The Immuno-Regulatory Effects of Schisandra chinensis and Its Constituents on Human Monocytic Leukemia Cells
Abstract
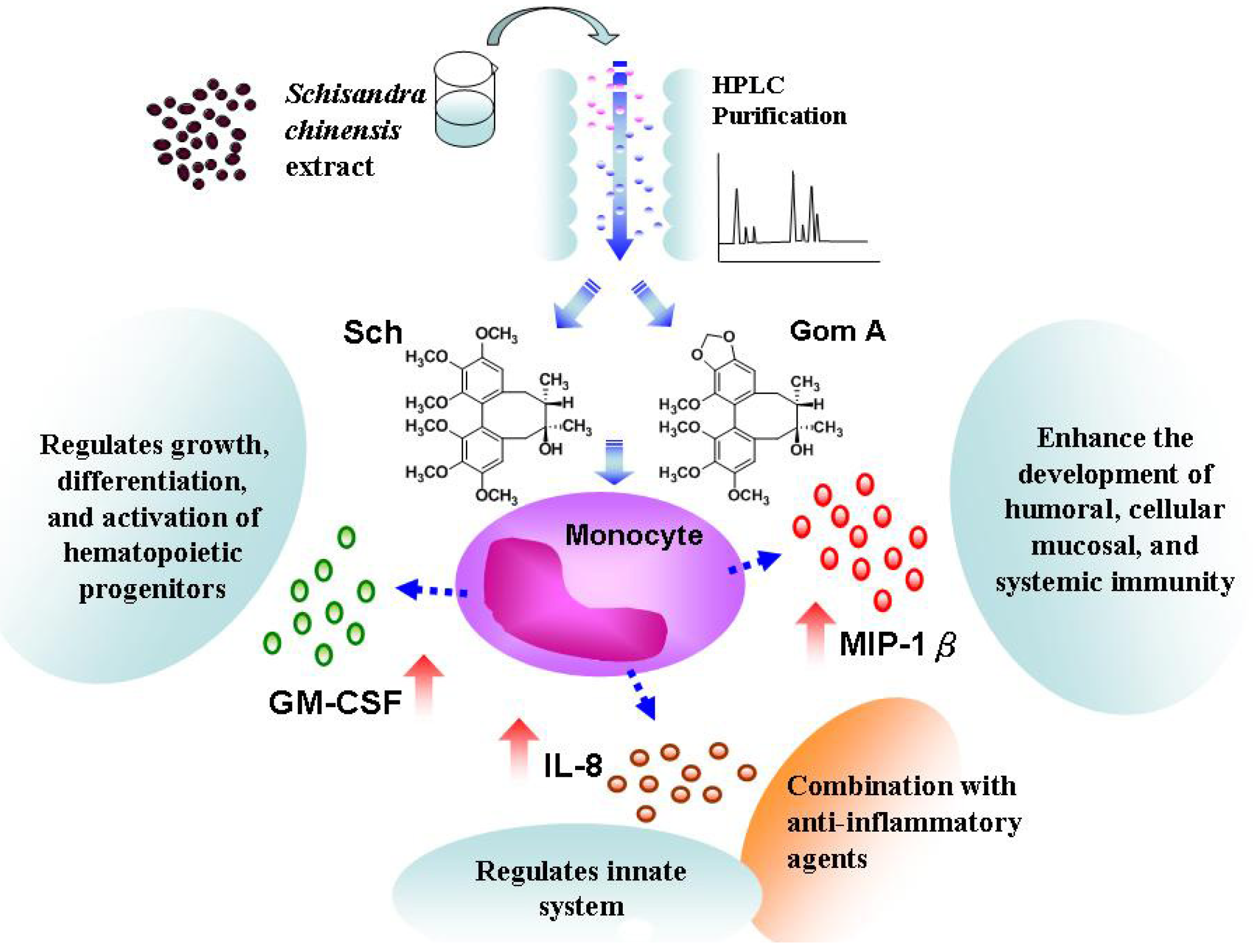
:1. Introduction
2. Results and Discussion
2.1. Analysis of Cytokine Release from Cells Treated with S. chinensis by Microparticle-Based Flow Cytometry
| Med a | LPS a | M-4 a | |
|---|---|---|---|
| IL-1β | ND | 8.20 ± 1.77 | ND |
| IL-2 | 0.52 ± 0.21 | ND | ND |
| IL-4 | ND | ND | ND |
| IL-5 | ND | ND | ND |
| IL-6 | ND | 11.19 ± 5.13 | ND |
| IL-7 | ND | ND | ND |
| IL-8 | 3.36 ± 0.48 | 4515.95 ± 445.08 * | 14.27 ± 5.93 *, # |
| IL-10 | ND | ND | ND |
| IL-12 (p70) | ND | ND | ND |
| IL-13 | ND | 0.68 ± 0.21 | ND |
| IL-17 | ND | 4.32 ± 0.59 | ND |
| G-CSF | 2.02 ± 0.49 | 5.37 ± 1.66 * | ND |
| GM-CSF | ND | 5.60 ± 1.45 | 27.91 ± 1.73 # |
| IFN-γ | 2.45 ± 0.83 | 31.13 ± 7.29 * | ND |
| MCP-1 (MCAF) | 2.78 ± 0.89 | 2159.28 ± 245.11 * | 7.30 ± 1.36 |
| MIP-1β | 6.62 ± 1.73 | 359094.48 ± 3286.85 * | 22.27 ± 2.97 *, # |
| TNF-α | ND | 173.18 ± 34.83 | ND |
2.2. Analysis of the Isolated Compounds from S. chinensis
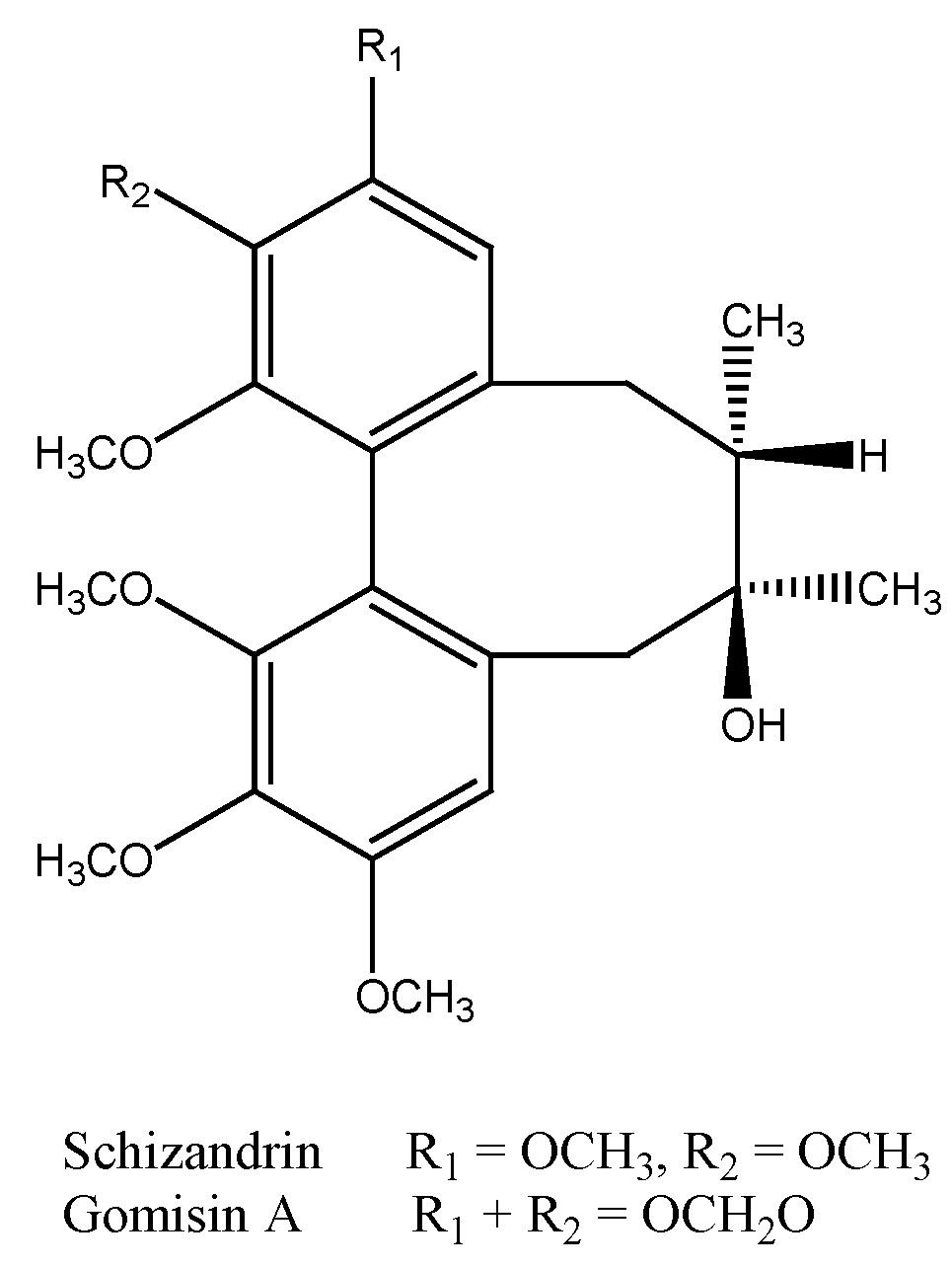
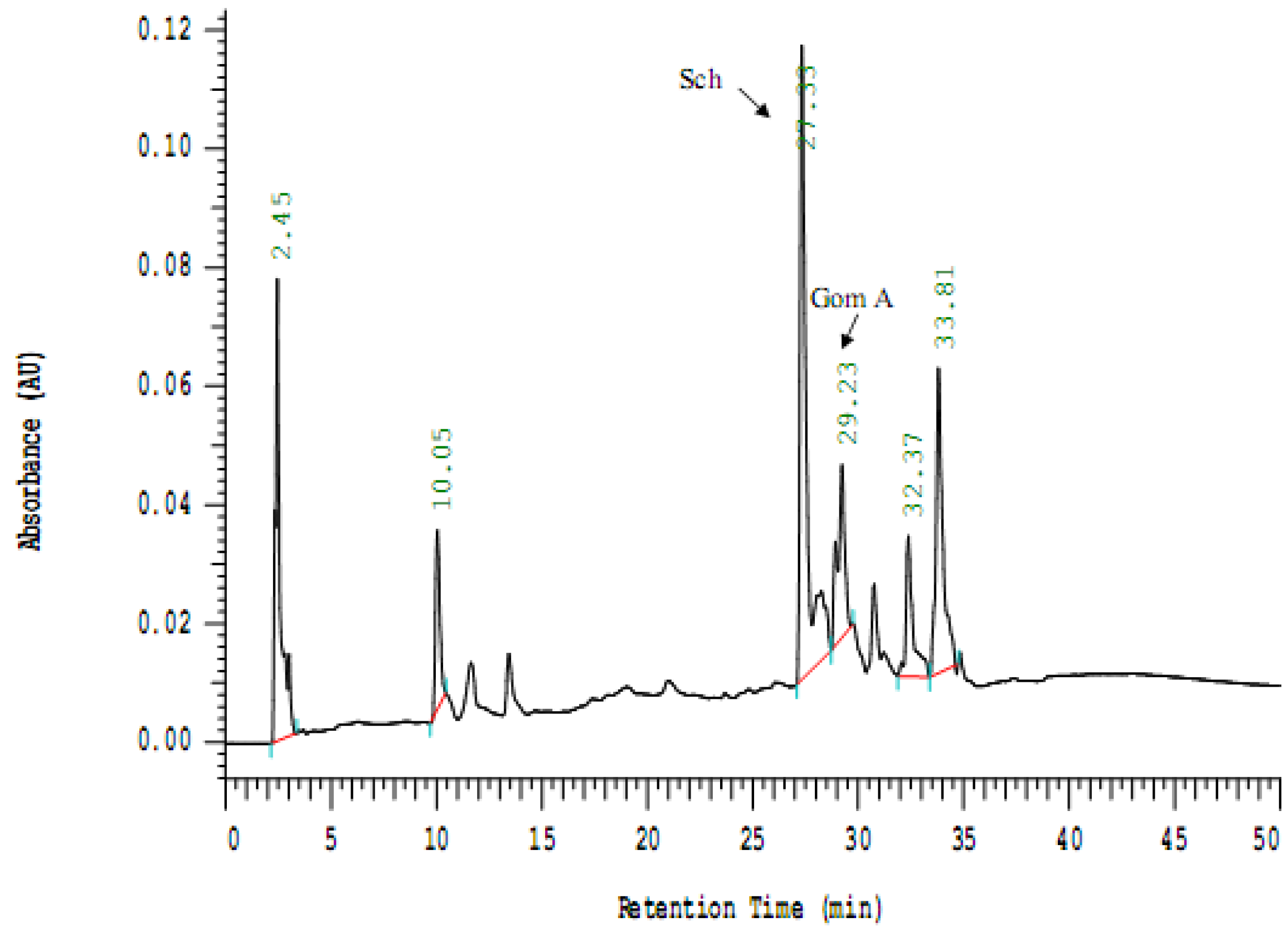
2.3. Cell Viability of S. chinensis and Its Constituents in THP-1 Cells
2.4. Analysis of Cytokine Release from Cells Treated with the Constituents of S. chinensis by Microparticle-Based Flow Cytometry
| Med | LPS | Sch a | Gom A a | |
|---|---|---|---|---|
| (10 ng/mL) | (100 μM) | (100 μM) | ||
| IL-8 | 6.843 ± 1.03 | 6493.99 ± 386.38 * | 136.21 ± 24.10 *, # | 67.15 ± 1.48 *, # |
| GM-CSF | ND | 8.71 ± 3.77 | 17.45 ± 21.16 # | 12.37 ± 5.21 # |
