3. Experimental
3.1. General
Melting points (mp) were determined on a Kofler block and are uncorrected. The reactions were monitored by TLC on Kieselgel-G (Merck Si 254 F) layers (0.25 mm thick); solvent systems (ss): (A) CH2Cl2/EtOAc (95:5 v/v), (B) CH2Cl2/EtOAc (80:20 v/v), (C) CH2Cl2/EtOAc (50:50 v/v). The spots were detected by spraying with 5% phosphomolybdic acid in 50% aqueous phosphoric acid. The Rf values were determined for the spots observed by illumination at 254 and 365 nm. Flash chromatography: Merck silica gel 60, 40-63 μm. All solvents were distilled prior to use. Reagents and materials were obtained from commercial suppliers and were used without purification.
Elementary analysis data were determined with a PerkinElmer CHN analyzer model 2400 and IR spectra were recorded on a BioRad FTS 60A FTIR spectrometer. NMR spectra were obtained at room temperature with a Bruker DRX 500 instrument. Chemical shifts are reported in ppm (δ scale), and coupling constants (J) in Hz. For the determination of multiplicities, the J-MOD pulse sequence was used.
Automated flow injection analyses were performed by using an HPLC/MSD system. The system comprised an Agilent 1100 micro vacuum degasser, a quaternary pump, a micro-well plate autoinjector and a 1946A MSD equipped with an electrospray ion source (ESI) operated in positive ion mode. The ESI parameters were: nebulizing gas N2, at 35 psi; drying gas N2, at 350 °C and 12 L/min; capillary voltage (VCap) 3000 V; fragmentor voltage 70 V. The MSD was operated in scan mode with a mass range of m/z 60−620. Samples (0.2 μL) with automated needle wash were injected directly into the solvent flow (0.3 mL/min) of CH3CN/H2O, 70:30 (v/v) supplemented with 0.1% formic acid. The system was controlled by Agilent LC/MSD Chemstation software.
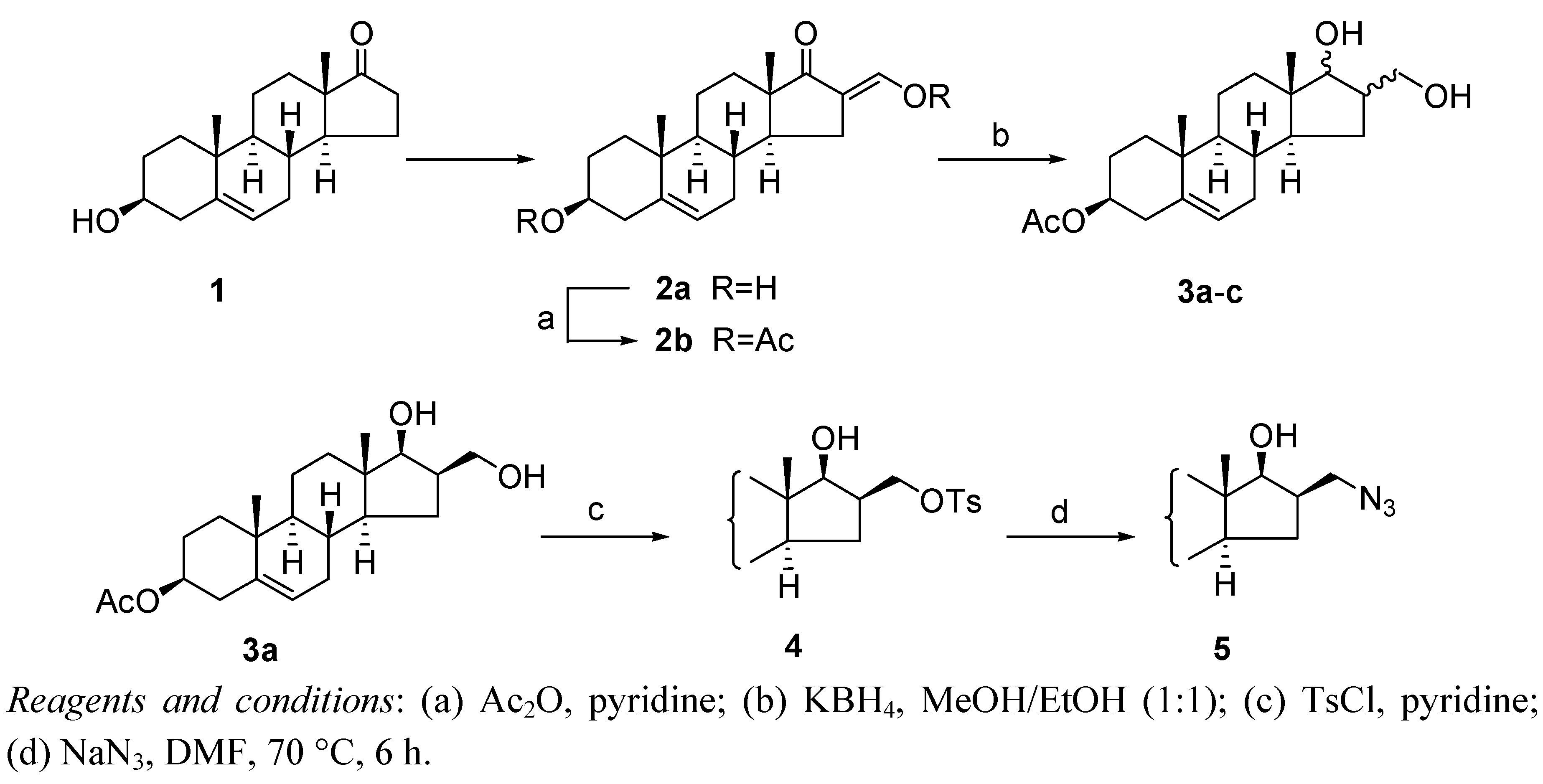
3.2. 3β-Acetoxy-16-acetoxymethylideneandrost-5-en-17-one (2b)
Compound
2a (19.9 g, 63 mmol) was dissolved in a mixture of pyridine (40 mL) and Ac
2O (40 mL), and the solution was stirred overnight, and then poured onto a mixture of ice and H
2SO
4 (18 mL). The precipitate was collected by filtration, washed to neutrality and dried, resulting in 23.8 g (94%) of
2b, mp 199-202 °C (lit. [
28] mp 198-200 °C),
Rf = 0.68 (ss A).
3.3. 3β-Acetoxy-16β-hydroxymethylandrost-5-en-17β-ol (3a)
Finely powdered
2b (23.8 g, 59.5 mmol) was suspended in a mixture of MeOH and EtOH (1:1, 500 mL), and KBH
4 (8 g, 148 mmol) was added in small portions. To maintain pH 6-8, the solution was repeatedly acidified as needed with MeOH/AcOH (1:1), using bromothymol blue as indicator. After completion of the reaction, the mixture was diluted with water and acidified with dilute HCl. The precipitate that formed was filtered off and washed with water to neutrality. The resulting crude product was purified by column chromatography, with CH
2Cl
2/EtOAc (8:2) as eluent, yielding
3a as a white solid (10.35 g, 48%), mp 197-199 °C (lit. [
28] mp 199-201 °C),
Rf = 0.44 (ss C). The spectroscopic data were consistent with those reported in the literature.
3.4. 3β-Acetoxy-16β-p-toluenesulfonyloxymethylandrost-5-en-17β-ol (4)
Compound 3a (7.25 g, 20 mmol) was dissolved in pyridine (50 mL), and a solution of p-toluene-sulfonyl chloride (7 g, 35 mmol) in pyridine (10 mL) was then added dropwise while cooling in ice. The reaction mixture was allowed to stand overnight, and was then poured into a mixture of ice and H2SO4 (20 mL). The precipitate that formed was filtered off and washed with water to neutrality. This substance was used in the subsequent step without further purification and characterization.
