Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-diene or 4,6-diene 20-ones and 4-Azasteroid 20-Oximes
Abstract
:1. Introduction
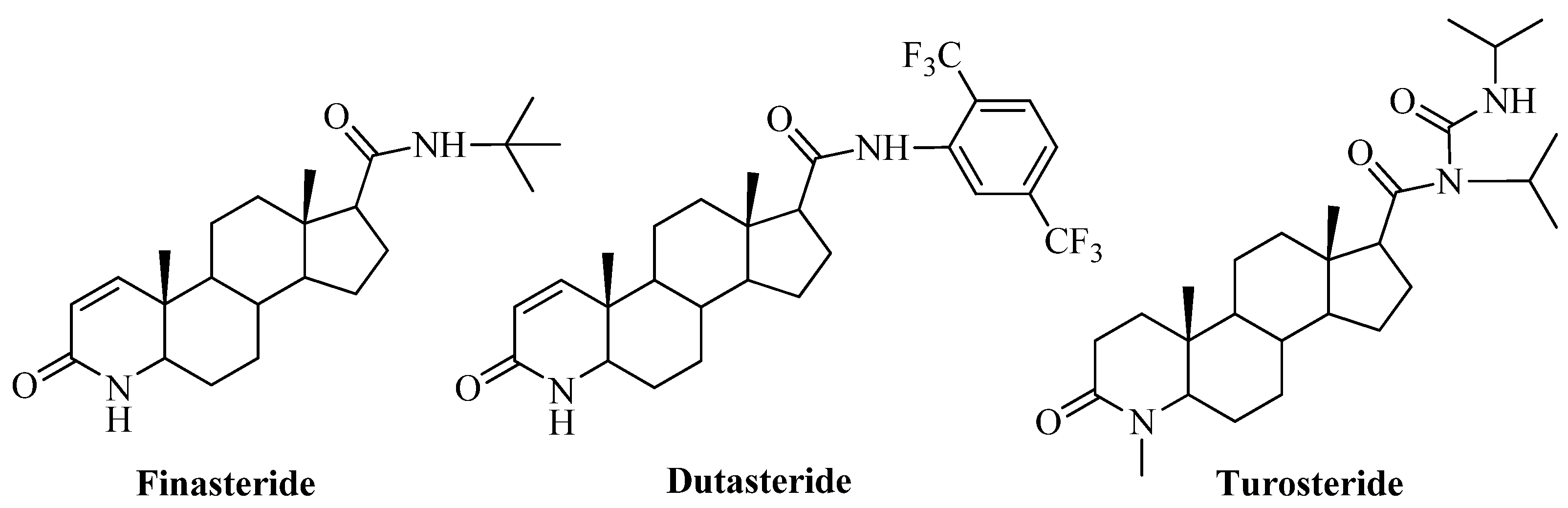
2. Results and Discussion
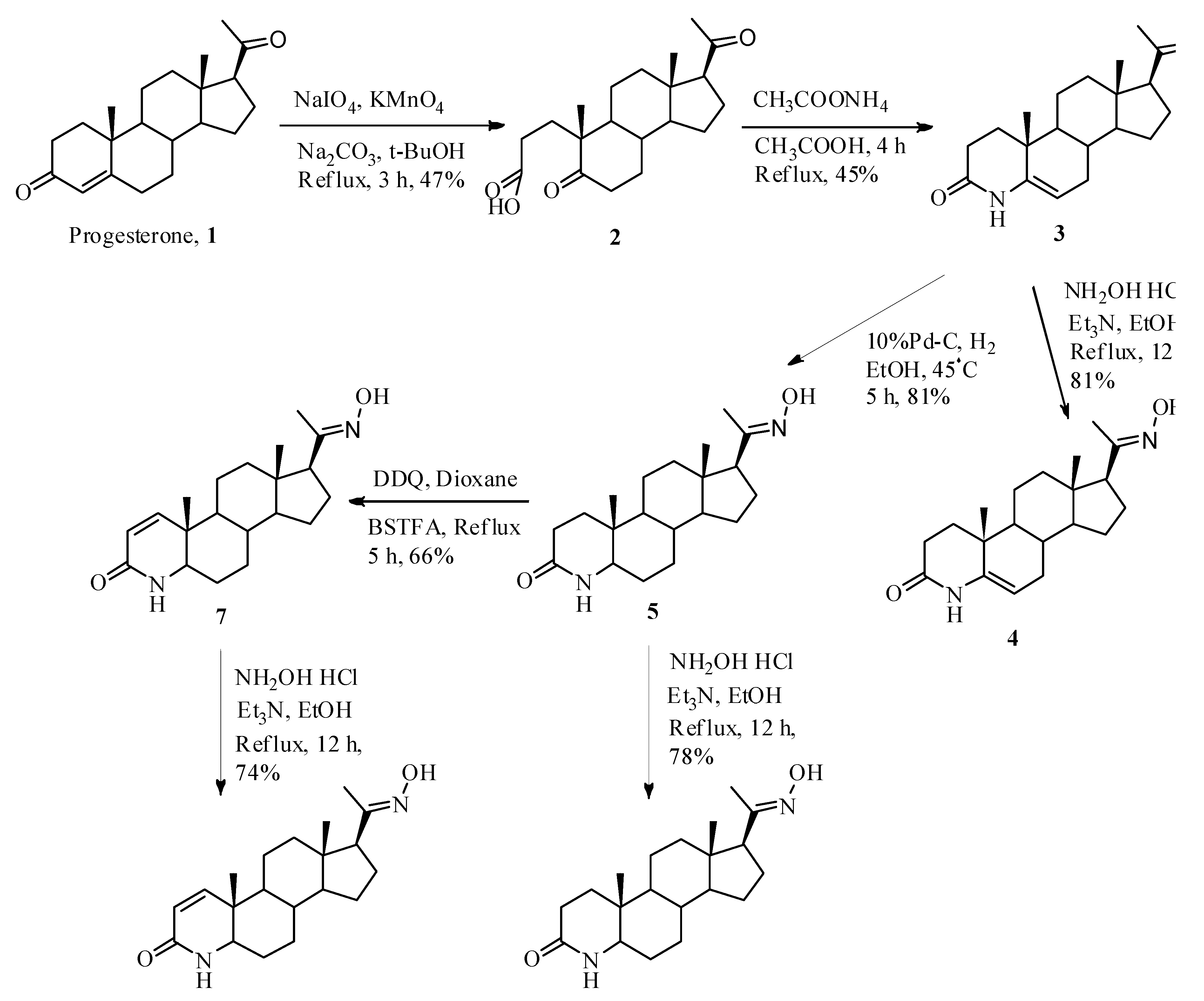
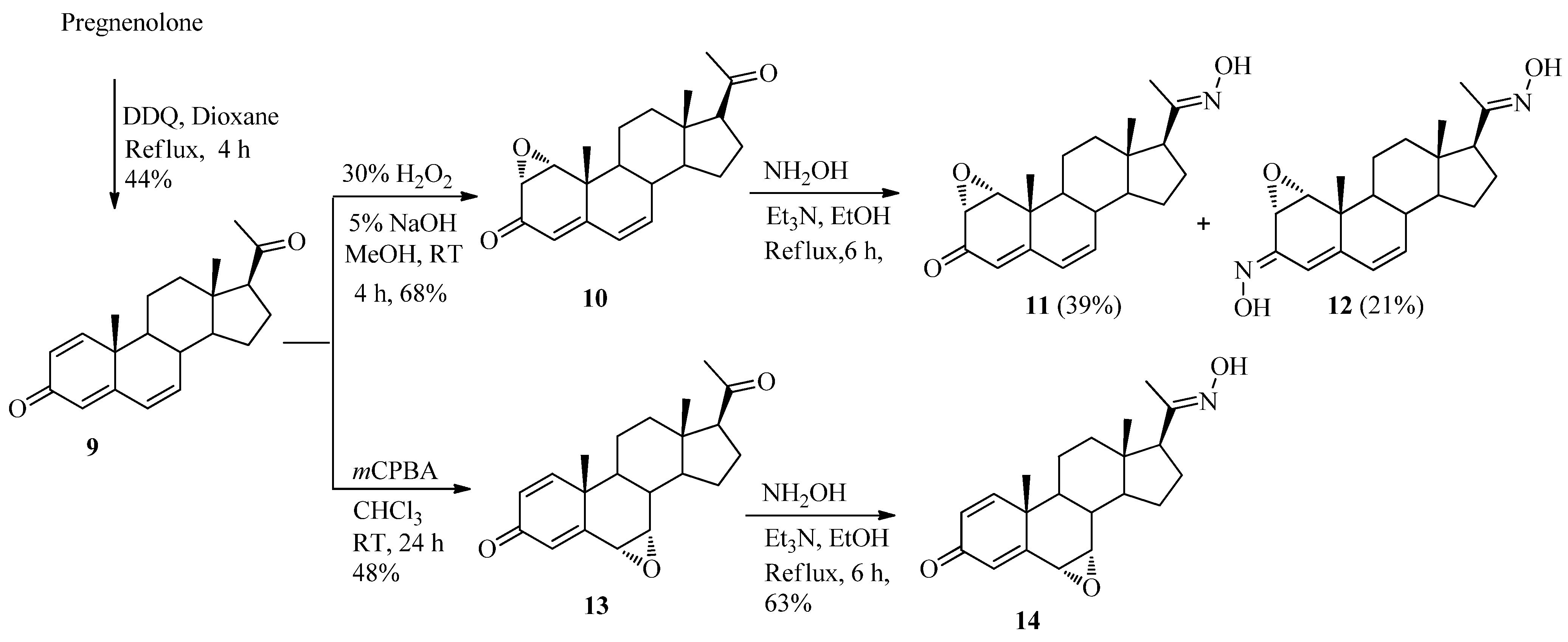
2.1. Chemistry
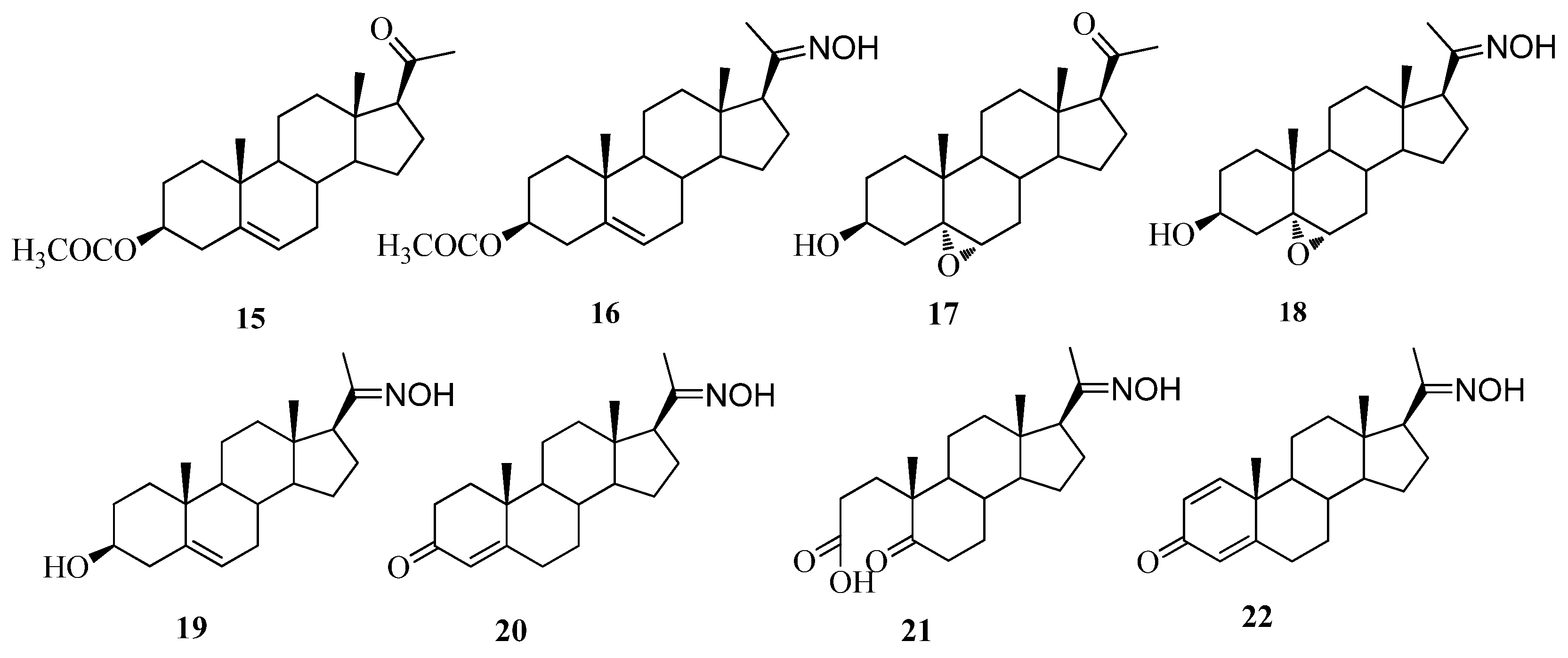
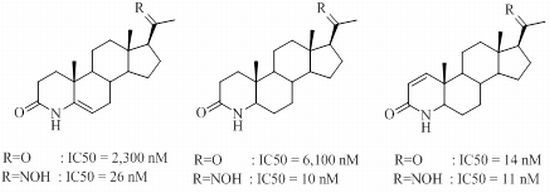
2.2. Testosterone 5α-Reductase Assay
| Compound No. | IC50 (M) | Compound No. | IC50 (M) |
|---|---|---|---|
| 1 | 6.5 × 10−9 | 13 | >1 × 10−5 |
| 2 | >1 × 10−5 | 15 | >1 × 10−5 |
| 3 | 2.3 × 10−6 | 16 | >1 × 10−5 |
| 4 | 2.6 × 10−8 | 17 | >1 × 10−5 |
| 5 | 6.1 × 10−6 | 18 | >1 × 10−5 |
| 6 | 1.0 × 10−8 | 19 | >1 × 10−5 |
| 7 | 1.4 × 10−8 | 20 | 1.4 × 10−8 |
| 8 | 1.1 × 10−8 | 21 | 2.1 × 10−6 |
| 9 | 7.6 × 10−7 | 22 | 1.4 × 10−7 |
| 10 | >1 × 10−5 | Finasteride | 1.2 × 10−9 |
3. Experimental
3.1. General Methods
3.2. Synthesis
3.2.1. 5,20-Dioxo-A-nor-3,5-secopregnane-3-oic acid (2)
3.2.2. 4-Aza-5-pregnene-3,20-dione (3)
3.2.3. 4-Aza-5-pregnene-3,20-dione-20-oxime (4)
3.2.4. 4-Aza-pregnane-3,20-dione (5)
3.2.5. 4-Aza-pregnane-3,20-dione-20-oxime (6)
3.2.6. 4-Aza-1-pregnene-3,20-dione (7)
3.2.7. 4-Aza-1-pregnene-3,20-dione-20-oxime (8)
3.2.8. 1,4,6-Pregnatriene-3,20-dione (9)
3.2.9. 1α,2α-Epoxypregna-4,6-diene-3,20-dione (10)
3.2.10. 1α,2α-Epoxypregna-4,6-diene-3,20-dione-20-oxime (11) and 1α,2α-epoxypregna-4,6-diene-3,20-dioxime (12)
3.2.11. 6α,7α-Epoxypregna-1,4-diene-3,20-dione (13)
3.2.12. 6α,7α-Epoxypregna-1,4-diene-3,20-dione-20-oxime (14)
3.3. Testosterone 5α-Reductase Assay
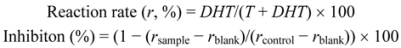