| MCP-1 (MCAF) | 5.87 ± 1.44 | 2092.61 ± 208.26 * | 9.20 ± 1.27 | 7.14 ± 2.84 |
| MIP-1β | 23.60 ± 3.67 | 19913.19 ± 3031.26 * | 24.49 ± 5.97 *, # | 21.30 ± 1.79 *, # |
2.5. Expression of IL-8, MIP-1β, and GM-CSF mRNA in S. chinensis and Its Constituents-Treated THP-1 Cells
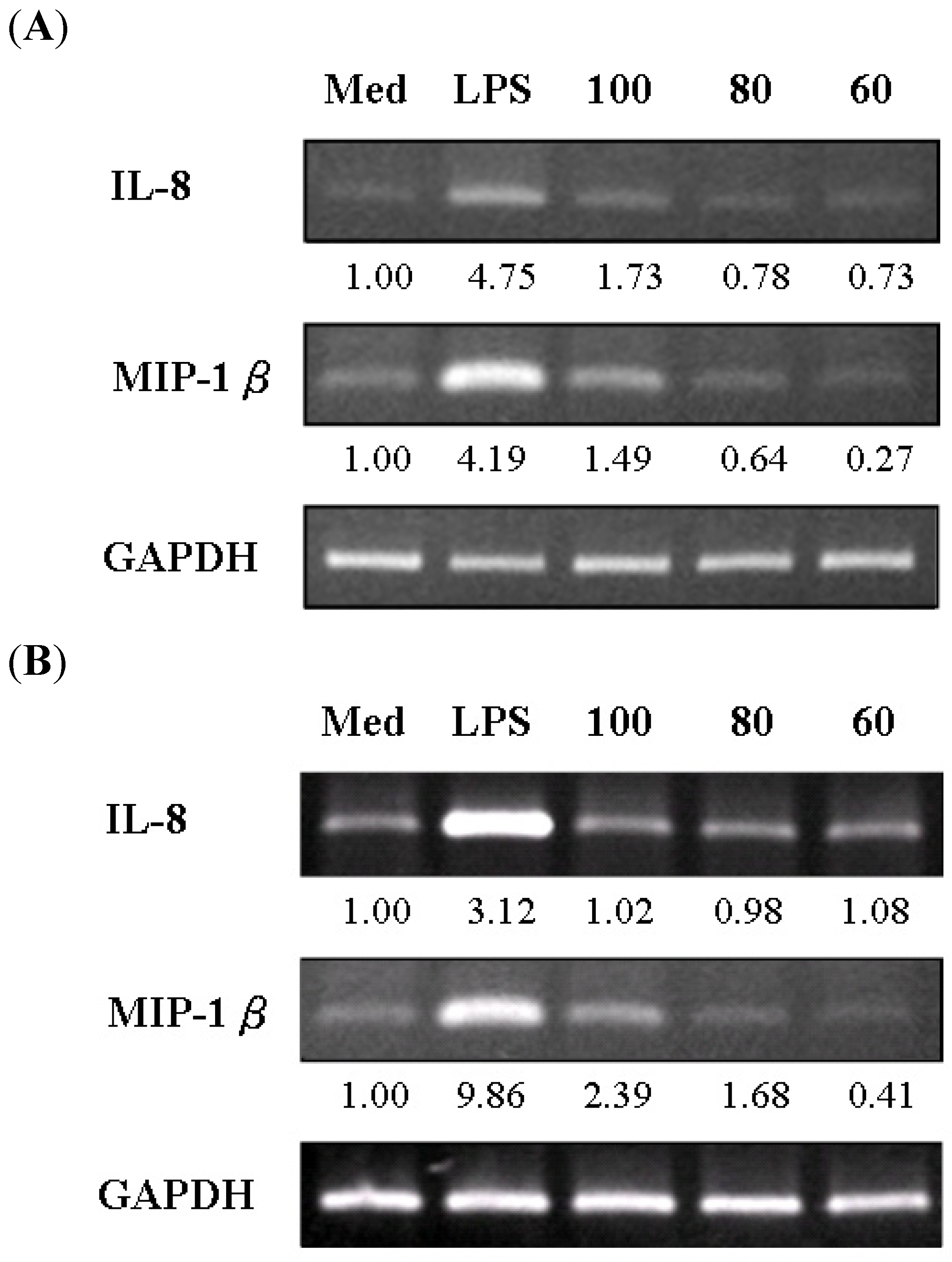
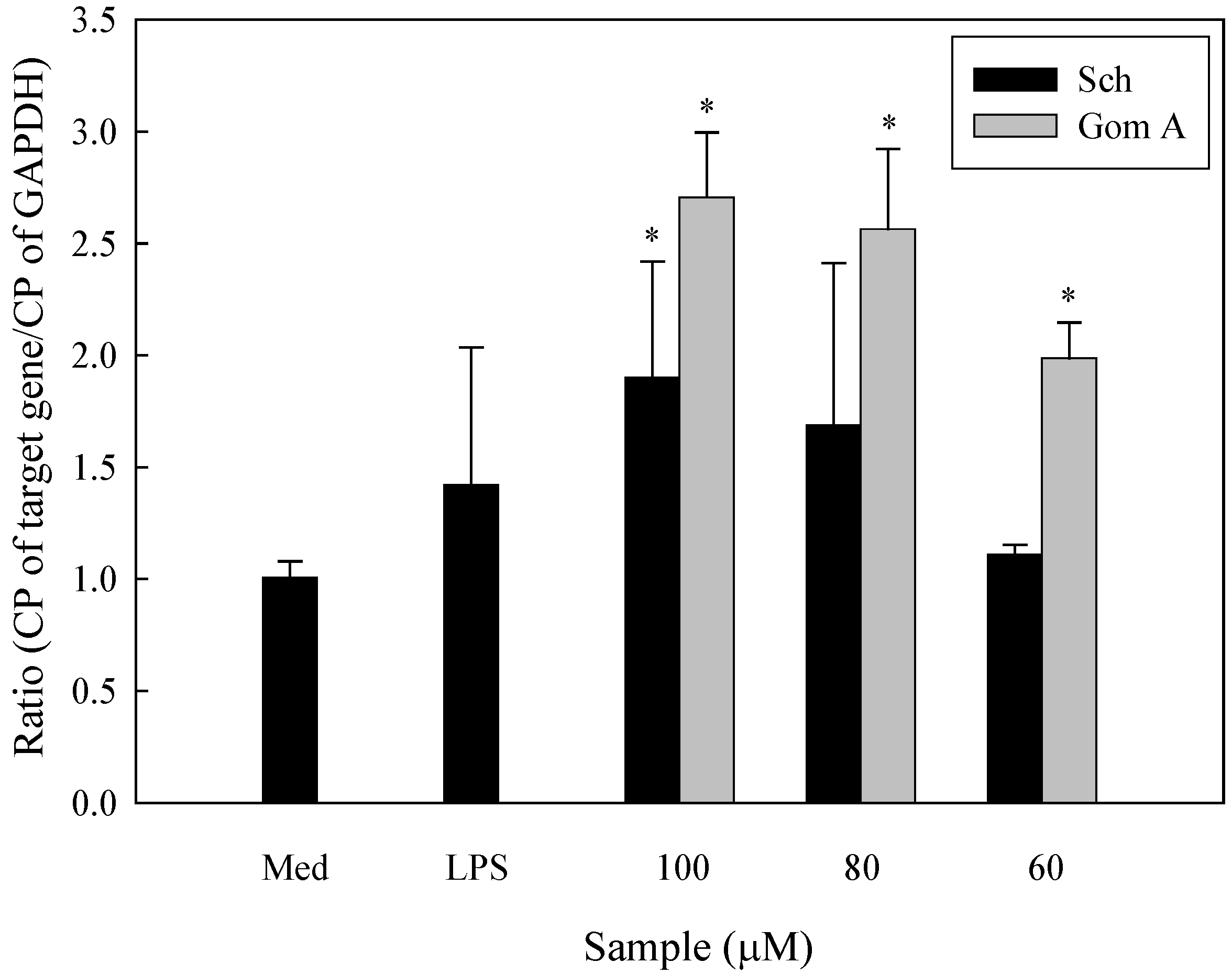
3. Experimental
3.1. Materials
3.2. Chemicals and Reagents
3.3. Isolation and Identification of Active Constituents of S. chinensis
3.4. HPLC Analysis
3.5. Preparation of Samples
3.6. Cells and Cell Cultures
3.7. MTT Assay
3.8. Cytokine Release from Cells by Microparticle-Based Flow Cytometric Analysis
3.9. RNA Isolation and Reverse Transcription
3.10. PCR Primers
3.11. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.12. Quantitative Real-Time PCR (qRT-PCR)
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Jinushi, M.; Tahara, H. Cytokine gene-mediated immunotherapy: current status and future perspectives. Cancer Sci. 2009, 100, 1389–1396. [Google Scholar] [CrossRef]
- Burgdorf, S.K.; Claesson, M.H.; Nielsen, H.J.; Rosenberg, J. Changes in cytokine and biomarker blood levels in patients with colorectal cancer during dendritic cell-based vaccination. Acta Oncol. 2009, 48, 1157–1164. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Fukuda, K.; Nakamura, Y.; Kumagai, N.; Nishida, T. Inhibition by triptolide of chemokine, proinflammatory cytokine, and adhesion molecule expression induced by lipopolysaccharide in corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3796–3800. [Google Scholar] [CrossRef]
