Studies on the Electrochemical Behavior of Thiazolidine and Its Applications Using a Flow–Through Chronoamperometric Sensor Based on a Gold Electrode
Abstract
:1. Introduction
2. Results and Discussion
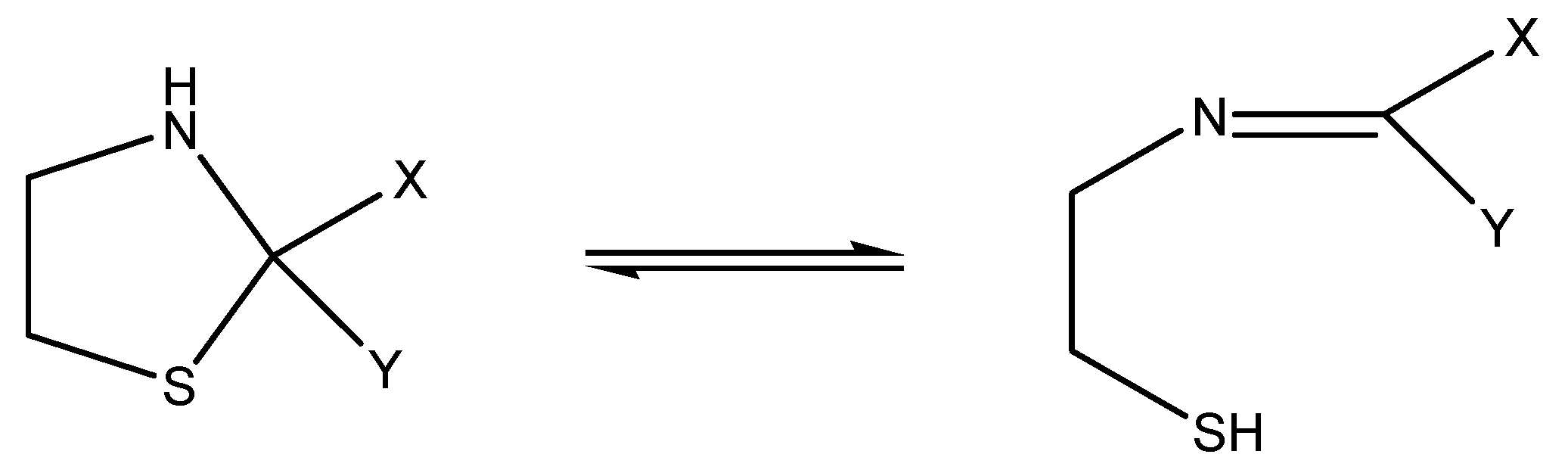
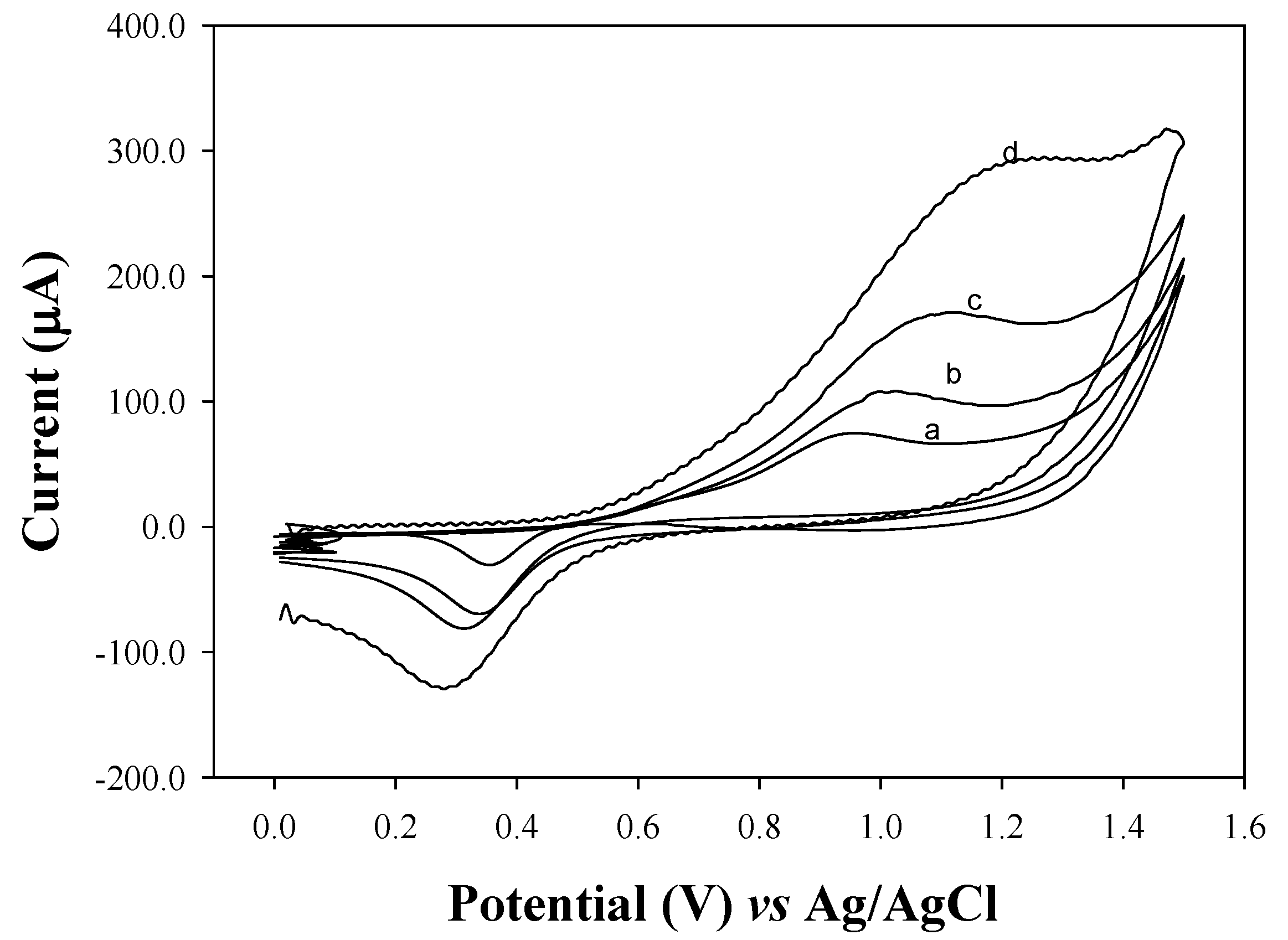
2.1. Electrochemical Behavior of Thiazolidine at an Au Electrode
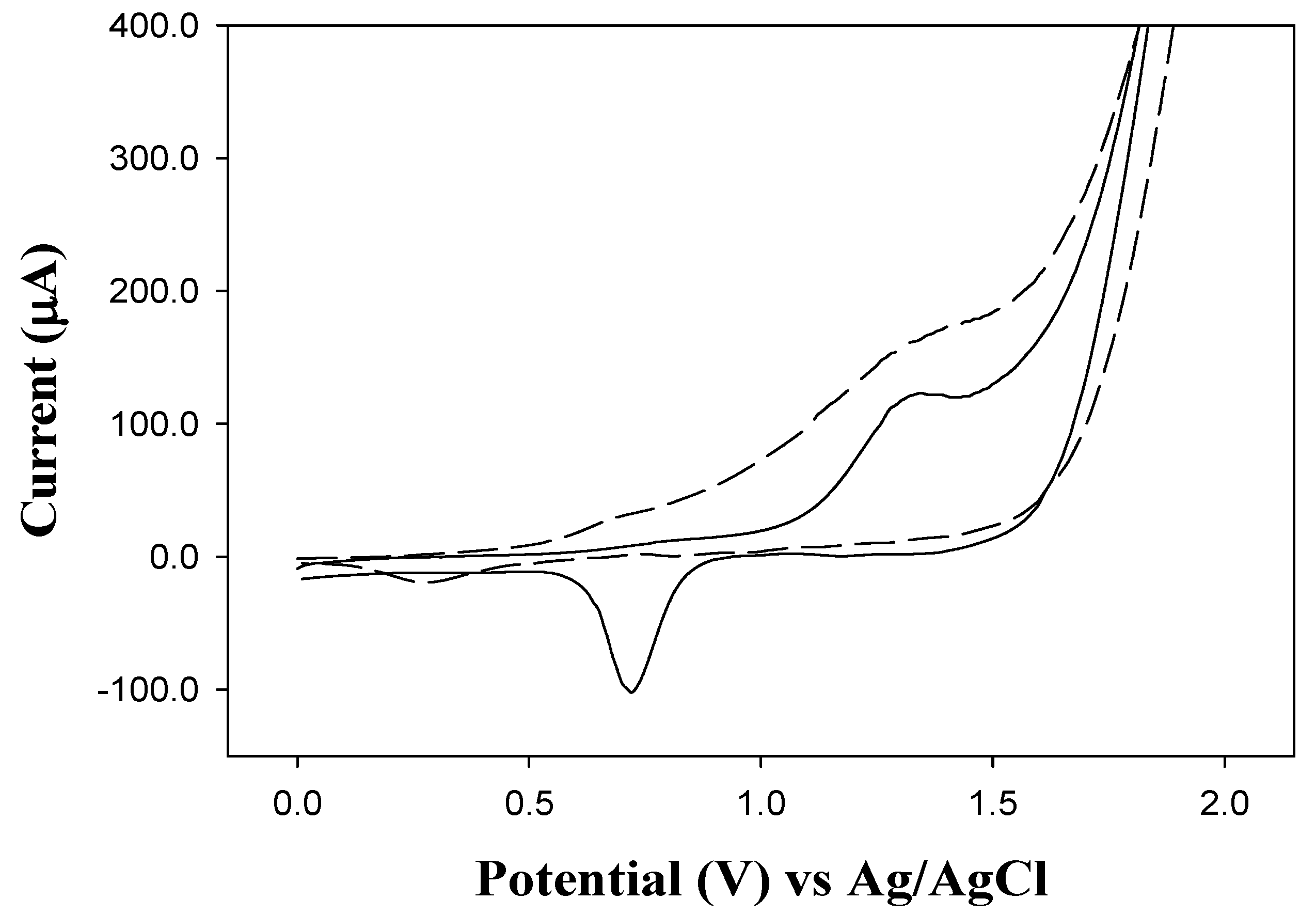
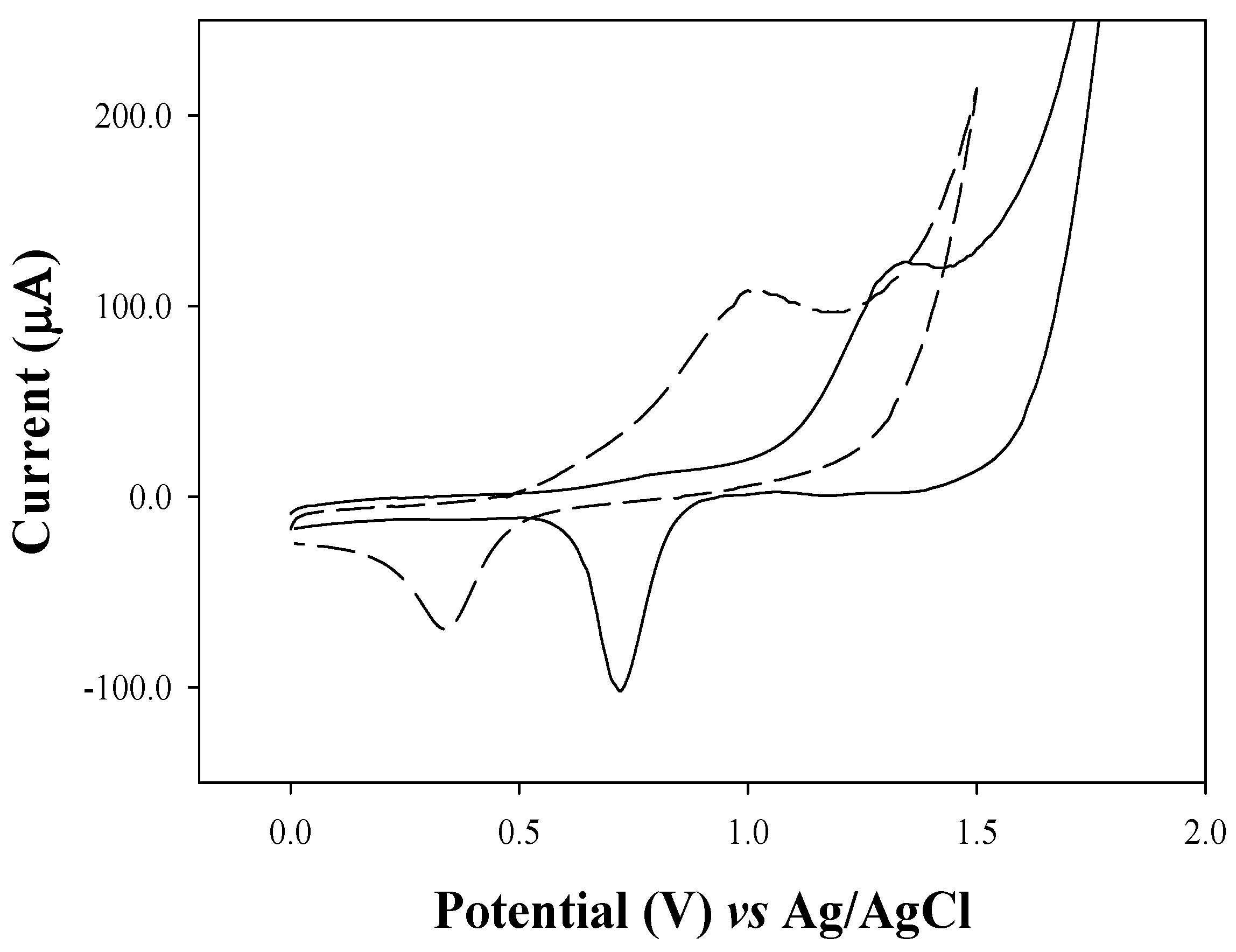
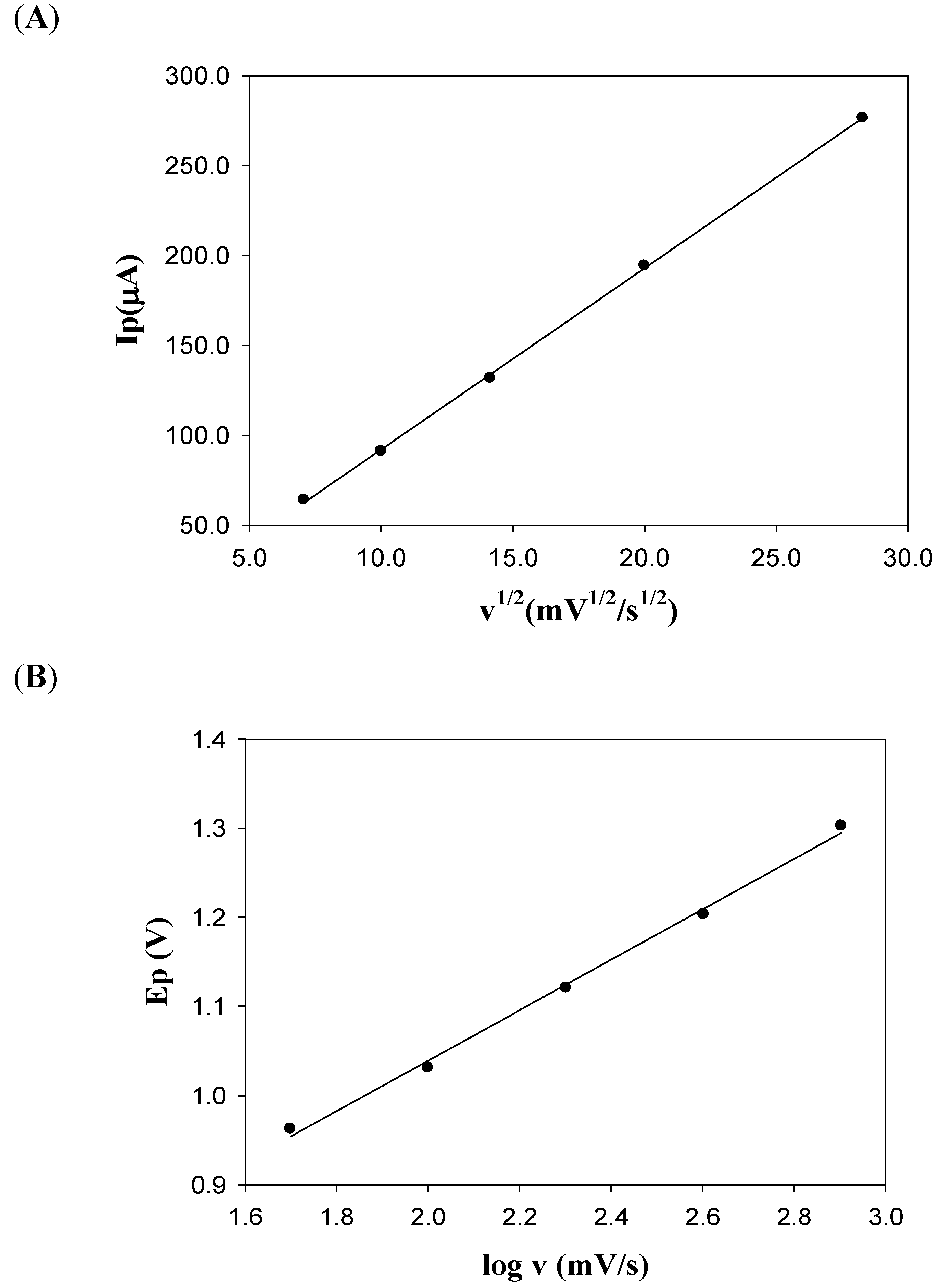
2.2. Cyclic Voltammetry (CV)
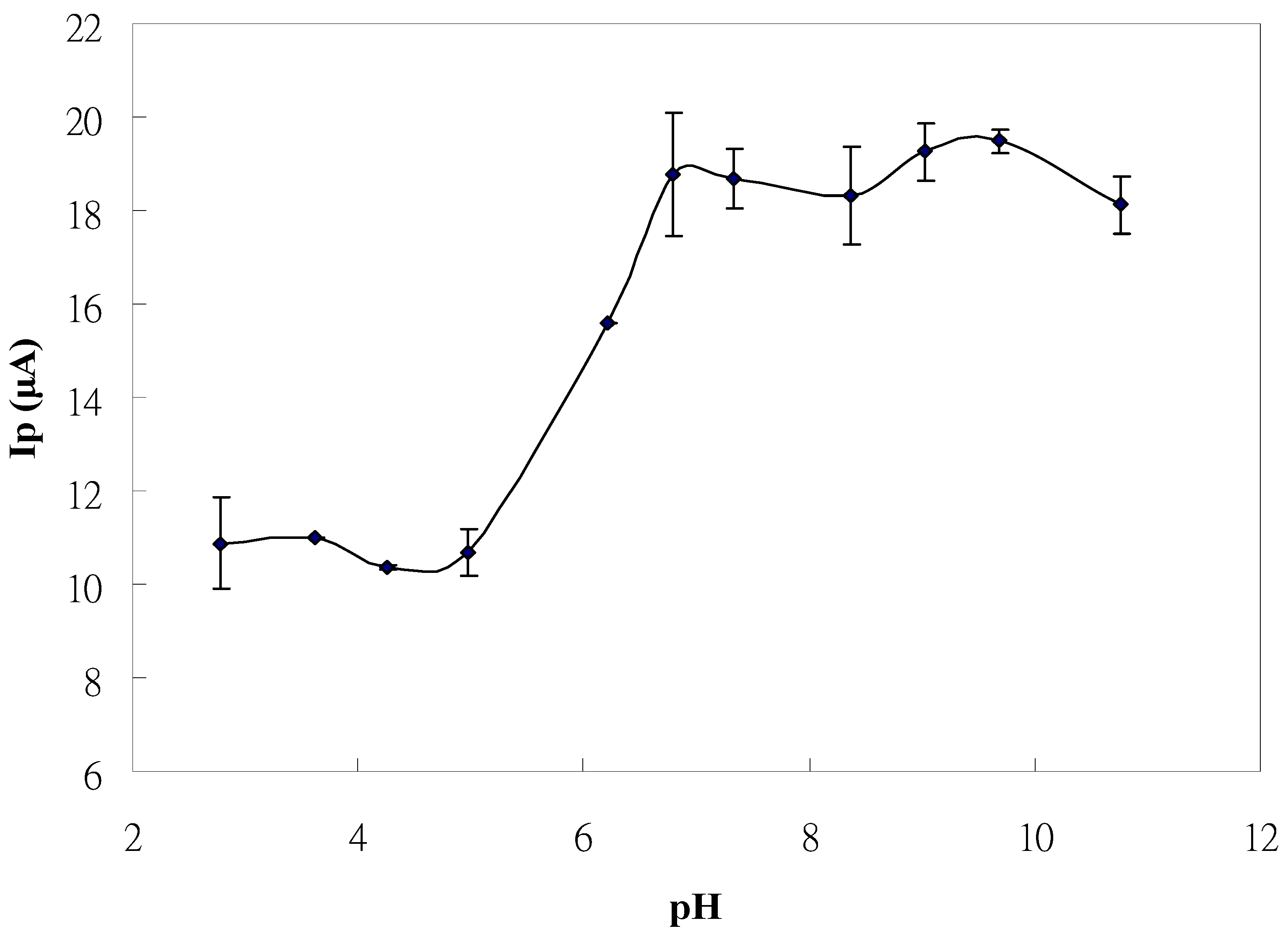
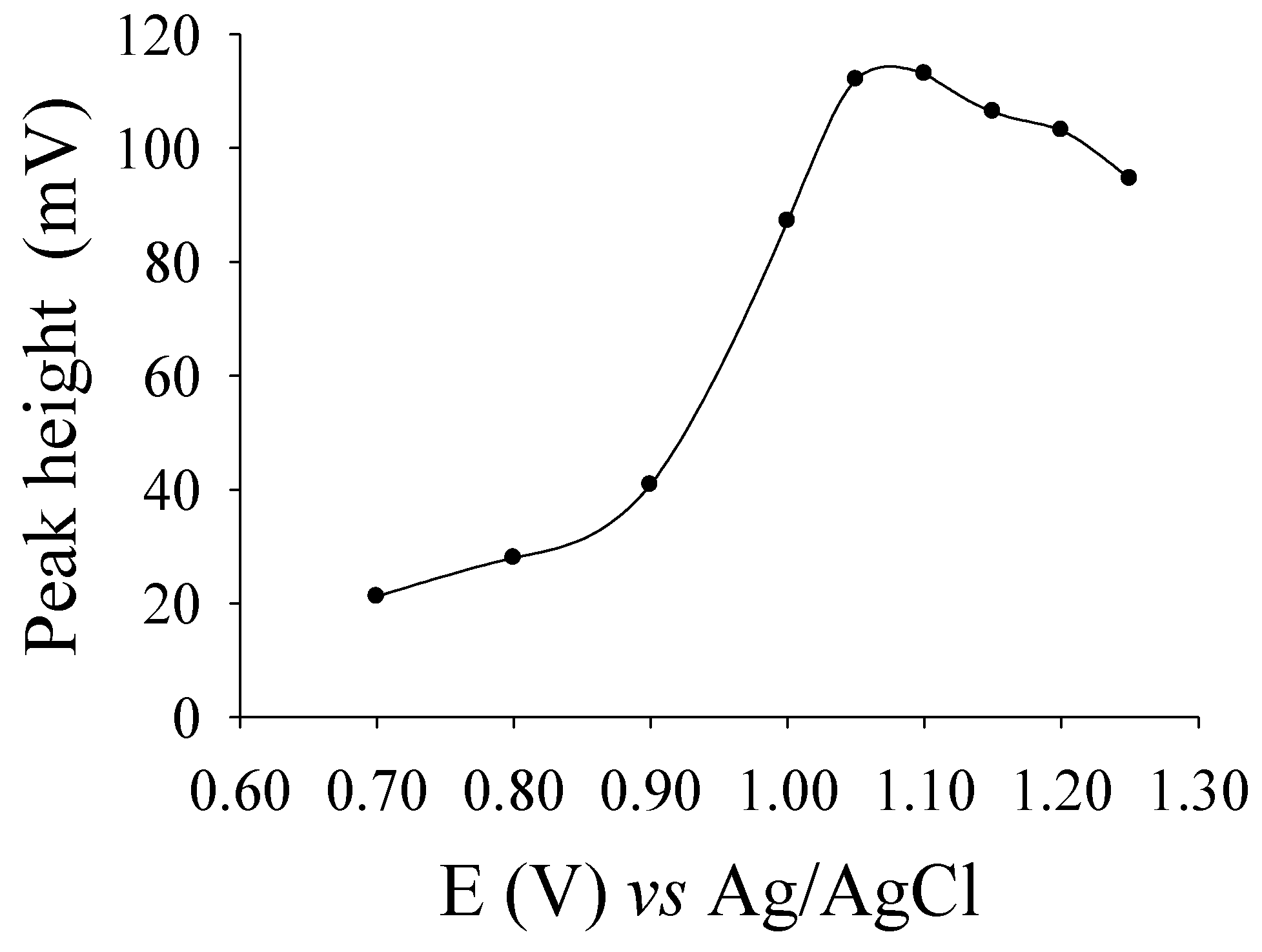
2.3. Optimum Conditions for Liquid Chromatography
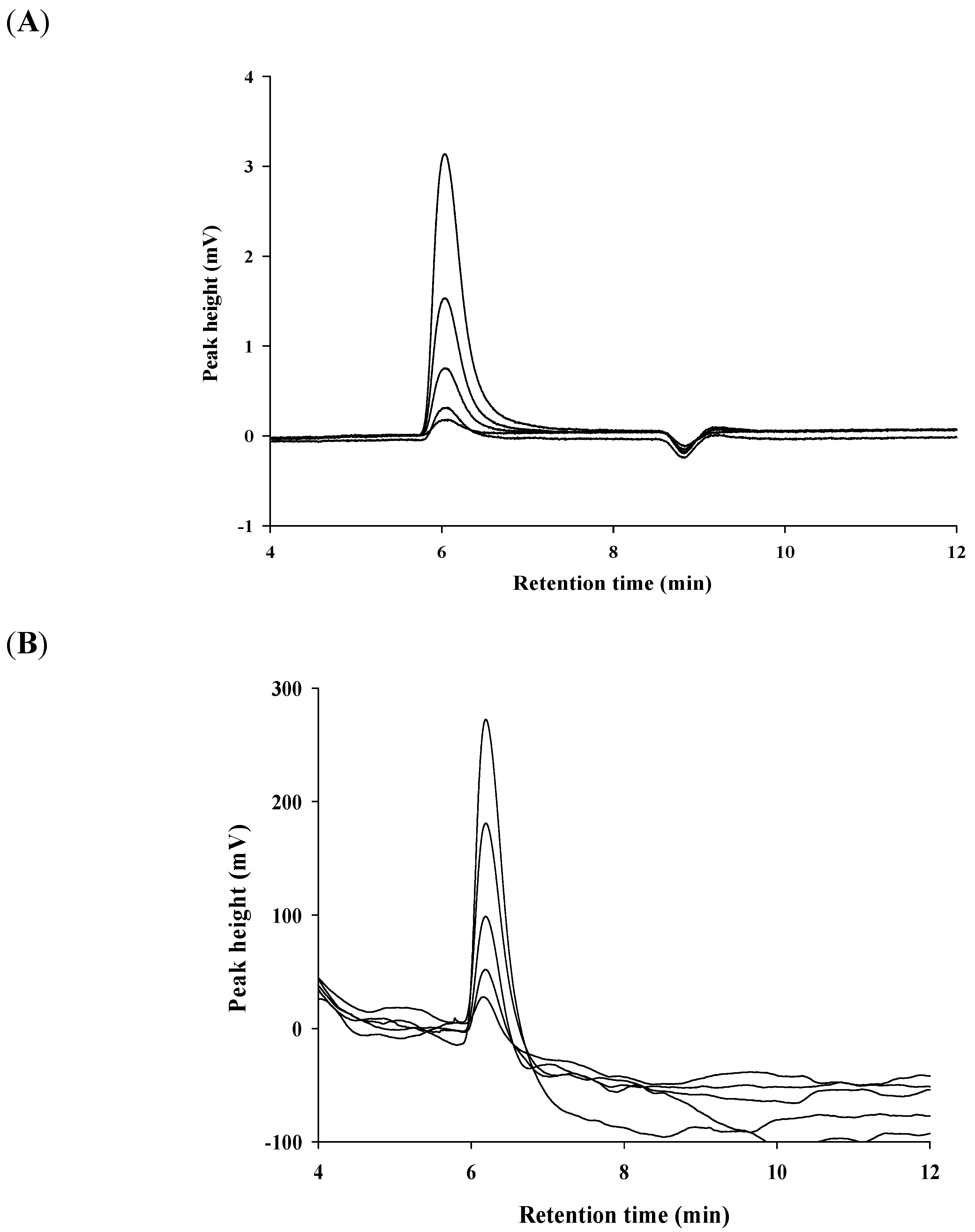
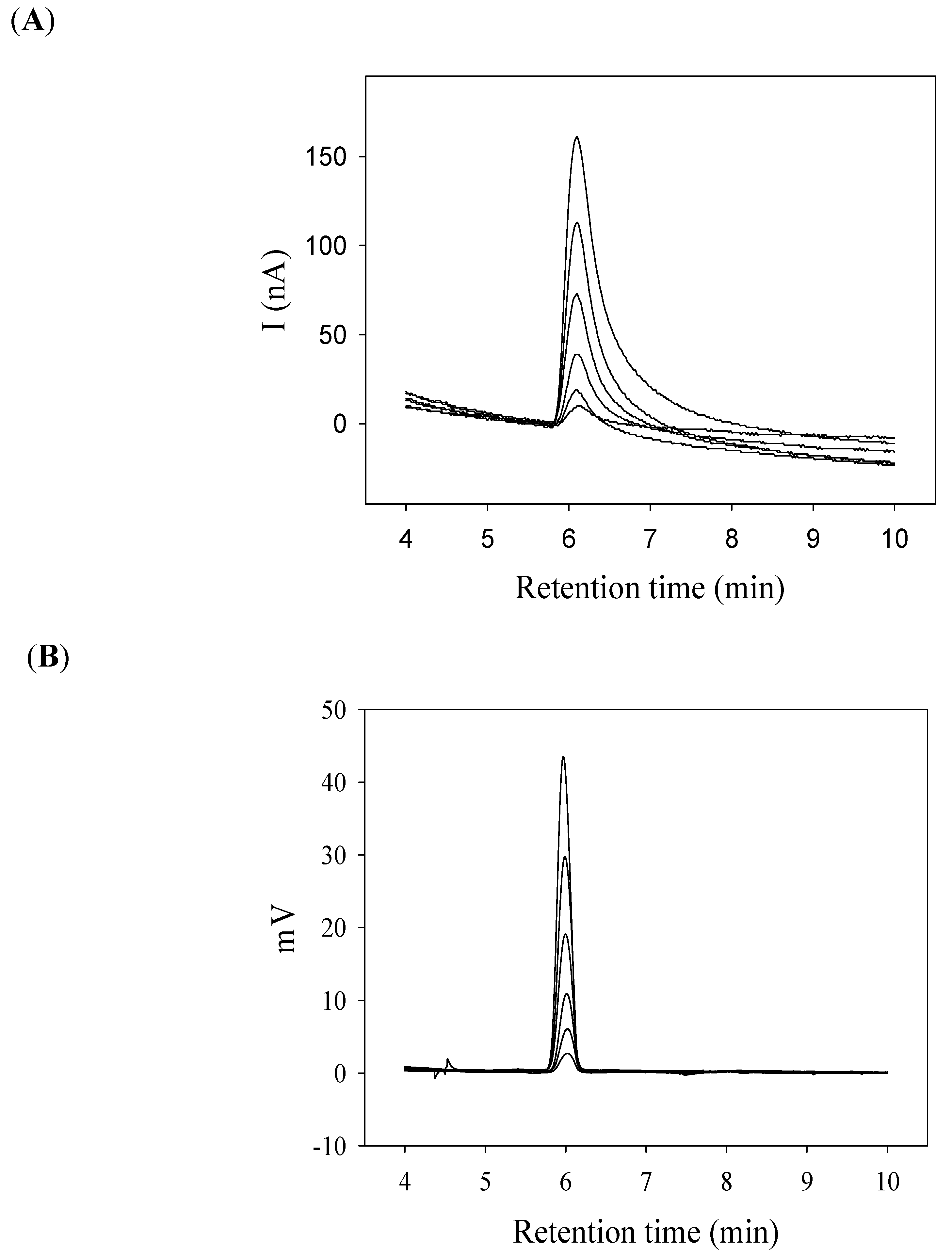
2.4. Sensitivity of Amperometric Detector and UV Detector for Liquid Chromatography
2.5. Application to Thiazolidine Using a Flow—through Chronoamperometric Sensor
3. Experimental
3.1. Materials and Apparatus
3.2. Procedures
3.2.1. Voltammetric Measurements
3.2.2. Determining Thiazolidine Using a Flow-through Chonoamperometric Detector
3.2.3. Determining Thiazolidine Using Liquid Chromatography with UV Detector
4. Conclusions