3.5. 3β-Acetoxy-16β-azidomethylandrost-5-en-17β-ol (5)
Sodium azide (1.8 g, 28 mmol) was added to a solution of 4 (5.8 g, 11 mmol) in DMF (80 mL). The reaction mixture was stirred at 70 °C for 6 h and was then poured into water. The precipitate that formed was allowed to stand overnight, and then filtered off and washed with water. Purification of the resulting crude product by column chromatography with CH2Cl2 as eluent afforded 5 as a white solid (3.75 g, 86%), mp 144-145 °C, Rf = 0.58 (ss A); 1H-NMR (CDCl3); δ [ppm] = 0.78 (s, 3H, 18-CH3), 0.95 (m, 1H), 1.03 (s, 3H, 19-CH3), 1.08-1.17 (overlapping m, 3H), 1.45 (m, 1H), 1.51-1.62 (overlapping m, 5H), 1.82-1.90 (overlapping m, 4H), 1.99 (m, 1H), 2.03 (s, 3H, Ac-CH3), 2.32 (m, 2H, 4-H2), 2.38 (m, 1H, 16-H), 3.31 (dd, 1H, J = 12.0 Hz, J = 6.5 Hz, 16a-H), 3.57 (dd, 1H, J = 12.0 Hz, J = 7.0 Hz, 16a-H), 3.79 (d, 1H, J = 10.0 Hz, 17-H), 4.60 (m, 1H, 3-H), 5.37 (d, 1H, J = 5.0 Hz, 6-H);13C-NMR (CDCl3); δ [ppm] = 12.1 (C-18), 19.3 (C-19), 20.5 (C-11), 21.4 (Ac-CH3), 27.7, 30.5, 31.1, 31.6, 36.6, 37.0, 37.4, 38.0, 39.9, 43.6, 49.9, 50.0, 53.3, 73.8 (C-3), 81.3 (C-17), 122.1 (C-6), 139.7 (C-5), 170.5 (Ac-CO); IR (neat, cm−1) 3526, 2945, 2909, 2112, 1717, 1439, 1365, 1256, 1032. ESI-MS: 388 (M+H)+; Anal. Calcd for C22H33N3O3 C, 68.19; H, 8.58; N, 10.84. Found: C, 68.01; H, 8.73; N, 11.04.
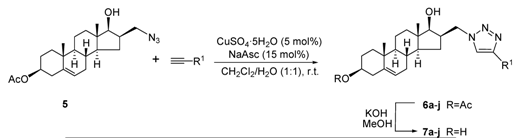
3.6. General Procedure for Preparation of 3β-acetoxy-16β-(4-phenyl-, substituted 4-phenyl- or 4-cycloalkyl-1H-1,2,3-triazol-1-ylmethyl)androst-5-en-17β-ols 6a-j
Compound 5 (387 mg, 1 mmol) was dissolved in CH2Cl2 (10 mL), and a solution of CuSO4·5H2O (12.5 mg, 5 mol %) and sodium ascorbate (30 mg, 15 mol %) in water (10 mL) was poured into the organic phase. The appropriate terminal alkyne (1.1 mmol) was added to the reaction mixture, which was then stirred for 1-4 h at ambient temperature. After the disappearance of the starting material (TLC monitoring), the two-phase solution was diluted with water (20 mL) and extracted with CH2Cl2 (2 × 20 mL). The combined organic layers were washed with water, dried over Na2SO4 and evaporated in vacuo. The resulting crude product was purified by flash chromatography with CH2Cl2/EtOAc (90:10), or CH2Cl2/EtOAc (80:20) as eluent.
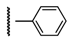
3β-Acetoxy-16β-(4-phenyl-1H-1,2,3-triazol-1-ylmethyl)androst-5-en-17β-ol (6a): Alkyne: phenyl-acetylene (0.12 mL). After purification, 6a was obtained as a white solid (435 mg, 89%), mp 251-252 °C, Rf = 0.30 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.80 (s, 3H, 18-CH3), 0.89 (m, 1H), 1.02 (s, 3H, 19-CH3), 1.11-1.20 (overlapping m, 3H), 1.37-1.59 (overlapping m, 6H), 1.80-1.91 (overlapping m, 4H), 1.97 (dd, 1H, J = 12.0 Hz, J = 2.5 Hz), 2.03 (s, 3H, Ac-CH3), 2.32 (m, 2H, 4-H2), 2.74 (m, 1H, 16-H), 3.88 (d, 1H, J = 10.0 Hz, 17-H), 4.32 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 4.58 (m, 1H, 3-H), 4.70 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.34 (br s, 1H, 6-H), 7.32 (t, 1H, J = 7.0 Hz, 4”-H), 7.41 (t, 2H, J = 7.0 Hz, 3”- and 5”-H), 7.82 (m, 3H, 2”-, 6”- and 5’-H); 13C-NMR (CDCl3); δ [ppm] = 12.3 (C-18), 19.3 (C-19), 20.5 (C-11), 21.4 (Ac-CH3), 27.7, 31.0, 31.1, 31.6, 36.6, 37.0, 37.3, 38.0, 41.3, 43.7, 49.9, 50.0, 52.0, 73.8 (C-3), 80.6 (C-17), 120.6 (C-5’), 122.0 (C-6), 125.7 (2C, C-2” and C-6”), 128.2 (C-4”), 128.8 (2C, C-3” and C-5”), 130.2 (C-1”), 139.7 (C-5), 147.2 (C-4’), 170.6 (Ac-CO); IR (neat, cm−1) 3404, 2941, 2910, 1732, 1373, 1242, 1034, 772, 706. ESI-MS: 490 (M+H)+; Anal. Calcd for C30H39N3O3: C, 73.59; H, 8.03; N, 8.58. Found: C, 73.70; H, 8.19; N, 8.76.
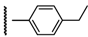
3β-Acetoxy-16β-[4-(4-ethylphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6b): Alkyne: 4-ethylphenylacetylene (0.15 mL). After purification, 6b was obtained as a white solid (470 mg, 91%), mp 249-250 °C, Rf = 0.36 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.79 (s, 3H, 18-CH3), 0.94 (m, 1H), 1.02 (s, 3H, 19-CH3), 1.09-1.21 (overlapping m, 3H), 1.25 (t, 3H, J = 7.5 Hz, Et-CH3), 1.41-1.62 (overlapping m, 6H), 1.79-1.92 (overlapping m, 4H), 1.95 (dd, 1H, J = 12.5 Hz, J = 2.5 Hz), 2.03 (s, 3H, Ac-CH3), 2.32 (m, 2H, 4-H2), 2.67 (q, 2H, J = 7.5 Hz, Et-CH2), 2.73 (m, 1H, 16-H), 3.87 (d, 1H, J = 10.0 Hz, 17-H), 4.30 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 4.58 (m, 1H, 3-H), 4.69 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.34 (br s, 1H, 6-H), 7.24 (d, 2H, J = 7.5 Hz, 3”- and 5”-H), 7.72 (d, 2H, J = 7.5 Hz, 2”- and 6”-H), 7.79 (s, 1H, 5’-H); 13C-NMR (CDCl3); δ [ppm] = 12.3 (C-18), 19.3 (C-19), 20.5 (C-11), 21.4 (Ac-CH3), 27.7, 28.6, 31.0, 31.1, 31.6, 36.6, 36.9, 37.0, 37.3, 38.0, 41.3, 43.7, 49.9, 50.0, 52.0, 73.8, 80.6, 120.3 (C-5’), 122.1 (C-6), 125.7 (2C, C-2” and C-6”), 127.7 (C-1”), 128.3 (2C, C-3” and C-5”), 139.7 (C-5), 144.4 (C-4”), 147.3 (C-4’), 170.5 (Ac-CO); IR (neat, cm−1) 3383, 2945, 2854, 1730, 1375, 1248, 1032, 976, 833, 613. ESI-MS: 518 (M+H)+; Anal. Calcd for C32H43N3O3: C, 74.24; H, 8.37; N, 8.12. Found: C, 74.38; H, 8.24; N, 8.19.
3β-Acetoxy-16β-[4-(3-tolyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6c): Alkyne: 3-tolyl-acetylene (0.14 mL). After purification, 6c was obtained as a white solid (458 mg, 91%), mp 232-233 °C, Rf = 0.36 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.79 (s, 3H, 18-CH3), 0.95 (m, 1H), 1.01 (s, 3H, 19-CH3), 1.08-1.21 (overlapping m, 3H), 1.33-1.61 (overlapping m, 6H), 1.79-1.90 (overlapping m, 4H), 1.95 (dd, 1H, J = 12.0 Hz, J = 3.0 Hz), 2.02 (s, 3H, Ac-CH3), 2.31 (m, 2H, 4-H2), 2.38 (s, 3H, 3”-CH3), 2.72 (m, 1H, 16-H), 3.87 (d, 1H, J = 10.0 Hz, 17-H), 4.30 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 4.58 (m, 1H, 3-H), 4.69 (dd, 1H, J = 14.0 Hz, J = 6.5 Hz, 16a-H), 5.33 (d, 1H, J = 4.0 Hz, 6-H), 7.13 (d, 1H, J = 7.0 Hz, 4”-H), 7.29 (t, 1H, J = 7.5 Hz, 5”-H), 7.57 (d, 1H, J = 7.5 Hz, 6”-H), 7.65 (s, 1H, 2”-H), 7.81 (s, 1H, 5’-H); 13C-NMR (CDCl3); δ [ppm] = 12.3 (C-18), 19.3 (C-19), 20.4 (C-11), 21.4 (Ac-CH3), 27.7, 29.6, 31.0, 31.1, 31.6, 36.6, 37.0, 37.3, 38.0, 41.3, 43.7, 49.8, 50.0, 52.0, 73.8 (C-3), 80.6 (C-17), 120.6 (C-5’), 122.0 (C-6), (122.7, 126.3, 128.7, 128.9): (4C, C-2”, C-4”, C-5” and C-6”), 130.2 (C-1”), 138.5 (C-3”), 139.7 (C-5), 147.3 (C-4’), 170.6 (Ac-CO); IR (neat, cm−1) 3406, 2922, 2850, 1731, 1364, 1244, 1024, 787, 696. ESI-MS: 504 (M+H)+; Anal. Calcd for C31H41N3O3: C, 73.92; H, 8.20; N, 8.34. Found: C, 73.80; H, 8.38; N, 8.49.