- Harzstark, A.L.; Small, E.J. Immunotherapeutics in development for prostate cancer. Oncologist 2009, 14, 391–398. [Google Scholar] [CrossRef]
- Covaleda, L.; Fuller, F.J.; Payne, S.L. EIAV S2 enhances pro-inflammatory cytokine and chemokine response in infected macrophages. Virology 2009, 397, 217–223. [Google Scholar]
- Chada, S.; Ramesh, R.; Mhashilkar, A.M. Cytokine- and chemokine-based gene therapy for cancer. Curr. Opin. Mol. Ther. 2003, 5, 463–474. [Google Scholar]
- Nakashima, E.; Oya, A.; Kubota, Y.; Kanada, N.; Matsushita, R.; Takeda, K.; Ichimura, F.; Kuno, K.; Mukaida, N.; Hirose, K.; et al. A candidate for cancer gene therapy: MIP-1 alpha gene transfer to an adenocarcinoma cell line reduced tumorigenicity and induced protective immunity in immunocompetent mice. Pharm. Res. 1996, 13, 1896–1901. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Ko, K.M. Schisandrin B protects myocardial ischemia-reperfusion injury partly by inducing Hsp25 and Hsp70 expression in rats. Mol. Cell. Biochem. 2004, 266, 139–144. [Google Scholar] [CrossRef]
- Madgula, V.L.; Avula, B.; Choi, Y.W.; Pullela, S.V.; Khan, I.A.; Walker, L.A.; Khan, S.I. Transport of Schisandra chinensis extract and its biologically-active constituents across Caco-2 cell monolayers - an in-vitro model of intestinal transport. J. Pharm. Pharmacol. 2008, 60, 363–370. [Google Scholar]
- Chiu, P.Y.; Mak, D.H.; Poon, M.K.; Ko, K.M. In vivo antioxidant action of a lignan-enriched extract of Schisandra fruit and an anthraquinone-containing extract of Polygonum root in comparison with schisandrin B and emodin. Planta Med. 2002, 68, 951–956. [Google Scholar] [CrossRef]