4. Conclusions
References and Notes
- Bruchovsky, N.; Wilson, J.D. The conversion of testosterone to 5-androstan-17-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 1968, 242, 2012–2021. [Google Scholar]
- Weisser, H.; Kreig, M. Kinetic analysis of androstenedione 5α-reductase in epithelium and stroma of human prostate. Steroids 1997, 62, 589–594. [Google Scholar] [CrossRef]
- Weisser, H.; Kreig, M. In vitro inhibition of androstenedione 5α-reduction by finasteride in epithelium and stroma of human benign prostatic hyperplasia. J. Steroid Biochem. Mol. Biol. 1998, 67, 49–55. [Google Scholar] [CrossRef]
- Abul-Hajj, Y.J. Stereospecificity of hydrogen transfer from NADPH by steroid △4-5α-and △4-5β-reductase. Steroids 1972, 20, 215–222. [Google Scholar] [CrossRef]
- Cabeza, M.; Flores, E.; Heuze, I.; Sanchez, M.; Bratoeff, E.; Ramirez, E.; Francolugo, V.A. Novel 17-substituted pregnadiene derivatives as 5α-reductase inhibitors and their binding affinity for the androgen receptor. Chem. Pharm. Bull. 2004, 52, 535–539. [Google Scholar] [CrossRef]
- Andersson, S.R.; Bishop, R.W.; Russell, D.W. Expression cloning and regulation of steroid 5α–reductase, an enzyme essential for male sexual differentiation. J. Biol. Chem. 1989, 264, 16249–16255. [Google Scholar]
- Andersson, S.; Russell, D.W. Structural and biochemical properties of cloned expressed human and rat steroid 5α-reductase. Proc. Natl. Acad. Sci. USA 1990, 87, 3640–3544. [Google Scholar] [CrossRef]
