Isolation and Purification of Oridonin from the Whole Plant of Isodon rubescens by High-Speed Counter-Current Chromatography
Abstract
:1. Introduction
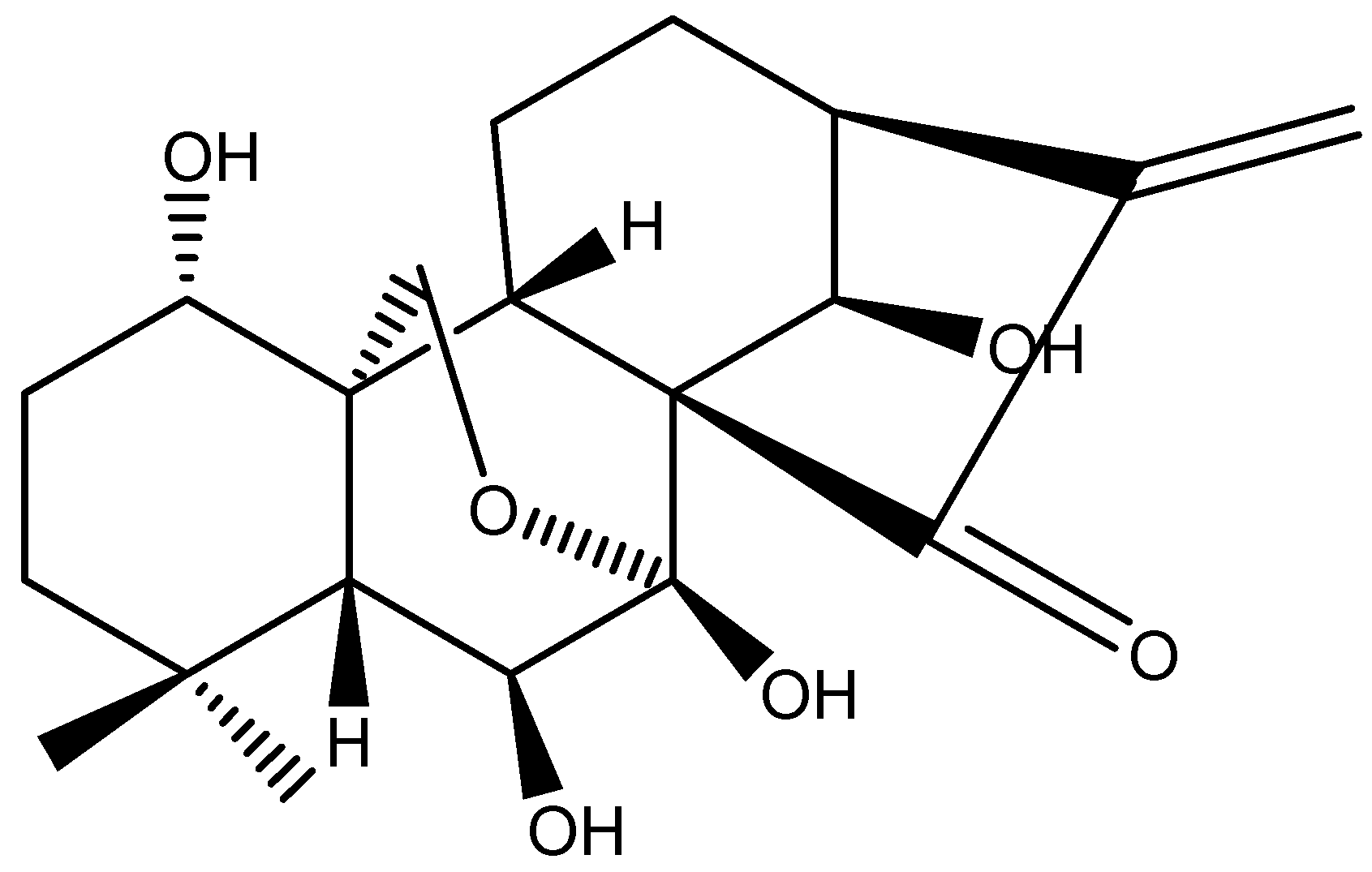
2. Results and Discussion
2.1. Optimization of HPLC Conditions
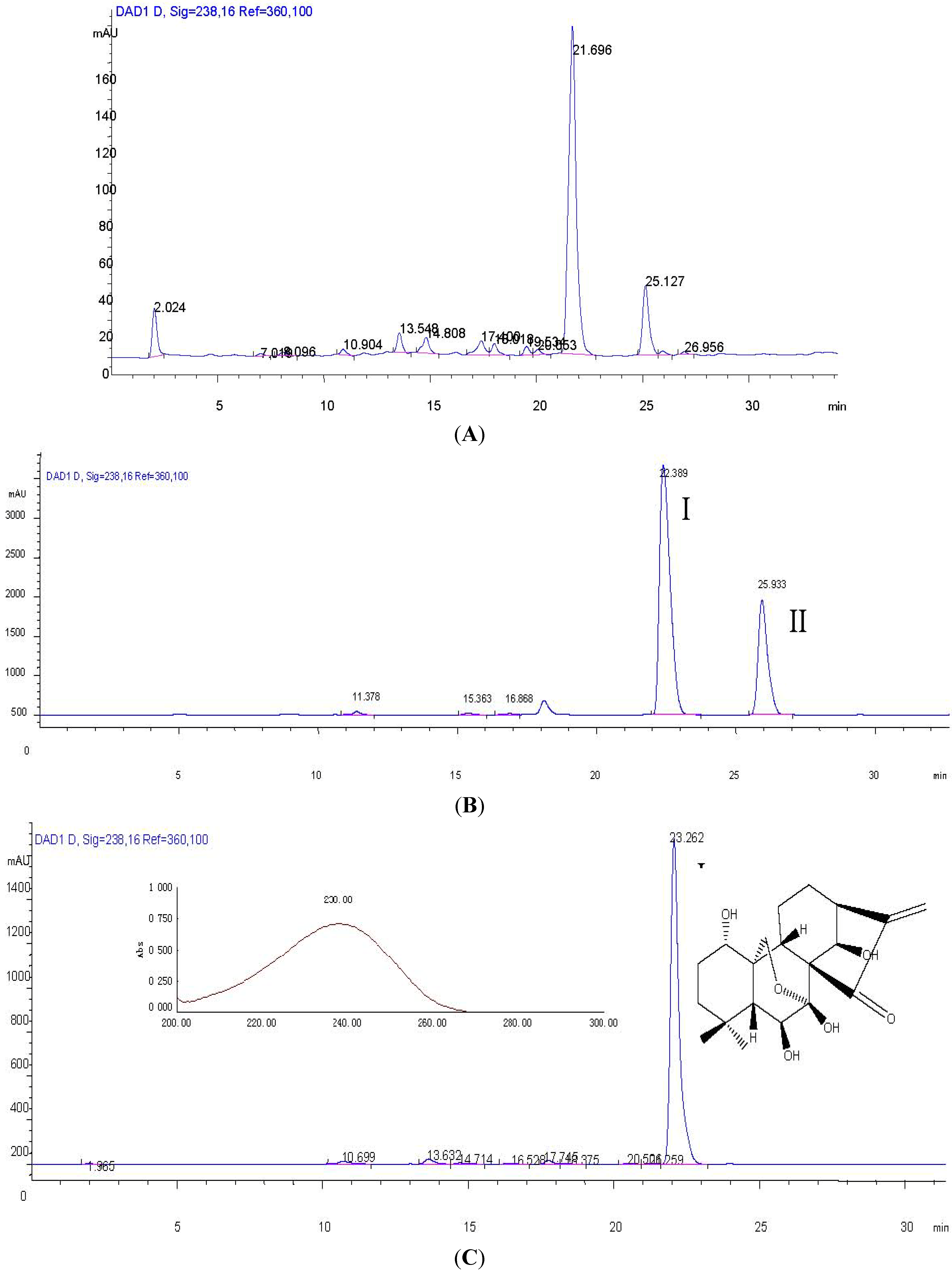
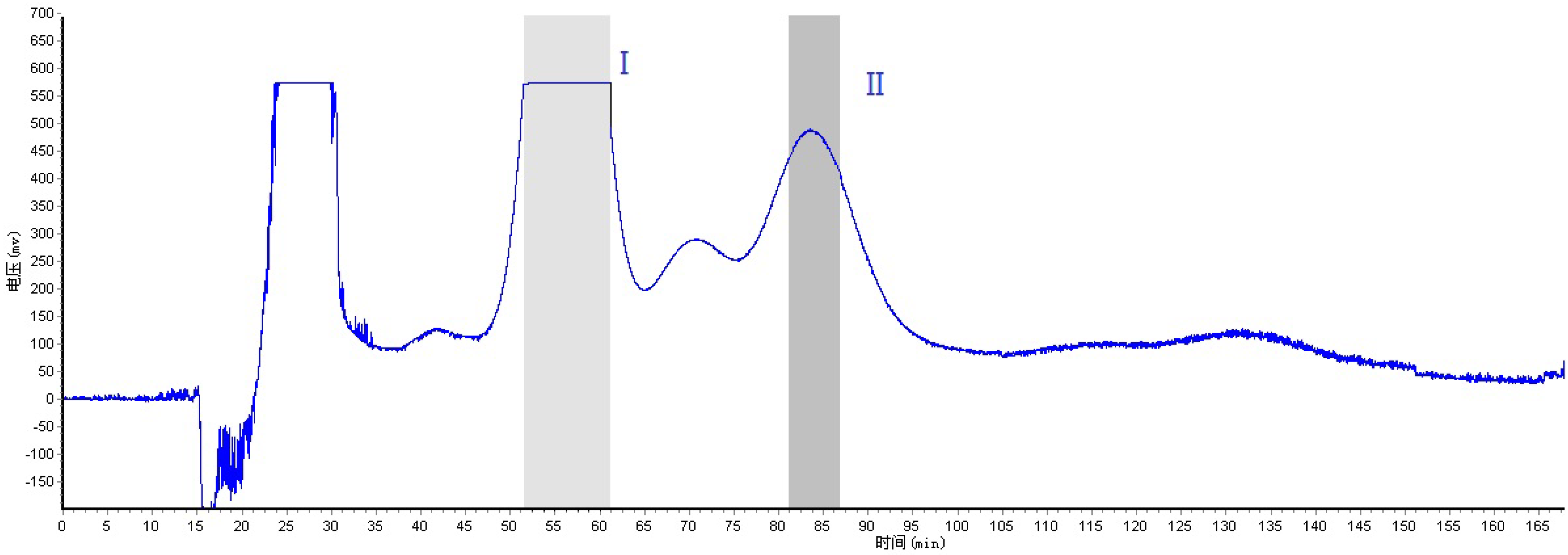
2.2. Optimization of HSCCC Conditions
| Solvent system | K-Value | |
|---|---|---|
| I | II | |
| n-hexane-ethyl acetate-methanol-water (1:5;1:5) | 2.95 | 2.53 |