- Ko, K.M.; Ip, S.P.; Poon, M.K.; Wu, S.S.; Che, C.T.; Ng, K.H.; Kong, Y.C. Effect of a lignan-enriched fructus schisandrae extract on hepatic glutathione status in rats: protection against carbon tetrachloride toxicity. Planta Med. 1995, 61, 134–137. [Google Scholar] [CrossRef]
- Nomura, M.; Nakachiyama, M.; Hida, T.; Ohtaki, Y.; Sudo, K.; Aizawa, T.; Aburada, M.; Miyamoto, K.I. Gomisin A, a lignan component of Schizandora fruits, inhibits development of preneoplastic lesions in rat liver by 3'-methyl-4-dimethylamino-azobenzene. Cancer Lett. 1994, 76, 11–18. [Google Scholar] [CrossRef]
- Zhu, M.; Yeung, R.Y.; Lin, K.F.; Li, R.C. Improvement of phase I drug metabolism with Schisandra chinensis against CCl4 hepatotoxicity in a rat model. Planta Med. 2000, 66, 521–525. [Google Scholar] [CrossRef]
- You, J.S.; Pan, T.L.; Hou, Y.C. Schisandra chinensis protects against adriamycin-induced cardiotoxicity in rats. Chang Gung Med. J. 2006, 29, 63–70. [Google Scholar]
- Chien, C.F.; Wu, Y.T.; Tsai, T.H. Biological analysis of herbal medicines used for the treatment of liver diseases. Biomed. Chromatogr. 2011, 25, 21–38. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Li Volti, G.; Lavazza, A.; Rezzani, R.; Rodella, L.F. Schisandrin B stimulates a cytoprotective response in rat liver exposed to mercuric chloride. Food Chem. Toxicol. 2009, 47, 2834–2840. [Google Scholar] [CrossRef]
- Ma, D.; Shan, A.; Li, J.; Zhao, Y.; Guo, X. Influence of an aqueous extract of Ligustrum lucidum and an ethanol extract of Schisandra chinensis on parameters of antioxidative metabolism and spleen lymphocyte proliferation of broilers. Arch. Anim. Nutr. 2009, 63, 66–74. [Google Scholar] [CrossRef]