3β-Acetoxy-16β-[4-(4-tert-butylphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6d): Alkyne: 4-tert-butylphenylacetylene (0.2 mL). After purification, 6d was obtained as a white solid (507 mg, 93%), mp 318-319 °C, Rf = 0.39 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.79 (s, 3H, 18-CH3), 0.88 (m, 1H), 1.02 (s, 3H, 19-CH3), 1.34 (s, 9H, 3 x tBu-CH3), 1.11-1.20 (overlapping m, 3H), 1.37-1.60 (overlapping m, 6H), 1.79-1.91 (overlapping m, 4H), 1.96 (dd, 1H, J = 11.5 Hz, J = 2.5 Hz), 2.03 (s, 3H, Ac-CH3), 2.31 (m, 2H, 4-H2), 2.75 (m, 1H, 16-H), 3.89 (d, 1H, J = 10.0 Hz, 17-H), 4.31 (m, 1H, 16a-H), 4.60 (m, 1H, 3-H), 4.72 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 5.34 (d, 1H, J = 4.0 Hz, 6-H), 7.45 (d, 2H, J = 6.0 Hz, 3”- and 5”-H), 7.78 (d, 2H, J = 6.0 Hz, 2”- and 6”-H), 7.87 (s, 1H, 5’-H); 13C-NMR (CDCl3); δ [ppm] = 12.3 (C-18), 19.3 (C-19), 20.5 (C-11), 21.4 (Ac-CH3), 27.7, 29.7, 30.9, 31.1, 31.3 (3C, 3 x tBu-CH3), 31.6, 36.6, 37.0, 37.3, 38.0, 41.3, 43.8, 49.9, 50.0, 52.1, 73.7 (C-3), 80.6 (C-17), 120.4 (C-5’), 122.0 (C-6), 125.5 (2C), 125.8 (2C), 127.3 (C-1”), 139.7 (C-5), 147.1 (C-4’), 151.4 (C-4”), 170.5 (Ac-CO); IR (neat, cm−1) 3441, 2949, 2903, 1730, 1454, 1366, 1247, 1034, 837, 810, 557. ESI-MS: 546 (M+H)+; Anal. Calcd for C34H47N3O3: C, 74.83; H, 8.68; N, 7.70. Found: C, 75.02; H, 8.61; N, 7.82.
3β-Acetoxy-16β-[4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6e): Alkyne: 4-methoxyphenylacetylene (145 mg). After purification, 6e was obtained as a white solid (447 mg, 86%), mp 241-242 °C, Rf = 0.34 (ss B); 1H-NMR (500 MHz, 10% MeOD/CDCl3); δ [ppm] = 0.81 (s, 3H, 18-CH3), 0.93 (m, 1H), 1.03 (s, 3H, 19-CH3), 1.09-1.21 (overlapping m, 3H), 1.33-1.62 (overlapping m, 6H), 1.77-1.90 (overlapping m, 4H), 1.97 (dd, 1H, J = 12.0 Hz, J = 2.5 Hz), 2.03 (s, 3H, Ac-CH3), 2.32 (m, 2H, 4-H2), 2.72 (m, 1H, 16-H), 3.82 (d, 1H, J = 10.0 Hz, 17-H), 3.86 (s, 3H, OCH3), 4.28 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 4.58 (m, 1H, 3-H), 4.70 (dd, 1H, J = 13.5 Hz, J = 6.0 Hz, 16a-H), 5.34 (br s, 1H, 6-H), 6.96 (d, 2H, J = 8.5 Hz, 3”- and 5”-H), 7.21 (d, 2H, J = 8.5 Hz, 2”- and 6”-H), 7.81 (s, 1H, 5’-H); IR (neat, cm−1) 3398, 2935, 2902, 1728, 1499, 1373, 1244, 1063, 839, 538. ESI-MS: 520 (M+H)+; Anal. Calcd for C31H41N3O4: C, 71.65; H, 7.95; N, 8.09. Found: C, 71.74; H, 7.76; N, 8.22.
3β-Acetoxy-16β-[4-(2-methoxyphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6f): Alkyne: 2-methoxyphenylacetylene (0.14 mL). After purification, 6f was obtained as a white solid (430 mg, 83%), mp 278-279 °C, Rf = 0.32 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.79 (s, 3H, 18-CH3), 0.94 (m, 1H), 1.01 (s, 3H, 19-CH3), 1.08-1.20 (overlapping m, 3H), 1.33-1.60 (overlapping m, 6H), 1.79-1.96 (overlapping m, 5H), 2.02 (s, 3H, Ac-CH3), 2.31 (m, 2H, 4-H2), 2.73 (m, 1H, 16-H), 3.87 (d, 1H, J = 10.0 Hz, 17-H), 3.92 (s, 3H, OCH3), 4.30 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 4.58 (m, 1H, 3-H), 4.70 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.34 (br s, 1H, 6-H), 6.95 (d, 1H, J = 8.0 Hz, 3”-H), 7.06 (t, 1H, J = 7.5 Hz, 5”-H), 7.29 (t, 1H, J = 7.5 Hz, 4”-H), 8.05 (s, 1H, 5’-H), 8.31 (d, 1H, J = 7.5 Hz, 6”-H); 13C-NMR (CDCl3); δ [ppm] = 12.3 (C-18), 19.3 (C-19), 20.5 (C-11), 21.4 (Ac-CH3), 27.7, 31.0, 31.1, 31.6, 36.6, 37.0, 37.3, 38.0, 41.3, 43.7, 49.9, 50.0, 51.8, 55.3 (OCH3), 73.8 (C-3), 80.6 (C-17), 110.7, 119.2 (C-1”), 120.9 (C-5’), 122.0 (C-6), 123.9, 127.5, 128.8, 139.7 (C-5), 142.7 (C-4’), 155.5 (C-2”), 170.6 (Ac-CO); IR (neat, cm−1) 3400, 2939, 2907, 1732, 1491, 1373, 1246, 1060, 974, 750, 679. ESI-MS: 520 (M+H)+; Anal. Calcd for C31H41N3O4: C, 71.65; H, 7.95; N, 8.09. Found: C, 71.76; H, 8.08; N, 8.01.
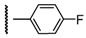
3β-Acetoxy-16β-[4-(4-fluorophenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6g): Alkyne: 4-fluorophenylacetylene (0.13 mL). After purification, 6g was obtained as a white solid (430 mg, 85%), mp 263-264 °C, Rf = 0.36 (ss B); 1H-NMR (10% MeOD/CDCl3); δ [ppm] = 0.82 (s, 3H, 18-CH3), 0.96 (m, 1H), 1.03 (s, 3H, 19-CH3), 1.10-1.22 (overlapping m, 3H), 1.40-1.63 (overlapping m, 6H), 1.78-1.91 (overlapping m, 4H), 1.97 (dd, 1H, J = 13.0 Hz, J = 2.5 Hz), 2.04 (s, 3H, Ac-CH3), 2.32 (m, 2H, 4-H2), 2.72 (m, 1H, 16-H), 3.84 (d, 1H, J = 10.0 Hz, 17-H), 4.30 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 4.58 (m, 1H, 3-H), 4.71 (dd, 1H, J = 13.5 Hz, J = 6.0 Hz, 16a-H), 5.34 (br s, 1H, 6-H), 7.11 (t, 2H, J = 7.5 Hz, 3”- and 5”-H), 7.76 (t, 2H, J = 7.5 Hz, 2”- and 6”-H), 7.88 (s, 1H, 5’-H); 13C-NMR (10% MeOD/CDCl3); δ [ppm] = 12.1 (C-18), 19.1 (C-19), 20.3 (C-11), 21.2 (Ac-CH3), 27.5, 30.9, 31.0, 31.4, 36.5, 36.8, 37.1, 37.8, 41.0, 43.5, 49.8, 49,9, 52.4, 73.9 (C-3), 80.3 (C-17), 115.7 (d, 2C, J = 21.5 Hz, C-3” and C-5”), 120.4 (C-5’), 122.0 (C-6), 126.5 (C-1”), 127.2 (d, 2C, J = 8 Hz, C-2” and C-6”), 139.5 (C-5), 146.4 (C-4’), 162.5 (d, J = 245 Hz, C-4”), 170.9 (Ac-CO); IR (neat, cm−1) 3412, 2945, 2912, 1730, 1460, 1243, 1062, 812, 524. ESI-MS: 508 (M+H)+; Anal. Calcd for C30H38FN3O3: C, 70.98; H, 7.55; N, 8.28. Found: C, 70.86; H, 7.64; N, 8.43.