| n-hexane-ethyl acetate-methanol-water (2.5:5;2.5:5) | 1.02 | 0.53 |
| n-hexane-ethyl acetate-methanol-water (2.8:5;2.8:5) | 0.79 | 0.48 |
| n-hexane-ethyl acetate-methanol-water (3.5:5;3.5:5) | 0.52 | 0.28 |
3. Experimental
3.1. Apparatus
3.2. Reagents and Materials
3.3. Preparation of the Sample
3.4. Selection of the Two-Phase-Solvent Systems
3.5. Preparation of Two-Phase Solvent System and Sample Solution
3.6. HSCCC Separation Procedure
3.7. HPLC Analysis and Identification of HSCCC Peak Fractions
3.8. Structure Identification
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Sartippour, M.R.; Seeram, N.P.; Heber, D.; Hardy, M.; Norris, A.; Lu, Q.; Zhang, L.; Lu, M.; Rao, J.Y.; Brooks, M.N. Rabddosia rubescens inhibits breast cancer growth and angiogenesis. Int. J. Oncol. 2005, 26, 121–129. [Google Scholar]
- China Pharmacopoeia Committee, Pharmacopoeia of the People’s Republic of China, 1st ed; China Chemical Industry Press: Beijing, China, 2010; pp. 106–107.