- Ko, K.M.; Chen, N.; Leung, H.Y.; Leong, E.P.; Poon, M.K.; Chiu, P.Y. Long-term schisandrin B treatment mitigates age-related impairments in mitochondrial antioxidant status and functional ability in various tissues, and improves the survival of aging C57BL/6J mice. Biofactors 2008, 34, 331–342. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; Wu, H. Non-thermal extraction of effective ingredients from Schisandra chinensis Baill and the antioxidant activity of its extract. Nat. Prod. Res. 2009, 23, 1390–1401. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Leung, H.Y.; Ko, K.M. Schisandrin B Enhances Renal Mitochondrial Antioxidant Status, Functional and Structural Integrity, and Protects against Gentamicin-Induced Nephrotoxicity in Rats. Biol. Pharm. Bull. 2008, 31, 602–605. [Google Scholar] [CrossRef]
- Choi, Y.W.; Takamatsu, S.; Khan, S.I.; Srinivas, P.V.; Ferreira, D.; Zhao, J.; Khan, I.A. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: Structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod. 2006, 69, 356–359. [Google Scholar] [CrossRef]
- Narimanian, M.; Badalyan, M.; Panosyan, V.; Gabrielyan, E.; Panossian, A.; Wikman, G.; Wagner, H. Impact of Chisan (ADAPT-232) on the quality-of-life and its efficacy as an adjuvant in the treatment of acute non-specific pneumonia. Phytomedicine 2005, 12, 723–729. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Zhang, Q.; Liu, J.Y.; Tan, R.X. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J. Ethnopharmacol. 2005, 98, 329–333. [Google Scholar] [CrossRef]
