An Electrochemical Synthesis of Functionalized Arylpyrimidines from 4-Amino-6-Chloropyrimidines and Aryl Halides
Abstract
:1. Introduction
2. Results and Discussion
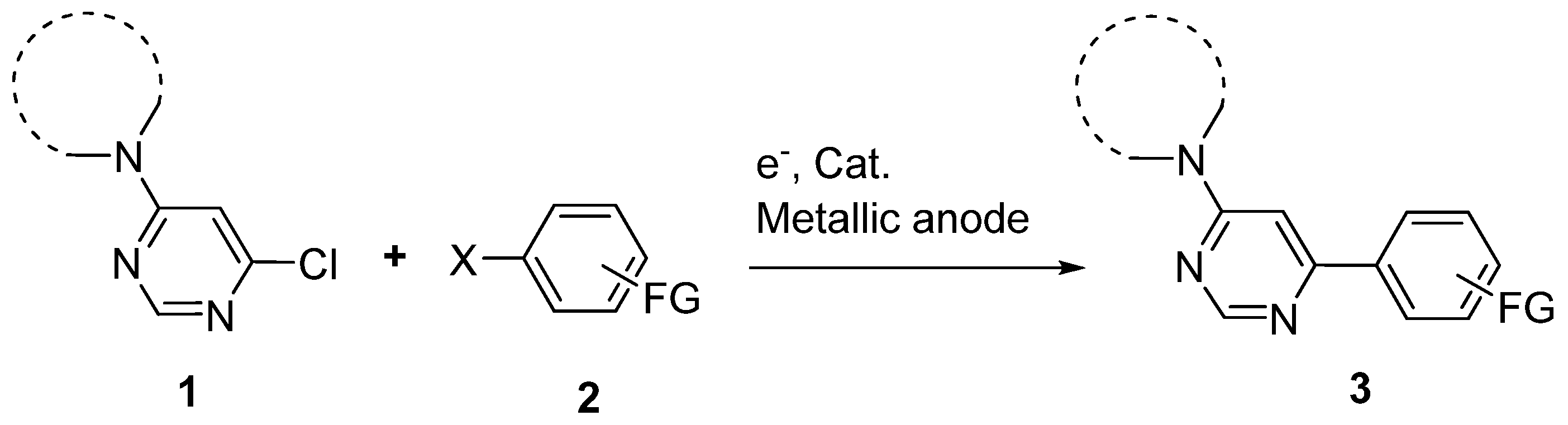
| Entry | Substrate | Halide | Reaction Time | Product | Isolated Yield (%) |
|---|---|---|---|---|---|
| 1 | 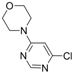 | 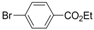 | 5 h |  3a 3a | 83 |
| 2 |  |  | 5 h | 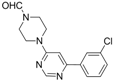 3b 3b | 78a |
| 3 | 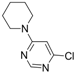 | 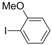 | 6 h | 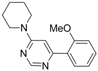 3c 3c | 48b |
| 4 |  |  | 5 h 30 |  3d 3d | 99b |
| 5 | 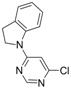 | 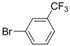 | 6 h 30 |  3e 3e | 77 |
| 6 |  | 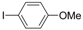 | 4 h 30 | 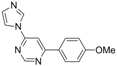 3f 3f | 36 |
| 7 | 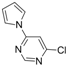 | 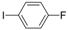 | 3 h 30 |  3g 3g | 34 |
| 8 |  | 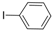 | 6 h 15 | 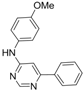 3h 3h | 41b |
| 9 | 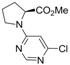 | 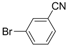 | 6h | 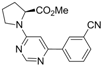 3i 3i | 41b |
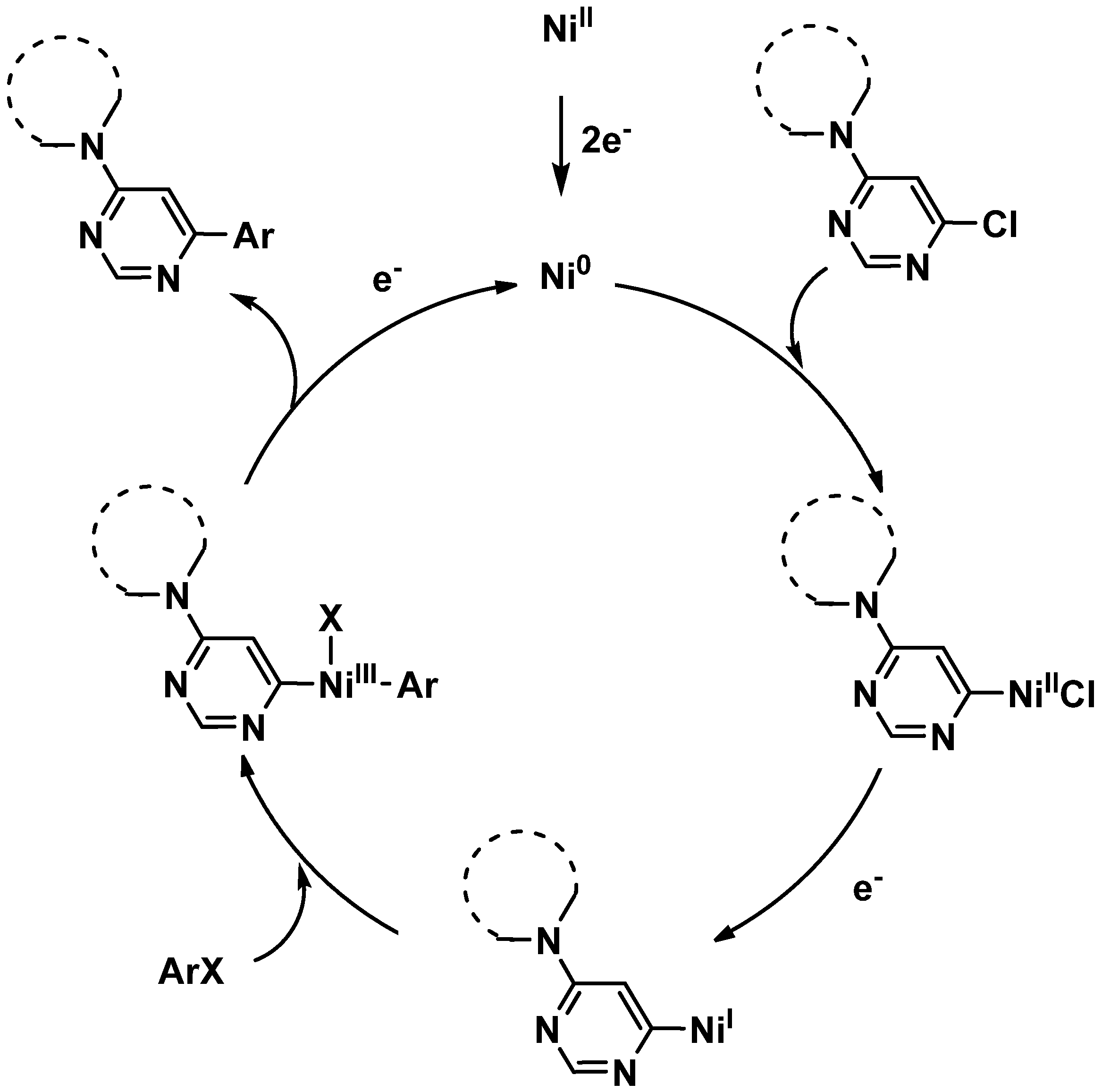
3. Experimental
3.1. General
3.2. Typical Procedure for the Cross-Coupling Reactions
4. Conclusions
References
- Fürstner, A. Carbon-carbon bond formations involving organochromium(III) reagents. Chem. Rev. 1999, 99, 991–1045. [Google Scholar] [CrossRef]
- Schröter, S.; Stock, C.; Bach, T. Regioselective cross-coupling reactions of multiple halogenated nitrogen-, oxygen-, and sulfur-containing heterocycles. Tetrahedron 2005, 61, 2245–2267. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar]
- Cahiez, G.; Moyeux, A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010, 110, 1435–1462. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K.; Smith, G.F. Heterocyclic Chemistry; Chapman & Hall: London, UK, 1995. [Google Scholar] [Green Version]
- Tang, G.; Kertesz, D.J.; Yang, M.; Lin, X.; Wang, Z.; Li, W.; Qiu, Z.; Chen, J.; Mei, J.; Chen, L.; et al. Exploration of piperidine-4-yl-aminopyrimidines as HIV-1 reverse transcriptase inhibitors. N-Phenyl derivatives with broad potency against resistant mutant viruses. Bioorg. Med. Chem. Lett. 2010, 20, 6020–6023. [Google Scholar]