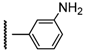
3β-Acetoxy-16β-[4-(3-aminophenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6h): Alkyne: 3-aminophenylacetylene (0.12 mL). After purification, 6h was obtained as a white solid (454 mg, 90%), mp 255-256 °C, Rf = 0.30 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.91 (m, 2H), 0.98 (s, 3H, 19-CH3), 1.02-1.15 (overlapping m, 3H), 1.38-1.56 (overlapping m, 6H), 1.74-1.90 (overlapping m, 4H), 1.97 (s, 3H, Ac-CH3), 2.25 (m, 2H, 4-H2), 2.64 (m, 1H, 16-H), 3.71 (d, 1H, J = 10.0 Hz, 17-H), 4.17 (t, 1H, J = 12.5 Hz, 16a-H), 4.43 (m, 1H, 3-H), 4.57 (dd, 1H, J = 13.5 Hz, J = 5.0 Hz, 16a-H), 5.02 (br s, 1H, OH), 5.30 (br s, 1H, 6-H), 5.66 (br s, 2H, NH2), 6.56 (d, 1H, J = 7.0 Hz, 4”-H), 6.98 (d, 1H, J = 7.0 Hz, 6”-H), 7.08 (t, 1H, J = 7.5 Hz, 5”-H), 7.14 (s, 1H, 2”-H), 8.41 (s, 1H, 5’-H); 13C-NMR (DMSO-d6); δ [ppm] = 12.4 (C-18), 19.0 (C-19), 20.1 (C-11), 21.0 (Ac-CH3), 27.3, 30.4, 30.7, 31.0, 36.1, 36.4, 36.8, 37.6, 40.3, 43.2, 49.1, 49.6, 52.2, 73.1 (C-3), 79.4 (C-17), 110.9, 113.7, 113.9, 121.1, 121.9, 129.3, 131.4, 139.4, 146.5, 147.8, 169.7 (Ac-CO); IR (neat, cm−1) 3340, 3228, 2941, 1732, 1454, 1242, 1069, 1034, 795. ESI-MS: 505 (M+H)+; Anal. Calcd for C30H40N4O3: C, 71.40; H, 7.99; N, 11.10. Found: C, 71.54; H, 7.86; N, 11.23.
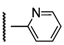
3β-Acetoxy-16β-[4-(2-pyridyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-en-17β-ol (6i): Alkyne: 2 pyridylacetylene (0.11 mL). After purification, 6i was obtained as a white solid (427 mg, 87%), mp 259-260 °C, Rf = 0.23 (ss C); 1H-NMR (CDCl3); δ [ppm] = 0.80 (s, 3H, 18-CH3), 0.93 (m, 1H), 1.01 (s, 3H, 19-CH3), 1.16-1.27 (overlapping m, 3H), 1.39-1.59 (overlapping m, 6H), 1.77-1.87 (overlapping m, 4H), 1.94 (dd, 1H, J = 12.5 Hz, J = 2.5 Hz), 2.01 (s, 3H, Ac-CH3), 2.30 (m, 2H, 4-H2), 2.72 (m, 1H, 16-H), 3.87 (d, 1H, J = 10.0 Hz, 17-H), 4.34 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 4.57 (m, 1H, 3-H), 4.75 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 5.32 (d, 1H, J = 3.0 Hz, 6-H), 7.24 (m, 1H, 4”-H), 7.79 (t, 1H, J = 7.5 Hz, 5”-H), 8.17 (d, 1H, J = 7.5 Hz, 6”-H), 8.30 (s, 1H, 5’-H), 8.54 (d, 1H, J = 3.0 Hz, 3”-H); 13C-NMR (CDCl3); δ [ppm] = 12.3 (C-18), 19.3 (C-19), 20.4 (C-11), 21.4 (Ac-CH3), 27.7, 31.0, 31.1, 31.6, 36.6, 37.0, 37.3, 38.0, 41.3, 43.7, 49.8, 50.0, 52.4, 73.8 (C-3), 80.5 (C-17), 120.4 (C-5’), 122.1 (C-6), 122.8, 123.1, 137.4, 139.6 (C-5), 147.3 (C-2”), 148.8 (C-6”), 149.9 (C-4’), 170.5 (Ac-CO); IR (neat, cm−1) 3395, 2932, 2911, 1731, 1435, 1364, 1240, 1032, 789, 540. ESI-MS: 491 (M+H)+; Anal. Calcd for C29H38N4O3: C, 70.99; H, 7.81; N, 11.42. Found: C, 71.16; H, 7.97; N, 11.61.
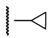
3β-Acetoxy-16β-(4-cyclopropyl-1H-1,2,3-triazol-1-ylmethyl)androst-5-en-17β-ol (6j): Alkyne: cyclo-propylacetylene (0.09 mL). After purification, 6j was obtained as a white solid (355 mg, 78%), mp 261-263 °C, Rf = 0.30 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.83 (m, 2H), 0.90-0.96 (overlapping m, 4H), 1.01 (s, 3H, 19-CH3), 1.08-1.17 (overlapping m, 3H), 1.41-1.61 (overlapping m, 5H), 1.76-1.95 (overlapping m, 5H), 2.02 (s, 3H, Ac-CH3), 2.31 (m, 2H, 4-H2), 2.63 (m, 1H, 16-H), 3.08 (br s, 1H, 1”-H), 3.84 (dd, 1H, J = 10.0 Hz, J = 3.5 Hz, 17-H), 4.19 (dd, 1H, J = 13.5 Hz, J = 6.5 Hz, 16a-H), 4.57-4.61 (overlapping m, 2H, 3- and 16a-H), 5.33 (d, 1H, J = 3.5 Hz, 6-H), 7.28 (s, 1H, 5’-H); 13C-NMR (CDCl3); δ [ppm] = 6.6, 7.7 (2C), 12.3 (C-18), 19.3 (C-19), 20.5 (C-11), 21.4 (Ac-CH3), 27.7, 31.0, 31.1, 31.6, 36.6, 37.0, 37.3, 38.0, 41.4, 43.7, 49.9, 50.0, 51.7, 73.8 (C-3), 80.5 (C-17), 120.5 (C-5’), 122.1 (C-6), 139.7 (C-5), 149.9 (C-4’), 170.5 (Ac-CO); IR (neat, cm−1) 3394, 2943, 1732, 1431, 1372, 1246, 1068, 1034, 814. ESI-MS: 454 (M+H)+; Anal. Calcd for C27H39N3O3: C, 71.49; H, 8.67; N, 9.26. Found: C, 71.61; H, 8.82; N, 9.57.
3.7. General procedure for preparation of 16β-(4-phenyl-, substituted 4-phenyl- or 4-cycloalkyl-1H-1,2,3-triazol-1-ylmethyl)androst-5-ene-3β,17β-diols 7a-j
Compounds 6a-j (0.5 mmol) were deacetylated by dissolving in MeOH (20 mL), adding KOH (150 mg, 2.7 mmol), stirring the mixture for 1 h at 50 °C, and then pouring into water (200 mL) and neutralizing with diluted HCl. The resulting precipitate was filtered off, washed with water and dried. The crude product obtained was purified by flash chromatography (silica gel) to afford 7a-j.
16β-(4-Phenyl-1H-1,2,3-triazol-1-ylmethyl)androst-5-ene-3β,17β-diol (7a): Eluent: CH2Cl2/EtOAc (75:25), yielding 7a as a white solid (184 mg, 82%), mp 264-265 °C, Rf = 0.45 (ss C); 1H-NMR (10% MeOD/CDCl3); δ [ppm] = 0.82 (s, 3H, 18-CH3), 0.94-0.98 (m, 2H), 1.02 (s, 3H, 19-CH3), 1.09-1.25 (overlapping m, 3H), 1.45-1.61 (overlapping m, 5H), 1.81-1.97 (overlapping m, 5H), 2.20-2.28 (m, 2H), 2.73 (m, 1H, 16-H), 3.47 (m, 1H, 3-H), 3.84 (d, 1H, J = 10.0 Hz, 17-H), 4.30 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 4.72 (dd, 1H, J = 13.5 Hz, J = 6.0 Hz, 16a-H), 5.31 (br s, 1H, 6-H), 7.34 (t, 1H, J = 7.5 Hz, 4”-H), 7.43 (t, 2H, J = 7.5 Hz, 3”- and 5”-H), 7.79 (d, 2H, J = 7.5 Hz, 2”- and 6”-H), 7.89 (s, 1H, 5’-H); IR (neat, cm−1) 3428, 2944, 2904, 1444, 1236, 1080, 827, 760, 691. ESI-MS: 448 (M+H)+; Anal. Calcd for C28H37N3O2: C, 75.13; H, 8.33; N, 9.39. Found: C, 75.27; H, 8.21; N, 9.56.