- Kang, O.H.; Chae, H.S.; Choi, J.H.; Choi, H.J.; Park, P.S.; Cho, S.H.; Lee, G.H.; So, H.Y.; Choo, Y.K.; Kweon, O.H.; Kwon, D.Y. Effects of the Schisandra fructus water extract on cytokine release from a human mast cell line. J. Med. Food 2006, 9, 480–486. [Google Scholar] [CrossRef]
- Smejkal, K.; Slapetova, T.; Krmencik, P.; Kubinova, R.; Suchy, P.; Dall'Acqua, S.; Innocenti, G.; Vanco, J.; Kalvarova, K.; Dvorska, M.; et al. Evaluation of the antiradical activity of Schisandra chinensis lignans using different experimental models. Molecules 2010, 15, 1223–1231. [Google Scholar] [CrossRef]
- Huyke, C.; Engel, K.; Simon-Haarhaus, B.; Quirin, K.W.; Schempp, C.M. Composition and biological activity of different extracts from Schisandra sphenanthera and Schisandra chinensis. Planta Med. 2007, 73, 1116–1126. [Google Scholar] [CrossRef]
- Prunet, C.; Montange, T.; Vejux, A.; Véjux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Néel, D.; et al. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A 2006, 69, 359–373. [Google Scholar]
- Mpiga, P.; Mansour, S.; Morisset, R.; Beaulieu, R.; Ravaoarinoro, M. Sustained interleukin-6 and interleukin-8 expression following infection with Chlamydia trachomatis serovar L2 in a HeLa/THP-1 cell co-culture model. Scand. J. Immunol. 2006, 63, 199–207. [Google Scholar] [CrossRef]
- Lillard, J.W., Jr.; Singh, U.P.; Boyaka, P.N.; Singh, S.; Taub, D.D.; McGhee, J.R. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood 2003, 101, 807–814. [Google Scholar] [CrossRef]