- Vidal, B.; Nueda, A.; Esteve, C.; Domenech, T.; Benito, S.; Reinoso, R.F.; Pont, M.; Calbet, M.; Lopez, R.; Cadavid, M.I.; et al. Discovery and characterization of 4’-(2-furyl)-N-pyridin-3-yl-4,5’ bipyrimidin-2’-amine (LAS38096), a potent, selective, and efficacious A2B adenosine receptor antagonist. J. Med. Chem. 2007, 50, 2732–2736. [Google Scholar]
- Folkes, A.J.; Ahmadi, K.; Alderton, W.K.; Alix, S.; Baker, S.J.; Box, G.; Chuckowree, I.S.; Clarke, P.A.; Depledge, P.; Eccles, S.A.; et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008, 51, 5522–5532. [Google Scholar] [CrossRef]
- Verheijen, J.C.; Richard, D.J.; Curran, K.; Kaplan, J.; Lefever, M.; Nowak, P.; Malwitz, D.J.; Brooijmans, N.; Toral-Barza, L.; Zhang, W.-G.; et al. Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J. Med. Chem. 2009, 52, 8010–8024. [Google Scholar] [CrossRef]
- Sutherlin, D.P.; Sampath, D.; Berry, M.; Castanedo, G.; Chang, Z.; Chuckowree, I.; Dotson, J.; Folkes, A.; Friedman, L.; Goldsmith, R.; et al. Discovery of (thienopyrimidin-2-yl)aminopyrimidines as potent, selective, and orally available pan-PI3-kinase and dual pan-PI3-kinase/mTOR inhibitors for the treatment of cancer. J. Med. Chem. 2010, 53, 1086–1097. [Google Scholar] [CrossRef]
- Stanetty, P.; Hattinger, G.; Schnürch, M.; Mihovilovic, M.D. Novel and efficient access to phenylamino-pyrimidine type protein kinase C inhibitors utilizing a Negishi cross-coupling strategy. J. Org. Chem. 2005, 70, 5215–5220. [Google Scholar] [CrossRef]
- Burger, M.T.; Knapp, M.; Wagman, A.; Ni, Z.-J.; Hendrickson, T.; Atallah, G.; Zhang, Y.; Frazier, K.; Verhagen, J.; Pfister, K.; et al. Synthesis and in vitro and in vivo evaluation of phosphoinositide-3-kinase inhibitors. ACS Med. Chem. Lett. 2011, 2, 34–38. [Google Scholar] [CrossRef]
- Kamenecka, T.; Jiang, R.; Song, X.; Duckett, D.; Chen, W.; Ling, Y.Y.; Habel, J.; Laughlin, J.D.; Chambers, J.; Figuera-Losada, M.; et al. Synthesis, biological evaluation, x-ray structure, and pharmacokinetics of aminopyrimidine c-jun-N-terminal kinase (JNK) inhibitors. J. Med. Chem. 2010, 53, 419–431. [Google Scholar] [CrossRef]