16β-[4-(4-Ethylphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7b): Eluent: CH2Cl2/ EtOAc (75:25), yielding 7b as a white solid (192 mg, 81%), mp 261-262 °C, Rf = 0.48 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.75 (s, 3H, 18-CH3), 0.83-0.88 (m, 2H), 0.94 (s, 3H, 19-CH3), 1.03-1.12 (overlapping m, 3H), 1.19 (t, 3H, J = 7.5 Hz, Et-CH3), 1.30-1.44 (overlapping m, 4H), 1.52 (m, 2H), 1.66 (m, 1H), 1.74-1.86 (overlapping m, 3H), 2.05-2.14 (m, 2H), 2.59 (q, 2H, J = 7.5 Hz, Et-CH2), 2.65 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.71 (dd, 1H, J = 9.5 Hz, J = 3.5 Hz, 17-H), 4.18 (m, 1H, 16a-H), 4.58 (overlapping m, 2H, 3-OH and 16a-H), 5.01 (d, 1H, J = 3.5 Hz, 17-OH), 5.21 (br s, 1H, 6-H), 7.26 (d, 2H, J = 7.5 Hz, 3”- and 5”-H), 7.74 (d, 2H, J = 7.5 Hz, 2”- and 6”-H), 8.51 (s, 1H, 5’-H); IR (neat, cm−1) 3383, 2943, 1440, 1240, 1082, 1051, 812, 738, 644. ESI-MS: 476 (M+H)+; Anal. Calcd for C30H41N3O2: C, 75.75; H, 8.69; N, 8.83. Found: C, 75.91; H, 8.87; N, 8.70.
16β-[4-(3-Tolyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7c): Eluent: CH2Cl2/EtOAc (75:25), yielding 7c as a white solid (203 mg, 88%), mp 237-238 °C, Rf = 0.44 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.86 (m, 2H), 0.94 (s, 3H, 19-CH3), 1.04-1.17 (overlapping m, 3H), 1.31-1.43 (overlapping m, 4H), 1.51 (m, 2H), 1.65 (m, 1H), 1.73-1.87 (overlapping m, 3H), 2.07-2.15 (m, 2H), 2.34 (s, 3H, 3”-CH3), 2.64 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.71 (dd, 1H, J = 9.5 Hz, J = 4.0 Hz, 17-H), 4.19 (m, 1H, 16a-H), 4.59 (overlapping m, 2H, 3-OH and 16a-H), 5.00 (d, 1H, J = 4.0 Hz, 17-OH), 5.21 (br s, 1H, 6-H), 7.12 (d, 1H, J = 7.0 Hz, 4”-H), 7.31 (t, 1H, J = 7.5 Hz, 5”-H), 7.62 (d, 1H, J = 7.0 Hz, 6”-H), 7.66 (s, 1H, 2”-H), 8.53 (s, 1H, 5’-H); IR (neat, cm−1) 3339, 3237, 2931, 1452, 1232, 1049, 787, 696. ESI-MS: 462 (M+H)+; Anal. Calcd for C29H39N3O2: C, 75.45; H, 8.52; N, 9.10. Found: C, 75.57; H, 8.67; N, 9.32.
16β-[4-(4-Tert-butylphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7d): Eluent: CH2Cl2/EtOAc (75:25), yielding 7d as a white solid (214 mg, 85%), mp 284-285 °C, Rf = 0.49 (ss C); 1H-NMR (10% MeOD/CDCl3); δ [ppm] = 0.75 (s, 3H, 18-CH3), 0.85-0.89 (m, 2H), 0.94 (s, 3H, 19-CH3), 1.27 (s, 9H, 3 x tBu-CH3), 0.99-1.13 (overlapping m, 3H), 1.40-1.53 (overlapping m, 5H), 1.70-1.89 (overlapping m, 5H), 2.14-2.21 (m, 2H), 2.66 (m, 1H, 16-H), 3.40 (m, 1H, 3-H), 3.76 (d, 1H, J = 10.0 Hz, 17-H), 4.21 (dd, 1H, J = 14.0 Hz, J = 8.5 Hz, 16a-H), 4.64 (dd, 1H, J = 13.5 Hz, J = 6.0 Hz, 16a-H), 5.24 (br s, 1H, 6-H), 7.38 (d, 2H, J = 8.0 Hz, 3”- and 5”-H), 7.64 (d, 2H, J = 8.0 Hz, 2”- and 6”-H), 7.79 (s, 1H, 5’-H); IR (neat, cm−1) 3477, 2949, 1460, 1215, 1070, 1047, 818, 559. ESI-MS: 504 (M+H)+; Anal. Calcd for C32H45N3O2: C, 76.30; H, 9.00; N, 8.34. Found: C, 76.17; H, 8.82; N, 8.56.
16β-[4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7e): Eluent: CH2Cl2/EtOAc (70:30), yielding 7e as a white solid (205 mg, 86%), mp 262-264 °C, Rf = 0.39 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.86-0.90 (m, 2H), 0.95 (s, 3H, 19-CH3), 1.03-1.18 (overlapping m, 3H), 1.33-1.43 (overlapping m, 4H), 1.52 (m, 2H), 1.66 (m, 1H), 1.75-1.87 (overlapping m, 3H), 2.08-2.14 (m, 2H), 2.64 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.71 (dd, 1H, J = 9.5 Hz, J = 3.0 Hz, 17-H), 3.79 (s, 3-H, 4”-OCH3), 4.17 (m, 1H, 16a-H), 4.58 (overlapping m, 2H, 3-OH and 16a-H), 4.98 (d, 1H, J = 3.0 Hz, 17-OH), 5.21 (br s, 1H, 6-H), 7.00 (d, 2H, J = 8.5 Hz, 3”- and 5”-H), 7.75 (d, 2H, J = 8.5 Hz, 2”- and 6”-H), 8.44 (s, 1H, 5’-H); IR (neat, cm−1) 3454, 3206, 2930, 1499, 1250, 1068, 1028, 833, 667. ESI-MS: 478 (M+H)+; Anal. Calcd for C29H39N3O3: C, 72.92; H, 8.23; N, 8.80. Found: C, 73.11; H, 8.05; N, 8.97
16β-[4-(2-Methoxyphenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7f): Eluent: CH2Cl2/EtOAc (70:30), yielding 7f as a white solid (208 mg, 87%), mp 219-220 °C, Rf = 0.46 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.86 (m, 2H), 0.94 (s, 3H, 19-CH3), 1.02-1.15 (overlapping m, 3H), 1.32-1.41 (overlapping m, 4H), 1.50 (m, 2H), 1.66 (m, 1H), 1.74-1.85 (overlapping m, 3H), 2.09-2.13 (m, 2H), 2.65 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.70 (dd, 1H, J = 9.5 Hz, J = 3.0 Hz, 17-H), 3.90 (s, 3H, 2”-OCH3), 4.22 (m, 1H, 16a-H), 4.59 (overlapping m, 2H, 3-OH and 16a-H), 4.97 (d, 1H, J = 3.0 Hz, 17-OH), 5.20 (br s, 1H, 6-H), 7.03 (t, 1H, J = 7.0 Hz, 5”-H), 7.10 (d, 1H, J = 8.0 Hz, 3”-H), 7.31 (t, 1H, J = 7.0 Hz, 4”-H), 8.13 (d, 1H, J = 7.5 Hz, 6”-H), 8.39 (s, 1H, 5’-H); IR (neat, cm−1) 3408, 3252, 2941, 1489, 1246, 1045, 1020, 752. ESI-MS: 478 (M+H)+; Anal. Calcd for C29H39N3O3: C, 72.92; H, 8.23; N, 8.80. Found: C, 73.10; H, 8.39; N, 8.59.
16β-[4-(4-Fluorophenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7g): Eluent: CH2Cl2/ EtOAc (70:30), yielding 7g as a white solid (212 mg, 91%), mp 282-283 °C, Rf = 0.43 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.87 (m, 2H), 0.95 (s, 3H, 19-CH3), 1.04-1.14 (overlapping m, 3H), 1.30-1.43 (overlapping m, 4H), 1.53 (m, 2H), 1.64 (m, 1H), 1.75-1.87 (overlapping m, 3H), 2.07-2.14 (m, 2H), 2.63 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.71 (d, 1H, J = 9.5 Hz, 17-H), 4.18 (m, 1H, 16a-H), 4.60 (m, 1H, 16a-H), 5.22 (br s, 1H, 6-H), 7.27 (t, 2H, J = 8.0 Hz, 3”- and 5”-H), 7.86 (t, 2H, J = 8.0 Hz, 2”- and 6”-H), 8.57 (s, 1H, 5’-H); IR (neat, cm−1) 3426, 2941, 1558, 1495, 1231, 1051, 817, 607. ESI-MS: 466 (M+H)+; Anal. Calcd for C28H36FN3O2: C, 72.23; H, 7.79; N, 9.02. Found: C, 72.44; H, 7.66; N, 8.84.