- Schweizerhof, M.; Stosser, S.; Kurejova, M.; Njoo, C.; Gangadharan, V.; Agarwal, N.; Schmelz, M.; Bali, K.K.; Michalski, C.W.; Brugger, S.; et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat. Med. 2009, 15, 802–807. [Google Scholar]
- Jaeger, K.; Scheinichen, D.; Heine, J.; Ruschulte, H.; Kuse, E.; Winkler, M.; Leuwer, M. GM-CSF increases in vitro the respiratory burst of human neutrophils after liver transplantation. Intens. Care Med. 1999, 25, 612–615. [Google Scholar] [CrossRef]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Osaki, M.; Kawamata, M.; Kato, T.; Okochi, H.; Ochiya, T. IFATS collection: In vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 2008, 26, 2705–2712. [Google Scholar] [CrossRef]
- Flohe, S.; Borgermann, J.; Dominguez, F.E.; Majetschak, M.; Lim, L.; Kreuzfelder, E.; Obertacke, U.; Nast-Kolb, D.; Schade, F.U. Influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) on whole blood endotoxin responsiveness following trauma, cardiopulmonary bypass, and severe sepsis. Shock 1999, 12, 17–24. [Google Scholar]
- Ikeya, Y.; Taguchi, H.; Sasaki, H.; Nakajima, K.; Yoshioka, I. 13C NMR magnetic resonance spectroscopy of dibenzocyclooctadiene lignans. Chem. Pharm. Bull. 1980, 27, 1383–1390. [Google Scholar]
- Kim, Y.; So, H.S.; Youn, M.J.; Kim, H.J.; Woo, W.H.; Shin, S.H.; Lee, I.; Moon, B.S.; Cho, K.H.; Park, R. Anti-inflammatory effect of Sasim extracts in PHA-stimulated THP-1 and peripheral blood mononuclear cells from cerebral infarction patients. J. Ethnopharmacol. 2007, 112, 32–39. [Google Scholar] [CrossRef]