Acknowledgments
References
- Liu, Y.; Jing, F.; Xu, Y.; Xie, Y.; Shi, F.; Fang, H.; Li, M.; Xu, W. Design, synthesis and biological activity of thiazolidine -4-carboxylic acid derivatives as novel influenza neuraminidase inhibitors. Bioorg. Med. Chem. 2011, 19, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Gududuru, V.; Hurh, E.; Dalton, J.T.; Miller, D.D. Polyamine conjugates of serine, 4-thiazolidinone and thiazolidine -4-carboxylic acid, Synthesis and growth inhibitory effects on human prostate cancer cell lines Abstracts of Papers. 229th ACS National Meeting, San Diego, CA, USA, 13–17 March 2005. [Google Scholar]
- Gouda, M.A.; Abu-Hashem, A.A. Synthesis, Characterization, Antioxidant and Antitumor Evaluation of Some New Thiazolidine and Thiazolidinone Derivatives. Arch. Pharm. 2011, 344, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Srivastava, S.D.; Srivastava, S.K. Synthesis, antimicrobial and antiinflammatory activities of 4-oxothiazolidines and their 5-arylidenes. Indian J. Chem. Sect B 2005, 44B, 1262–1266. [Google Scholar] [CrossRef]
- Zimenkovsky, B.S.; Lesyk, R.B.; Lukyanchuk, V.D.; Shtoyko, N.Y.; Nektegayev, I.O.; Roman, A.M.; Kazmirchuk, G.V.; Nekhlopochin, O.S. Study of trends in interaction between structure and biological activity among thiazolidine derivatives. Fiz. Akt. Rech. 2002, 2, 58–64. [Google Scholar]
- Bhaskar, V.H.; Kumar, M.; Sangameswaran, B.; Balakrishnan, B.R. Antimicrobial activity of some 4- thiazolidine derivatives. Pharmacist 2007, 2, 1–5. [Google Scholar]
- Dashevs’kii, A.M.; Zagorii, V.A.; Buts’ka, V.E. Screening of antioxidant activity of some thiazolidine derivatives. Farm. Zh. 2005, 1, 57–64. [Google Scholar]
- Ma, L.; Xie, C.; Ma, Y.; Liu, J.; Xiang, M.; Ye, X.; Zheng, H.; Chen, Z.; Xu, Q.; Chen, T. Synthesis and Biological Evaluation of Novel 5-Benzylidenethiazolidine-2,4-dione Derivatives for the Treatment of Inflammatory Diseases. J. Med. Chem. 2011, 54, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Shimazaki, N. Anti-cancer pharmaceutical compositions containing benzimidazole thiazolidinedione derivatives - PPAR agonists and RXR agonists and methods for treating patients with cancer. U.S. Pat. Appl. Publ. Pub. No: US 2009/0028868 A1.Pub., 29 January 2009. [Google Scholar]
