indium(III) with bis(2-ethylhexyl) phosphinic acid in toluene. J. Chem. Eng. Jpn. 1999, 32, 366–369. [Google Scholar] [CrossRef]
- Fleitlikh, I.Y.; Pashkov, G.L.; Stoyanov, E.S.; Makarov, I.V.; Kholkin, A.I.; Nikiforova, L.K.; Grigorieva, N.A.; Pavlenko, N.I.; Kolesnichenko, G.V. Extraction of indium from sulfuric acid solutions by mixtures of di-(2-ethylhexyl)phosphoric and octanoic acids. Solvent Extr. Ion Exch. 2002, 20, 765–776. [Google Scholar] [CrossRef]
- Coello, J.; Gené, J.; Iturriaga, H. Atomic absorption determination of indium after extraction with di-(2-ethylhexyl)phosphoric acid. Mikrochim. Acta 1986, 88, 221–230. [Google Scholar] [CrossRef]
- Nishihama, S.; Hino, A.; Hirai, T.; Komasawa, I. Extraction and separation of gallium and indium from aqueous chloride solution using several organophosphorus compounds as extractants. J. Chem. Eng. Jpn. 1998, 31, 818–827. [Google Scholar] [CrossRef]
- Sato, T. The extraction of indium(III), lanthanum(III) and bismuth(III) from sulphuric acid solutions by di-(2-ethylhexyl)-phosphoric acid. J. Inorg. Nucl. Chem. 1975, 37, 1485–1488. [Google Scholar] [CrossRef]
- Sato, T.; Sato, K. Liquid-liquid extraction of indium (III) from aqueous acid solutions by acid organophosphorus compounds. Hydrometallurgy 1992, 30, 367–383. [Google Scholar] [CrossRef]
- Baes, C.F., Jr. The extraction of metallic species by dialkylphosphoric acids. J. Inorg. Nucl. Chem. 1962, 24, 707–720. [Google Scholar] [CrossRef]
- Juang, R.-S.; Su, J.-Y. Thermodynamic equilibria of the extraction of cobalt(II) from sulfate solutions with bis(2-ethylhexyl)phosphoric acid. Ind. Eng. Chem. Res. 1992, 31, 2395–2400. [Google Scholar] [CrossRef]
- Sato, T. The Extraction of thorium from sulfuric acid by di(2-ethylhexyl)phosphoric acid. J. Inorg. Nucl. Chem. 1965, 27, 1395–1403. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibra in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966; p. 437. [Google Scholar]
- Kolařik, Z.; Grimm, R. Acidic organophosphorus extractants—XXIV: The polymerization behaviour of Cu(II), Cd(II), Zn(II) and Co(II) complexes of di(2-ethylhexyl) phosphoric acid in fully loaded organic phases. J. Inorg. Nucl. Chem. 1976, 38, 1721–1727. [Google Scholar] [CrossRef]
- Liem, D.H. High-speed computers as a supplement to graphical methods. 12. application of LETAGROP to data for liquid-liquid distribution equilibria. Acta Chem. Scand. 1971, 25, 1521–1534. [Google Scholar] [CrossRef]
- Komasawa, I.; Otake, T.; Higaki, Y. Equilibrium studies of the extraction of divalent metals from nitrate media with di-(2ethylhexyl) phosphoric acid. J. Inorg. Nucl. Chem. 1981, 43, 3351–3356. [Google Scholar] [CrossRef]
- Ferguson, R.C.; Dobud, P.; Tuck, D.G. Co-ordination compounds of indium. Part VII. The stability of indium(III) nitrate complexes in aqueous solution. J. Chem. Soc. (A) 1968, 1058–1160. [Google Scholar]
- McDowell, W.J.; Perdue, P.T.; Case, G.N. Purification of di(2-ethylhexyl)phosphoric acid. J. Inorg. Nucl. Chem. 1976, 38, 2127–2129. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, H.-S.; Tsai, T.-H. Extraction Equilibrium of Indium(III) from Nitric Acid Solutions by Di(2-ethylhexyl)phosphoric Acid Dissolved in Kerosene. Molecules 2012, 17, 408-419. https://doi.org/10.3390/molecules17010408
Tsai H-S, Tsai T-H. Extraction Equilibrium of Indium(III) from Nitric Acid Solutions by Di(2-ethylhexyl)phosphoric Acid Dissolved in Kerosene. Molecules. 2012; 17(1):408-419. https://doi.org/10.3390/molecules17010408
Chicago/Turabian StyleTsai, Hung-Sheng, and Teh-Hua Tsai. 2012. "Extraction Equilibrium of Indium(III) from Nitric Acid Solutions by Di(2-ethylhexyl)phosphoric Acid Dissolved in Kerosene" Molecules 17, no. 1: 408-419. https://doi.org/10.3390/molecules17010408
APA StyleTsai, H.-S., & Tsai, T.-H. (2012). Extraction Equilibrium of Indium(III) from Nitric Acid Solutions by Di(2-ethylhexyl)phosphoric Acid Dissolved in Kerosene. Molecules, 17(1), 408-419. https://doi.org/10.3390/molecules17010408