- Liu, H.; Altenbach, R.J.; Carr, T.L.; Chandran, P.; Hsieh, G.C.; Lewis, L.G.R.; Manelli, A.M.; Milicic, I.; Marsh, K.C.; Miller, T.R.; et al. cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), a new histamine H4R antagonist that blocks pain responses against carrageenan-induced hyperalgesia. J. Med Chem. 2008, 51, 7094–7098. [Google Scholar] [CrossRef]
- Wang, H.-L.; Katon, J.; Balan, C.; Bannon, A.W.; Bernard, C.; Doherty, E.M.; Dominguez, C.; Gavva, N.R.; Gore, V.; Ma, V.; et al. Novel vanilloid receptor-1 antagonists: 3. The identification of a second-generation clinical candidate with improved physicochemical and pharmacokinetic properties. J. Med. Chem. 2007, 50, 3528–3539. [Google Scholar]
- Tamayo, N.; Liao, H.; Stec, M.M.; Wang, X.; Chakrabarti, P.; Retz, D.; Doherty, E.M.; Surapaneni, S.; Tamir, R.; Bannon, A.W.; et al. Design and synthesis of peripherally restricted transient receptor potential vanilloid 1 (TRPV1) antagonists. J. Med. Chem. 2008, 51, 2744–2757. [Google Scholar]
- Schomaker, J.M.; Delia, T.J. Arylation of halogenated pyrimidines via Suzuki coupling. J. Org. Chem. 2001, 66, 7125–7128. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. 2002, 41, 4179–4211. [Google Scholar]
- Frisch, A.C.; Beller, M. Catalysts for cross-coupling reactions with non-activated alkyl halides. Angew. Chem. Int. Ed. 2005, 44, 674–688. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar]
- Chaussard, J.; Folest, J.-C.; Nédélec, J.-Y.; Périchon, J.; Sibille, S.; Troupel, M. Use of sacrificial anodes in electrochemical functionalization of organic halides. Synthesis 1990, 5, 369–381. [Google Scholar]
- Sengmany, S.; Léonel, E.; Polissaint, F.; Nédélec, J.-Y.; Pipelier, M.; Thobie-Gautier, C.; Dubreuil, D. Preparation of functionalized aryl- and heteroarylpyridazines by nickel-catalyzed electrochemical cross-coupling reactions. J. Org. Chem. 2007, 72, 5631–5636. [Google Scholar]
- Urgin, K.; Barhdadi, R.; Condon, S.; Léonel, E.; Pipelier, M.; Blot, V.; Thobie-Gautier, C.; Dubreuil, D. Some mechanistic aspects of a nickel-catalyzed electrochemical cross-coupling between aryl halides and substituted chloropyridazines. Electrochimica Acta 2010, 55, 4495–4500. [Google Scholar]
- Gosmini, C.; Nédélec, J.-Y.; Périchon, J. Electrochemical cross-coupling between functionalized aryl halides and 2-chloropyrimidine or 2-chloropyrazine catalyzed by nickel 2,2’-bipyridine complex. Tetrahedron Lett. 2000, 41, 201–203. [Google Scholar] [CrossRef]