- Russell, D.W.; Wilson, J.D. Steroid 5alpha-reductase: Two genes/two enzymes. Ann. Rev. Biochem. 1994, 63, 25–61. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Silver, R.I.; Guileyardo, J.M.; Casey, M.; McConnel, J.D.; Russell, D.W. Tissue distribution and ontogeny of steroid 5alpha-reductase isozyme expression. J. Clin. Invest. 1993, 92, 903–910. [Google Scholar] [CrossRef]
- Hartmann, R.W.; Hector, M.; Haidar, S.; Ehmer, P.B.; Reichert, W.; Jose, J. Synthesis and evaluation of novel steroidal oxime inhibitors of P450 17 (17α-hydroxylase/C17,20-lyase) and 5α-reductase types 1 and 2. J. Med. Chem. 2000, 43, 4266–4277. [Google Scholar] [CrossRef]
- Ramírez, E.; Cabeza, M.; Heuze, I.; Gutierrez, E.; Bratoeff, E.; Membrillo, M.; Lira, A. Synthesis and pharmacological evaluation of new 16-methyl pregnenolone derivatives. Chem. Pharm. Bull. 2002, 50, 15–20. [Google Scholar] [CrossRef]
- Flores, E.; Bratoeff, E.; Cabeza, M.; Ramirez, E.; Quiroz, A.; Heuze, I. Steroid 5-reductase inhibitors. Mini Rev. Med. Chem. 2003, 3, 225–237. [Google Scholar] [CrossRef]
- Audia, J.E.; Lawhorn, D.E.; Deeter, J.B. Synthesis of the individual enantiomers of the benzoquinolinone human type 1 steroid 5α-reductase inhibitors LY191704 and LY266111. Tetrahedron Lett. 1993, 34, 7001–7004. [Google Scholar] [CrossRef]
- Jones, C.D.; Audia, J.E.; Lawhorn, D.E.; McQuaid, L.A.; Neubauer, B.L.; Pike, A.J.; Pennington, P.A.; Stamm, N.; Toomey, R.E.; Hirsch, K.S. Nonsteroidal inhibitors of human type I steroid 5α-reductase. J. Med. Chem. 1993, 36, 421–423. [Google Scholar] [CrossRef]
- Kurup, A.; Garg, R.; Hansch, C. Comparative QSAR analysis of 5α-reductase inhibitors. Chem. Rev. 2000, 100, 909–924. [Google Scholar]