- Miller, D.D.; Dalton, J.T.; Gududuru, V.; Hurh, E. Preparation of thiazolidinone amides, thiazolidine carboxylic acid amides, and serine amides, including polyamine conjugates thereof, as selective antitumor agents for treating melanoma. U.S. Pat. Appl. Publ. Publ. Pub. No: US 2007/0155807 A1.Pub., 5 July 2007. [Google Scholar]
- Fujiwara, K.; Shimazaki, N. Anti-cancer pharmaceutical composition. PCT Int. Appl. WO 2007/091622 A1., 16 August 2007. [Google Scholar]
- Rida, S.M.; El-Hawash, S.A.M.; Fahmy, H.T.Y.; Hazzaa, A.A.; El-Meligy, M.M.M. Synthesis of novel benzofuran and related benzimidazole derivatives for evaluation of in vitro anti-HIV-1, anticancer and antimicrobial activities. Arch. Pharm. Res. 2006, 29, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Osman, A.N.; Noaman, E.; Heiba, H.I.; Zaher, N.H. The synthesis of some new sulfur heterocyclic compounds as potential radioprotective and anticancer agents. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 1935–1950. [Google Scholar] [CrossRef]
- Kamins’kii, D.V.; Roman, O.M.; Atamanyuk, D.V.; Lesik, R.B. 5-Ylidene-2-thioxo-4-thiazolidone-3-succinic acids and their derivatives: Synthesis, anticancer activity, and QSAR analysis. Zh. Org. Khi. 2006, 4, 41–48. [Google Scholar]
- Song, B.A.; Chen, C.J.; Yang, S.; Jin, L.H.; Xue, W.; Zhang, S.M.; Zou, Z.H.; Hu, D.Y.; Liu, G. Synthesis, structure and antitumor activity of 2-alkylthio-5-(3,4,5-trimethoxyphenyl)-1,3,4-thiadiazole compounds. Hua Xue Xue Bao 2005, 63, 1720–1726. [Google Scholar]
- Tkachenko, Y.V.; Lesyk, R.B.; Lukyanchuk, V.D. Comparative evaluation of thiazolidine derivatives. Farm. Zh. 2004, 5, 88–93. [Google Scholar]
- Fuchigami, T. Selective anodic monofluorination of sulfur-containing heterocycles: Potent applications towards pharmaceuticals. Phosphorus, Sulfur Silicone Relat. Elem. 1997, 120 & 121, 343–344. [Google Scholar] [CrossRef]
- Sarkar, A.; Banerjee, P.; Hossain, S.U.; Bhattacharya, S.; Bhattacharya, S.C. Role of hydrogen bonding in the spectroscopic properties of thiazolidinedione derivatives in homogeneous solvents. Spectrochim. Acta Part A 2009, 72 A, 1097–1102. [Google Scholar] [CrossRef]