- Sun, H.; Huang, S.; Han, Q. Diterpenoids from isodon species and their bioactivity. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef]
- Liu, L.M.; Chen, L.; Wang, R.H.; Wu, P. Studies on chemical constituents of Qinpi. Zhong Cao Yao 2001, 32, 1073–1074. [Google Scholar]
- Liu, L.M.; Wang, R.H.; Chen, L.; Wu, P.; Wang, L. Study on chemical constituents of bark of Fraxinus rhynchophylla. Zhong Cao Yao 2003, 34, 889–890. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar]
- Zhao, H.Y.; Jiang, J.G. Application of chromatography technology in the separation of active components from nature derived drugs. Mini Rev. Med. Chem. 2010, 10, 1223–1234. [Google Scholar] [CrossRef]
- Oka, F.; Oka, H.; Ito, Y. Systematic search for suitable two-phase solvent systems for high-speed countercurrent chromatography. J. Chromatogr. 1991, 538, 99–108. [Google Scholar] [CrossRef]
- Fujita, E. Terpenoids, part XV. Structure and absolute configuration of of oridonin isolated from Isodon japonicus and Isodon trichocarpus. J. Chem. Soc. (C) 1970, 21, 1674–1677. [Google Scholar]
- Sun, H.D.; Lin, Z.W.; Qin, C.Q.; Chao, J.H.; Zhao, Q.Z. Studies on the chemical constituents of antitumor plant Rabdosia rubescens (Hemsl.) Hara. Yunnan Zhi Wu Yan Jiu 1981, 3, 95–100. [Google Scholar]
- Sample Availability: Samples of the compound are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, F.; Bai, Y.; Wang, J.; Wei, J.; Yu, C.; Li, S.; Yang, W.; Han, C. Isolation and Purification of Oridonin from the Whole Plant of Isodon rubescens by High-Speed Counter-Current Chromatography. Molecules 2011, 16, 7949-7957. https://doi.org/10.3390/molecules16097949
He F, Bai Y, Wang J, Wei J, Yu C, Li S, Yang W, Han C. Isolation and Purification of Oridonin from the Whole Plant of Isodon rubescens by High-Speed Counter-Current Chromatography. Molecules. 2011; 16(9):7949-7957. https://doi.org/10.3390/molecules16097949
Chicago/Turabian StyleHe, Fa, Yuhua Bai, Jing Wang, Jing Wei, ChunYue Yu, Sen Li, Weili Yang, and Chenghua Han. 2011. "Isolation and Purification of Oridonin from the Whole Plant of Isodon rubescens by High-Speed Counter-Current Chromatography" Molecules 16, no. 9: 7949-7957. https://doi.org/10.3390/molecules16097949
APA StyleHe, F., Bai, Y., Wang, J., Wei, J., Yu, C., Li, S., Yang, W., & Han, C. (2011). Isolation and Purification of Oridonin from the Whole Plant of Isodon rubescens by High-Speed Counter-Current Chromatography. Molecules, 16(9), 7949-7957. https://doi.org/10.3390/molecules16097949