16β-[4-(3-Aminophenyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7h): Eluent: CH2Cl2/ EtOAc (50:50), yielding 7h as a white solid (190 mg, 82%), mp 227-228 °C, Rf = 0.22 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.89 (m, 2H), 0.95 (s, 3H, 19-CH3), 1.05-1.13 (overlapping m, 3H), 1.34-1.52 (overlapping m, 6H), 1.66 (m, 1H), 1.76-1.89 (overlapping m, 3H), 2.07-2.12 (m, 2H), 2.63 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.70 (dd, 1H, J = 9.5 Hz, J = 3.0 Hz, 17-H), 4.18 (m, 1H, 16a-H), 4.57 (overlapping m, 2H, 3-OH and 16a-H), 4.98 (d, 1H, J = 3.0 Hz, 17-OH), 5.13 (br s, 2H, NH2), 5.21 (br s, 1H, 6-H), 6.51 (d, 1H, J = 7.0 Hz, 4”-H), 6.92 (d, 1H, J = 7.0 Hz, 6”-H), 7.05 (t, 1H, J = 7.0 Hz, 5”-H), 7.08 (s, 1H, 2”-H), 8.39 (s, 1H, 5’-H); IR (neat, cm−1) 3558, 3373, 2936, 1585, 1439, 1066, 1046, 790, 586. ESI-MS: 463 (M+H)+; Anal. Calcd for C28H38N4O2: C, 72.69; H, 8.28; N, 12.11. Found: C, 72.86; H, 8.45; N, 12.39.
16β-[4-(2-Pyridyl)-1H-1,2,3-triazol-1-ylmethyl]androst-5-ene-3β,17β-diol (7i): Eluent: CH2Cl2/ EtOAc (50:50), yielding 7i as a white solid (202 mg, 90%), mp 240-241 °C, Rf = 0.26 (ss C); 1H-NMR (10% MeOD/CDCl3); δ [ppm] = 0.76 (s, 3H, 18-CH3), 0.85 (m, 2H), 0.95 (s, 3H, 19-CH3), 1.04-1.14 (overlapping m, 3H), 1.37-1.53 (overlapping m, 6H), 1.60 (m, 1H), 1.79-1.91 (overlapping m, 3H), 2.13-2.20 (m, 2H), 2.64 (m, 1H, 16-H), 3.41 (m, 1H, 3-H), 3.76 (d, 1H, J = 10.0 Hz, 17-H), 4.27 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 4.67 (dd, 1H, J = 13.5 Hz, J = 6.0 Hz, 16a-H), 5.24 (br s, 1H, 6-H), 7.21 (m, 1H, 4”-H), 7.76 (t, 1H, J = 7.5 Hz, 5”-H), 8.09 (d, 1H, J = 7.5 Hz, 6”-H), 8.22 (s, 1H, 5’-H), 8.46 (d, 1H, J = 3.0 Hz, 3”-H); IR (neat, cm−1) 3331, 2929, 1599, 1460, 1263, 1072, 787, 577. ESI-MS: 449 (M+H)+; Anal. Calcd for C27H36N4O2: C, 72.29; H, 8.09; N, 12.49. Found: C, 72.40; H, 8.22; N, 12.41.
16β-(4-Cyclopropyl-1H-1,2,3-triazol-1-ylmethyl)androst-5-ene-3β,17β-diol (7j): Eluent: CH2Cl2/ EtOAc (75:25), yielding 7j as a white solid (170 mg, 83%), mp 235-236 °C, Rf = 0.47 (ss C); 1H-NMR (DMSO-d6); δ [ppm] = 0.67 (m, 2H), 0.72 (s, 3H, 18-CH3), 0.81-0.89 (overlapping m, 4H), 0.94 (s, 3H, 19-CH3), 1.02-1.13 (overlapping m, 3H), 1.31-1.53 (overlapping m, 6H), 1.66 (m, 1H), 1.77-1.91 (overlapping m, 3H), 2.08-2.14 (m, 2H), 2.57 (m, 1H, 16-H), 3.24 (m, 1H, 3-H), 3.67 (dd, 1H, J = 9.5 Hz, J = 3.5 Hz, 17-H), 4.05 (m, 1H, 16a-H), 4.46 (m, 1H, 16a-H), 4.61 (br s, 1H, 3-OH), 4.95 (d, 1H, J = 3.5 Hz, 17-OH), 5.23 (br s, 1H, 6-H), 7.79 (s, 1H, 5’-H); IR (neat, cm−1) 3401, 3251, 2937, 1433, 1219, 1049, 808, 588. ESI-MS: 412 (M+H)+; Anal. Calcd for C25H37N3O2: C, 72.95; H, 9.06; N, 10.21. Found: C, 73.19; H, 9.27; N, 10.36.
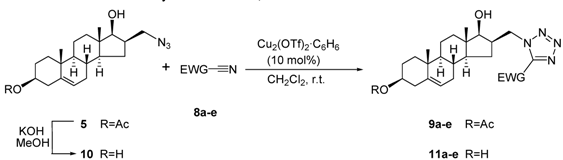
3.8. General procedure for preparation of 3β-acetoxy-16β-(5-substituted-1H-tetrazol-1-ylmethyl)-androst-5-en-17β-ols 9a-e
Compound 5 (387 mg, 1 mmol) was dissolved in CH2Cl2 (5 mL), and Cu2(OTf)2·C6H6 (50 mg, 10 mol %) was added as catalyst. The appropriate nitrile (1.1 mmol) was added to the reaction mixture, which was then stirred for 48 h at ambient temperature. The progress of the reactions was monitored by TLC, and the solvent was then evaporated in vacuo. The resulting crude product was purified by flash chromatography with CH2Cl2/EtOAc (95:5) as eluent.
3β-Acetoxy-16β-(5-methoxycarbonyl-1H-tetrazol-1-ylmethyl)androst-5-en-17β-ol (9a): Nitrile: methyl cyanoformate (8a, 0.09 mL) was added to the mixture. After purification, 9a was obtained as a white solid (310 mg, 66%), mp 168-171 °C, Rf = 0.47 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.85 (s, 3H, 18-CH3), 0.90-0.97 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.09-1.22 (overlapping m, 3H), 1.44-1.58 (overlapping m, 5H), 1.71 (m, 1H), 1.83-1.86 (overlapping m, 3H), 1.94 (m, 1H), 2.01 (s, 3H, Ac-CH3), 2.30 (m, 2H, 4-H2), 2.86 (m, 1H, 16-H), 3.84 (d, 1H, J = 9.5 Hz, 17-H), 4.05 (s, 3H, OCH3), 4.57 (m, 1H, 3-H), 4.66 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 5.04 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.34 (d, 1H, J = 4.0 Hz, 6-H); 13C-NMR (CDCl3); δ [ppm] = 11.8 (C-18), 18.9 (C-19), 20.1 (C-11), 21.0 (Ac-CH3), 27.3, 30.0, 30.8, 31.1, 36.2, 36.6, 36.7, 37.6, 39.8, 43.4, 49.4, 49.6, 51.0, 53.3 (OCH3), 73.4 (C-3), 80.5 (C-17), 121.6 (C-6), 139.4 (C-5), 145.4 (C-5’), 156.8, 170.2 (Ac-CO); IR (neat, cm−1) 3501, 2934, 1742, 1703, 1427, 1254, 1032, 826, 691. ESI-MS: 473 (M+H)+; Anal. Calcd for C25H36N4O5: C, 63.54; H, 7.68; N, 11.86. Found: C, 63.75; H, 7.57; N, 12.03.
3β-Acetoxy-16β-(5-ethoxycarbonyl-1H-tetrazol-1-ylmethyl)androst-5-en-17β-ol (9b): Nitrile: ethyl cyanoformate (8b, 0.11 mL) was added to the mixture. After purification, 9b was obtained as a white solid (350 mg, 72%), mp 172-174 °C, Rf = 0.59 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.84 (s, 3H, 18-CH3), 0.91-0.96 (overlapping m, 2H), 1.01 (s, 3H, 19-CH3), 1.08-1.16 (overlapping m, 2H), 1.21 (m, 1H), 1.45 (t, 3H, J = 7.0 Hz, OEt-CH3), 1.47-1.58 (overlapping m, 5H), 1.70 (m, 1H), 1.82-1.86 (overlapping m, 3H), 1.92 (m, 1H), 2.01 (s, 3H, Ac-CH3), 2.30 (m, 2H, 4-H2), 2.85 (m, 1H, 16-H), 3.84 (d, 1H, J = 9.5 Hz, 17-H), 4.51 (q, 2H, J = 7.0 Hz, OEt-CH2), 4.56 (m, 1H, 3-H), 4.66 (dd, 1H, J = 13.5 Hz, J = 8.5 Hz, 16a-H), 5.03 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.33 (d, 1H, J = 4.0 Hz, 6-H); 13C-NMR (CDCl3); δ [ppm] = 12.2 (C-18), 14.0, 19.3 (C-19), 20.4 (C-11), 21.4 (Ac-CH3), 27.6, 30.3, 31.1, 31.5, 36.6, 36.9, 37.1, 38.0, 40.2, 43.8, 49.7, 50.0, 51.3, 63.4, 73.7 (C-3), 80.8 (C-17), 121.9 (C-6), 139.7 (C-5), 145.9 (C-5’), 156.8, 170.5 (Ac-CO); IR (neat, cm−1) 3428, 2918, 1743, 1721, 1470, 1240, 1020, 854. ESI-MS: 487 (M+H)+; Anal. Calcd for C26H38N4O5: C, 64.18; H, 7.87; N, 11.51. Found: C, 64.32; H, 7.67; N, 11.66.