- Lazier, C.B.; Thomas, L.N.; Douglas, R.C.; Vessey, J.P.; Rittmaster, R.S. Dutasteride, the dual 5α-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate 2004, 58, 130–144. [Google Scholar] [CrossRef]
- Sudduth, S.L.; Koronkowski, M.J. Finasteride, The first 5 alpha-reductase inhibitor. Pharmacotherapy 1993, 309–325. [Google Scholar] [Green Version]
- Guarna, A.; Belle, C.; Machetti, F.; Occhiato, E.G.; Payne, H.P.; Cassiani, A.; Danza, G.; De Bellis, A.; Dini, S.; Marrucci, A.; Serio, M. 19-Nor-10-azasterides: A novel class of inhibitors for human steroid 5α-reductase 1 and 2. J. Med. Chem. 1997, 40, 1112–1129. [Google Scholar] [CrossRef]
- Scarpi, D.; Occhiato, E.G.; Danza, G.; Sero, M.; Guarna, A. Synthesis of 17β-N-substituted 19-nor-10-aza steroids as inhibitors of human 5α-reductases I and II. Bioorg. Med. Chem. 2002, 10, 3455–3461. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Kollár, L. Transition-mediated-catalyzed reactions in steroid synthesis. Chem. Rev. 2003, 103, 4095–4129. [Google Scholar] [CrossRef]
- Holt, D.A.; Levy, M.A.; Oh, H.J.; Erb, J.M.; Heaslip, J.I.; Brandt, M.; Lan-Hargest, H.Y.; Metcalf, B.W. Inhibition of steroid 5 alpha-reductase by unsaturated 3-carboxysteroids. J. Med. Chem. 1990, 33, 943–950. [Google Scholar] [CrossRef]
- Victor, P.O.; Cabeza, M.; Bratoeff, E.; Heuze, I.; Sanchez, M.; Ramirez, E.; Elia, N.R. New 5α-reductase inhibitors: In vitro and in vivo effects. Steroids 2005, 70, 217–224. [Google Scholar] [CrossRef]
- Levina, I.S,; Pokrovskaya, E.V.; Kulikova, L.E.; Kmernitzky, A.V.; Kachala, V.V.; Smirnov, A.N. 3- and 19-Oximes of 16α,17α-cyclohexanoprogesterone derivatives: Synthesis and interactions with progesterone receptor and other proteins. Steroids 2008, 75, 815–827. [Google Scholar]