- Uchoa, F.D.T.; Cattani, V.B.; Lima, M.C.A.; Galdino, S.L.; Pitta, I.R.; Dalla, C.T. Development and application of LC-UV method for the quantification of the anti-inflammatory thiazolidinone PG15 in rat plasma. J. Braz. Chem. Soc. 2008, 19, 1553–1559. [Google Scholar] [CrossRef]
- Shin, H.S.; Ahn, H.S.; Lee, B.H. Determination of thiazolidine-4-carboxylates in urine by chloroformate derivatization and gas chromatography-electron impact mass spectrometry. J. Mass. Spectrom. 2007, 42, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, Z.J. Pulse voltammetric determination of sulfur-containing organic compounds. Contemp. Electroanal. Chem., [Proc. ElectroFinnAnalysis Int. Conf. Electroanal. Chem.], 1988, 395-9. Plenum: New York, NY, USA, 1990; CODEN:57JFA4. [Google Scholar]
- Barus, C.; Gros, P.; Comtat, M.; Daunes-Marion, S.; Tarroux, R. Electrochemical behaviour of N-acetyl-l-cysteine on gold electrode—A tentative reaction mechanism. Electrochim. Acta 2007, 52, 7978–7985. [Google Scholar]
- Tu, A.J.; Vandeberg, P.J.; Johnson, D.C. Evaluation of EQCM Data from a Study of Cysteine Adsorption on Gold Electrodes in Acidic Media. Anal. Chem. 1995, 67, 552–556. [Google Scholar]