- Le Gall, E.; Gosmini, C.; Nédélec, J.-Y.; Périchon, J. Cobalt-catalyzed electrochemical cross-coupling of functionalized phenyl halides with 4-chloroquinoline derivatives. Tetrahedron Lett. 2001, 42, 267–269. [Google Scholar]
- Sander, K.; Kottke, T.; Tanrikulu, Y.; Proschak, E.; Weizel, L.; Schneider, E.H.; Seifert, R.; Schneider, G.; Stark, H. 2,4-Diaminopyrimidines as histamine H4 receptor ligands—Scaffold optimization and pharmacological characterization. Bioorg. Med. Chem. 2009, 17, 7186–7196. [Google Scholar] [CrossRef]
- Nédélec, J.-Y.; Périchon, J.; Troupel, M. Organic electroreductive coupling reactions using transition metal complexes as catalysts. Top. Curr. Chem. 1997, 185, 141–173. [Google Scholar] [CrossRef]
- De Lamo Marin, S.; Martens, T.; Mioskowski, C.; Royer, J. Efficient N-p-methoxyphenyl amine deprotection through anodic oxidation. J. Org. Chem. 2005, 70, 10592–10595. [Google Scholar] [CrossRef]
- Troupel, M.; Rollin, Y.; Sock, O.; Meyer, G.; Périchon, J. Electrochimie de complexes du nickel associés à la 2,2’-bipyridine dans le solvant N-méthylpyrrolidinone. New J. Chem. 1986, 10, 593–599. [Google Scholar]
- Hartung, C.G.; Backes, A.C.; Felber, B.; Missioy, A.; Philipp, A. Efficient microwave-assisted synthesis of highly functionalized pyrimidine derivatives. Tetrahedron 2006, 62, 10055–10064. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sengmany, S.; Le Gall, E.; Léonel, E. An Electrochemical Synthesis of Functionalized Arylpyrimidines from 4-Amino-6-Chloropyrimidines and Aryl Halides. Molecules 2011, 16, 5550-5560. https://doi.org/10.3390/molecules16075550
Sengmany S, Le Gall E, Léonel E. An Electrochemical Synthesis of Functionalized Arylpyrimidines from 4-Amino-6-Chloropyrimidines and Aryl Halides. Molecules. 2011; 16(7):5550-5560. https://doi.org/10.3390/molecules16075550
Chicago/Turabian StyleSengmany, Stéphane, Erwan Le Gall, and Eric Léonel. 2011. "An Electrochemical Synthesis of Functionalized Arylpyrimidines from 4-Amino-6-Chloropyrimidines and Aryl Halides" Molecules 16, no. 7: 5550-5560. https://doi.org/10.3390/molecules16075550
APA StyleSengmany, S., Le Gall, E., & Léonel, E. (2011). An Electrochemical Synthesis of Functionalized Arylpyrimidines from 4-Amino-6-Chloropyrimidines and Aryl Halides. Molecules, 16(7), 5550-5560. https://doi.org/10.3390/molecules16075550