- Ling, Y.; Li, J.; Liu, Y.; Wang, X.; Klus, G.T.; Marat, K.; Nnane, I.P.; Brodie, A.M.H. Synthesis and in vivo acting of some epimeric 20α-hydroxy, 20-oxime and aziridine pregnene derivatives as inhibitors of human 17α-hydroxylase/C17,20-lyase and 5α-reductase. Bioorg. Med. Chem. 1998, 6, 1683–1693. [Google Scholar] [CrossRef]
- Li, X.; Singh, S.M.; Labrie, I. Synthesis and in vitro activity of 17beta-(N-alkyl/arylformamido)-and 17beta-[N-alkyl/aryl)alkyl/arylamido]-4-methyl-4-aza-3-oxo-5alpha-androstan-3-ones as inhibitors of human 5alpha-reductases and antagonists of the androgen receptor. J. Med. Chem. 1995, 38, 1158–1173. [Google Scholar] [CrossRef]
- Kim, E.; Ma, E. Epoxidation and reduction of DHEA, 1,4,6-androstatriene-3-one and 4,6-androstadien-3β,17β-diol. Molecules 2005, 10, 572–582. [Google Scholar] [CrossRef]
- Pouzar, V.; Cerny, I. Preparation and properties of 3-O-(3-carboxyethyl)oxime derivatives of steroid hormones. Steroids 1996, 61, 89–93. [Google Scholar] [CrossRef]
- Szentesi, A.; Gergely, P.; Horvath, P.; Maho, S.; Matyus, P.; Szasz, G. Determination of circular dichroism and ultraviolet spectral parameters of norgestimate- and other △4-3-ketosteroid oxime isomers via normal phase HPLC method. Curr. Med. Chem. 2001, 8, 1341–1347. [Google Scholar]
- Kim, S.; Ma, E. Synthesis of pregnane derivatives, their cytotoxicity on LNCap and PC-3 cells, and screening on 5α-reductase inhibitory activity. Molecules 2009, 14, 4655–4668. [Google Scholar] [CrossRef]