3β-Acetoxy-16β-(5-benzyloxycarbonyl-1H-tetrazol-1-ylmethyl)androst-5-en-17β-ol (9c): Nitrile: benzyl cyanoformate (8c 0.16 mL) was added to the mixture. After purification, 9c was obtained as a white solid (340 mg, 62%), mp 153-156 °C, Rf = 0.21 (ss A); 1H-NMR (CDCl3); δ [ppm] = 0.78 (s, 3H, 18-CH3), 0.88-0.95 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.08-1.15 (overlapping m, 3H), 1.43-1.57 (overlapping m, 5H), 1.66 (m, 1H), 1.78-1.86 (overlapping m, 3H), 1.96 (m, 1H), 2.01 (s, 3H, Ac-CH3), 2.31 (m, 2H, 4-H2), 2.79 (m, 1H, 16-H), 3.76 (d, 1H, J = 9.5 Hz, 17-H), 4.59-4.63 (overlapping m, 2H, 3- and 16a-H), 5.00 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.33 (d, 1H, J = 3.0 Hz, 6-H), 5.46 (dd, 2H, J = 21.5 Hz, J = 12.0 Hz, OCH2Ph), 7.37 (m, 3H, 3”-, 4”- and 5”-H), 7.46 (d, 2H, J = 7.0 Hz, 2”- and 6”-H); 13C-NMR (CDCl3); δ [ppm] = 12.1 (C-18), 19.3 (C-19), 20.4 (C-11), 21.4 (Ac-CH3), 27.6, 30.2, 31.1, 31.5, 36.6, 36.9, 37.1, 38.0, 40.2, 43.7, 49.7, 50.0, 51.3, 68.7, 73.7 (C-3), 80.8 (C-17), 122.0 (C-6), 128.7 (2C), 128.9 (2C), 129.0 (C-4”), 134.0 (C-1”), 139.7 (C-5), 145.9 (C-5’), 156.6, 170.5 (Ac-CO); IR (neat, cm−1) 3525, 2945, 1733, 1703, 1454, 1256, 1026, 748, 697. ESI-MS: 549 (M+H)+; Anal. Calcd for C31H40N4O5: C, 67.86; H, 7.35; N, 10.21. Found: C, 67.98; H, 7.52; N, 10.12
3β-Acetoxy-16β-(5-acetyl-1H-tetrazol-1-ylmethyl)androst-5-en-17β-ol (9d): Nitrile: acetyl cyanide (8d, 0.08 mL) was added to the mixture. After purification, 9d was obtained as a white solid (260 mg, 57%), mp 191-193 °C, Rf = 0.33 (ss A); 1H-NMR (CDCl3); δ [ppm] = 0.82 (s, 3H, 18-CH3), 0.91-0.97 (overlapping m, 2H), 1.01 (s, 3H, 19-CH3), 1.09-1.19 (overlapping m, 3H), 1.44-1.59 (overlapping m, 5H), 1.72 (m, 1H), 1.81-1.87 (overlapping m, 3H), 1.95 (m, 1H), 2.01 (s, 3H, Ac-CH3), 2.31 (m, 2H, 4-H2), 2.53 (s, 3H, 5’Ac-CH3), 2.84 (m, 1H, 16-H), 3.81 (d, 1H, J = 9.5 Hz, 17-H), 4.56 (m, 1H, 3-H), 4.64 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 5.02 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.33 (d, 1H, J = 3.5 Hz, 6-H); 13C-NMR (CDCl3); δ [ppm] = 11.9 (C-18), 19.1 (C-19), 20.2 (C-11), 20.6, 21.0 (Ac-CH3), 27.4, 30.2, 30.9, 31.3, 36.4, 36.8, 37.0, 37.8, 39.9, 43.6, 49.5, 49.9, 51.2, 73.5 (C-3), 80.7 (C-17), 121.7 (C-6), 139.6 (C-5), 147.7 (C-5’), 170.4 (Ac-CO), 190.4; IR (neat, cm−1) 3512, 2931, 1740, 1709, 1486, 1259, 1023, 896, 682. ESI-MS: 457 (M+H)+; Anal. Calcd for C25H36N4O4: C, 65.76; H, 7.95; N, 12.27. Found: C, 65.61; H, 8.06; N, 12.51.
3β-Acetoxy-16β-(5-benzoyl-1H-tetrazol-1-ylmethyl)androst-5-en-17β-ol (9e): Nitrile: benzoyl cyanide (8e, 145 mg) was added to the mixture. After purification, 9e was obtained as a white solid (280 mg, 54%), mp 182-185 °C, Rf = 0.30 (ss A); 1H-NMR (CDCl3); δ [ppm] = 0.81 (s, 3H, 18-CH3), 0.88-0.95 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.08-1.12 (overlapping m, 2H), 1.23 (m, 1H), 1.43-1.61 (overlapping m, 5H), 1.73-1.86 (overlapping m, 4H), 1.93 (m, 1H), 2.01 (s, 3H, Ac-CH3), 2.30 (m, 2H, 4-H2), 2.88 (m, 1H, 16-H), 3.82 (d, 1H, J = 9.5 Hz, 17-H), 4.57 (m, 1H, 3-H), 4.64 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 5.03 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.33 (d, 1H, J = 4.0 Hz, 6-H), 7.54 (t, 2H, J = 7.5 Hz, 3”- and 5”-H), 7.69 (t, 1H, J = 7.5 Hz, 4”-H), 8.33 (d, 2H, J = 7.5 Hz, 2”- and 6”-H); 13C-NMR (CDCl3); δ [ppm] = 11.8 (C-18), 18.9 (C-19), 20.1 (C-11), 21.0 (Ac-CH3), 27.3, 30.0, 30.7, 31.1, 36.2, 36.6, 36.8, 37.6, 39.9, 43.4, 49.4, 49.6, 50.8, 73.4 (C-3), 80.7 (C-17), 121.6 (C-6), 128.4 (2C), 130.6 (2C), 134.5 (C-4”), 134.7 (C-1”), 139.3 (C-5), 149.5 (C-5’), 170.2 (Ac-CO), 181.8; IR (neat, cm−1) 3533, 2938, 1728, 1702, 1595, 1265, 1026, 921, 692. ESI-MS: 519 (M+H)+; Anal. Calcd for C30H38N4O4: C, 69.47; H, 7.38; N, 10.80. Found: C, 69.63; H, 7.21; N, 10.91.
3.9. 16β-Azidomethylandrost-5-ene-3β,17β-diol (10)
Compound 5 (1.94 g, 5 mmol) was dissolved in MeOH (80 mL), and KOH (750 mg, 13.5 mmol) was added. The mixture was stirred for 1 h at room temperature, and then poured into water (800 mL) and neutralized with diluted HCl. The resulting precipitate was filtered off, washed with water and dried. The crude product obtained was purified by flash chromatography to afford 10 as a white solid (1.43 g, 83%), mp 154-157 °C, Rf = 0.19 (ss A); 1H-NMR (CDCl3); δ [ppm] = 0.78 (s, 3H, 18-CH3), 0.93 (m, 1H), 1.02 (s, 3H, 19-CH3), 1.05-1.15 (overlapping m, 3H), 1.42-1.60 (overlapping m, 6H), 1.84-1.89 (overlapping m, 4H), 1.99 (m, 1H), 2.25-2.32 (m, 2H, 4-H2), 2.37 (m, 1H, 16-H), 3.29 (dd, 1H, J = 12.0 Hz, J = 6.5 Hz, 16a-H), 3.52 (m, 1H, 3-H), 3.57 (dd, 1H, J = 12.5 Hz, J = 7.5 Hz, 16a-H), 3.78 (dd, 1H, J = 9.5 Hz, J = 5.0 Hz, 17-H), 5.34 (d, 1H, J = 4.5 Hz, 6-H); 13C-NMR (CDCl3); δ [ppm] = 12.1 (C-18), 19.4 (C-19), 20.6 (C-11), 30.6, 31.2, 31.6, 31.7, 36.6, 37.2, 37.5, 39.9, 42.2, 43.6, 50.0, 50.1, 53.3, 71.6 (C-3), 81.3 (C-17), 121.2 (C-6), 140.9 (C-5); IR (neat, cm−1) 3519, 2941, 2904, 2115, 1452, 1374, 1246, 1028. ESI-MS: 346 (M+H)+; Anal. Calcd for C20H31N3O2: C, 69.53; H, 9.04; N, 12.16. Found: C, 69.38; H, 9.16; N, 12.35.