- Gopichandran, N.; Ekbote, U.V.; Walker, J.J.; Brooke, D.; Orsi, N.M. Multiplex determination of murine seminal fluid cytokine profiles. Reproduction 2006, 131, 613–621. [Google Scholar] [CrossRef]
- Vignali, D.A. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 2000, 243, 243–255. [Google Scholar] [CrossRef]
- Choi, Y.G.; Bae, E.J.; Kim, D.S.; Park, S.H.; Kwon, S.B.; Na, J.I.; Park, K.C. Differential regulation of melanosomal proteins after hinokitiol treatment. J. Dermatol. Sci. 2006, 43, 181–188. [Google Scholar] [CrossRef]
- Sample Availability: Please contact the corresponding author.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, R.-D.; Mao, Y.-W.; Leu, S.-J.; Huang, C.-Y.; Lee, M.-H. The Immuno-Regulatory Effects of Schisandra chinensis and Its Constituents on Human Monocytic Leukemia Cells. Molecules 2011, 16, 4836-4849. https://doi.org/10.3390/molecules16064836
Lin R-D, Mao Y-W, Leu S-J, Huang C-Y, Lee M-H. The Immuno-Regulatory Effects of Schisandra chinensis and Its Constituents on Human Monocytic Leukemia Cells. Molecules. 2011; 16(6):4836-4849. https://doi.org/10.3390/molecules16064836
Chicago/Turabian StyleLin, Rong-Dih, Yi-Wen Mao, Sy-Jye Leu, Ching-Yi Huang, and Mei-Hsien Lee. 2011. "The Immuno-Regulatory Effects of Schisandra chinensis and Its Constituents on Human Monocytic Leukemia Cells" Molecules 16, no. 6: 4836-4849. https://doi.org/10.3390/molecules16064836
APA StyleLin, R.-D., Mao, Y.-W., Leu, S.-J., Huang, C.-Y., & Lee, M.-H. (2011). The Immuno-Regulatory Effects of Schisandra chinensis and Its Constituents on Human Monocytic Leukemia Cells. Molecules, 16(6), 4836-4849. https://doi.org/10.3390/molecules16064836