- Brandt, M.; Greway, A.T.; Holt, D.A.; Metcalf, B.W.; Levy, M. Studies on the mechanism of steroid 5α-reductase inhibition by 3-carboxy A-ring aryl steroids. J. Steroid Biochem. Mol. Biol. 1990, 37, 575–579. [Google Scholar] [CrossRef]
- Holt, D.A.; Levy, M.A.; Ladd, D.L.; Oh, H.J.; Erb, J.M.; Heaslip, J.I.; Brandt, M.; Metcalf, B.W. Steroidal A ring aryl carboxylic acids: A new class of steroid 5α-reductase inhibitors. J. Med. Chem. 1990, 33, 937–942. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.Y.; Son, H.K.; Song, H.K.; Ha, J.; Lee, S.S.; Lee, S.K. Testosterone 5α-reductase inhibitors, menaquinone 7 produced by a Bacillus and phenazine methosulfate. Biol. Pharm. Bull. 1999, 22, 1396–1399. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, S.; Kim, Y.-u.; Ma, E. Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-diene or 4,6-diene 20-ones and 4-Azasteroid 20-Oximes. Molecules 2012, 17, 355-368. https://doi.org/10.3390/molecules17010355
Kim S, Kim Y-u, Ma E. Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-diene or 4,6-diene 20-ones and 4-Azasteroid 20-Oximes. Molecules. 2012; 17(1):355-368. https://doi.org/10.3390/molecules17010355
Chicago/Turabian StyleKim, Sujeong, Yong-ung Kim, and Eunsook Ma. 2012. "Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-diene or 4,6-diene 20-ones and 4-Azasteroid 20-Oximes" Molecules 17, no. 1: 355-368. https://doi.org/10.3390/molecules17010355
APA StyleKim, S., Kim, Y.-u., & Ma, E. (2012). Synthesis and 5α-Reductase Inhibitory Activity of C21 Steroids Having 1,4-diene or 4,6-diene 20-ones and 4-Azasteroid 20-Oximes. Molecules, 17(1), 355-368. https://doi.org/10.3390/molecules17010355