3.10. General procedure for preparation of 16β-(5-substituted-1H-tetrazol-1-ylmethyl)androst-5-ene-3β,17β-diols 11a-e
Compound 10 (345 mg, 1 mmol) was dissolved in CH2Cl2 (5 mL), and Cu2(OTf)2·C6H6 (50 mg, 10 mol %) was added as catalyst. The appropriate nitrile (1.1 mmol) was added to the reaction mixture, which was then stirred for 48 h at ambient temperature. The progress of the reactions was monitored by TLC, and the solvent was then evaporated in vacuo. The resulting crude product was purified by flash chromatography with CH2Cl2/EtOAc (85:15) as eluent.
16β-(5-Methoxycarbonyl-1H-tetrazol-1-ylmethyl)androst-5-ene-3β,17β-diol (11a): Nitrile: methyl cyanoformate (8a, 0.09 mL) was added to the mixture. After purification, 11a was obtained as a white solid (255 mg, 59%), mp 183-185 °C, Rf = 0.19 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.86 (s, 3H, 18-CH3), 0.92-0.96 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.07-1.14 (overlapping m, 3H), 1.46-1.58 (overlapping m, 5H), 1.73 (m, 1H), 1.83-1.87 (overlapping m, 3H), 1.95 (m, 1H), 2.25-2.31 (overlapping m, 2H), 2.87 (m, 1H, 16-H), 3.51 (m, 1H, 3-H), 3.85 (dd, 1H, J = 9.5 Hz, J = 3.5 Hz, 17-H), 4.06 (s, 3H, OCH3), 4.67 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 5.04 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.33 (d, 1H, J = 4.5 Hz, 6-H); ESI-MS: 431 (M+H)+; Anal. Calcd for C23H34N4O4: C, 64.16; H, 7.96; N, 13.01. Found: C, 64.43; H, 7.80; N, 13.19.
16β-(5-Ethoxycarbonyl-1H-tetrazol-1-ylmethyl)androst-5-ene-3β,17β-diol (11b): Nitrile: ethyl cyanoformate (8b, 0.11 mL) was added to the mixture. After purification, 11b was obtained as a white solid (285 mg, 64%), mp 176-179 °C, Rf = 0.27 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.86 (s, 3H, 18-CH3), 0.93-0.96 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.07-1.15 (overlapping m, 3H), 1.46 (t, 3H, J = 7.0 Hz, OEt-CH3), 1.49-1.59 (overlapping m, 5H), 1.72 (m, 1H), 1.82-1.86 (overlapping m, 3H), 1.96 (m, 1H), 2.23-2.30 (overlapping m, 2H), 2.86 (m, 1H, 16-H), 3.51 (m, 1H, 3-H), 3.85 (dd, 1H, J = 9.0 Hz, J = 3.0 Hz, 17-H), 4.53 (q, 2H, J = 7.0 Hz, OEt-CH2), 4.67 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 5.04 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 5.32 (d, 1H, J = 3.5 Hz, 6-H); ESI-MS: 445 (M+H)+; Anal. Calcd for C24H36N4O4: C, 64.84; H, 8.16; N, 12.60. Found: C, 64.97; H, 8.36; N, 12.84.
16β-(5-Benzyloxycarbonyl-1H-tetrazol-1-ylmethyl)androst-5-ene-3β,17β-diol (11c): Nitrile: benzyl cyanoformate (8c, 0.16 mL) was added to the mixture. After purification, 11c was obtained as a white solid (268 mg, 53%), mp 178-181 °C, Rf = 0.38 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.79 (s, 3H, 18-CH3), 0.87-0.94 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.05-1.15 (overlapping m, 3H), 1.42-1.55 (overlapping m, 5H), 1.67 (m, 1H), 1.78-1.85 (overlapping m, 3H), 1.91 (m, 1H), 2.24-2.32 (overlapping m, 2H), 2.80 (m, 1H, 16-H), 3.51 (m, 1H, 3-H), 3.76 (dd, 1H, J = 9.5 Hz, J = 3.0 Hz, 17-H), 4.63 (dd, 1H, J = 13.5 Hz, J = 8.0 Hz, 16a-H), 5.01 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 5.32 (d, 1H, J = 4.0 Hz, 6-H), 5.47 (q, 2H, J = 12.0 Hz, OCH2Ph), 7.38 (m, 3H, 3”-, 4”- and 5”-H), 7.48 (d, 2H, J = 6.5 Hz, 2”- and 6”-H); ESI-MS: 507 (M+H)+; Anal. Calcd for C29H38N4O4: C, 68.75; H, 7.56; N, 11.06. Found: C, 68.88; H, 7.74; N, 10.89.
16β-(5-Acetyl-1H-tetrazol-1-ylmethyl)androst-5-ene-3β,17β-diol (11d): Nitrile: acetyl cyanide (8d, 0.08 mL) was added to the mixture. After purification, 11d was obtained as a white solid (195 mg, 47%), mp 199-202 °C, Rf = 0.41 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.84 (s, 3H, 18-CH3), 0.93-0.97 (overlapping m, 2H), 1.02 (s, 3H, 19-CH3), 1.08-1.16 (overlapping m, 3H), 1.47-1.58 (overlapping m, 5H), 1.74 (m, 1H), 1.82-1.87 (overlapping m, 3H), 1.96 (m, 1H), 2.26-2.32 (overlapping m, 2H), 2.55 (s, 3H, 5’Ac-CH3), 2.86 (m, 1H, 16-H), 3.51 (m, 1H, 3-H), 3.83 (d, 1H, J = 9.5 Hz, 17-H), 4.66 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 5.03 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.32 (d, 1H, J = 3.5 Hz, 6-H); ESI-MS: 415 (M+H)+; Anal. Calcd for C23H34N4O3: C, 66.64; H, 8.27; N, 13.52. Found: C, 66.48; H, 8.38; N, 13.74.
16β-(5-Benzoyl-1H-tetrazol-1-ylmethyl)androst-5-ene-3β,17β-diol (11e): Nitrile: benzoyl cyanide (8e, 145 mg) was added to the mixture. After purification, 11e was obtained as a white solid (215 mg, 45%), mp 196-200 °C, Rf = 0.34 (ss B); 1H-NMR (CDCl3); δ [ppm] = 0.83 (s, 3H, 18-CH3), 0.91-0.96 (overlapping m, 2H), 1.03 (s, 3H, 19-CH3), 1.07-1.14 (overlapping m, 3H), 1.46-1.57 (overlapping m, 5H), 1.71 (m, 1H), 1.81-1.86 (overlapping m, 3H), 1.94 (m, 1H), 2.23-2.31 (overlapping m, 2H), 2.87 (m, 1H, 16-H), 3.51 (m, 1H, 3-H), 3.84 (d, 1H, J = 9.5 Hz, 17-H), 4.66 (dd, 1H, J = 13.5 Hz, J = 7.5 Hz, 16a-H), 5.03 (dd, 1H, J = 13.5 Hz, J = 7.0 Hz, 16a-H), 5.32 (d, 1H, J = 4.0 Hz, 6-H), 7.56 (t, 2H, J = 7.5 Hz, 3”- and 5”-H), 7.70 (t, 1H, J = 7.5 Hz, 4”-H), 8.34 (d, 2H, J = 7.5 Hz, 2”- and 6”-H); ESI-MS: 477 (M+H)+; Anal. Calcd for C28H36N4O3: C, 70.56; H, 7.61; N, 11.76. Found: C, 70.77; H, 7.45; N, 11.89.
3.11. Determination of Antiproliferative Activities
Human cancer cell lines were purchased from ECACC (Salisbury, UK). HeLa (cervix adenocarcinoma), A2780 (ovarian carcinoma) and MCF7 (breast adenocarcinoma) cells were cultivated in minimal essential medium supplemented with 10% fetal bovine serum, 1% non-essential amino acids and an antibiotic-antimycotic mixture.
Near-confluent cancer cells were seeded onto a 96-well microplate (5000/well) and attached to the bottom of the well overnight. On the second day, new medium containing the tested compound (at 10 or 30 µM, 200 μL) was added. After incubation for 72 h at 37 °C in humidified air containing 5% CO
2, the living cells were assayed by the addition of 5 mg/mL MTT solution (20 μL). MTT was converted by intact mitochondrial reductase and precipitated as blue crystals during a 4 h contact period. The medium was then removed and the precipitated crystals were dissolved in 100 μL DMSO during a 60 min period of shaking at 25 °C. Finally, the reduced MTT was assayed at 545 nm, using a microplate reader; wells with untreated cells were utilized as controls [
31]. All
in vitro experiments were carried out on two microplates with at least five parallel wells. Cisplatin was used as positive control. Stock solutions of the tested substances (10 mM) were prepared with DMSO. The DMSO content of the medium (0.1% or 0.3%) did not have any significant effect on the cell proliferation.