- Damani, L.A. Metabolism of sulphur functional groups. In Sulphur-Containing Drugs and Related Organic Compounds Chemistry, Biochemistry and Toxicology; Ellis Horwood Limited: Chichester, UK, 1989; Volume 1, Part B; p. 234. [Google Scholar]
- Albert, J.F. Synthetic Organic Electrochemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1989; p. 279. [Google Scholar]
- Wang, L.H.; Hsia, H.C.; Lan, Y.Z. Design of a Flow—Through Polarographic Sensor Based on Metal Films for Determination N-nitrosodiethanolamine in Rabbit Biological Fluids. Sensors 2006, 6, 1555–1567. [Google Scholar] [CrossRef]
Sample Availability: Contact the authors. |
| Supporting electrolytes | Thiazolidine | |||
|---|---|---|---|---|
| Au | Pt | |||
| Potential (V) | Current (μA) | Potential (V) | Current (μA) | |
| Bu4NBF4/100% CH3CN | ND | ND | ND | ND |
| Et4NBF4/100% CH3CN | ND | ND | ND | ND |
| Bu4NClO4/100% CH3CN | ND | ND | ND | ND |
| Et4NClO4/100% CH3CN | ND | ND | ND | ND |
| LiClO4/100% CH3CN | ND | ND | ND | ND |
| LiClO4/100% MeOH | ND | ND | ND | ND |
| Et4NBF4/30% CH3CN | 1.344 | 162.7 | ND | ND |
| Et4NClO4/30% CH3CN | 1.336 | 86.69 | ND | ND |
| LiClO4/30% CH3CN | 1.308 | 85.31 | ND | ND |
| LiClO4/30% MeOH | 1.341 | 122.1 | 1.332 | 155.9 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.-H.; Li, W.-J. Studies on the Electrochemical Behavior of Thiazolidine and Its Applications Using a Flow–Through Chronoamperometric Sensor Based on a Gold Electrode. Molecules 2011, 16, 7608-7620. https://doi.org/10.3390/molecules16097608
Wang L-H, Li W-J. Studies on the Electrochemical Behavior of Thiazolidine and Its Applications Using a Flow–Through Chronoamperometric Sensor Based on a Gold Electrode. Molecules. 2011; 16(9):7608-7620. https://doi.org/10.3390/molecules16097608
Chicago/Turabian StyleWang, Lai-Hao, and Wen-Jie Li. 2011. "Studies on the Electrochemical Behavior of Thiazolidine and Its Applications Using a Flow–Through Chronoamperometric Sensor Based on a Gold Electrode" Molecules 16, no. 9: 7608-7620. https://doi.org/10.3390/molecules16097608
APA StyleWang, L.-H., & Li, W.-J. (2011). Studies on the Electrochemical Behavior of Thiazolidine and Its Applications Using a Flow–Through Chronoamperometric Sensor Based on a Gold Electrode. Molecules, 16(9), 7608-7620. https://doi.org/10.3390/molecules16097608




