On the π Coordination of Organometallic Fullerene Complexes
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
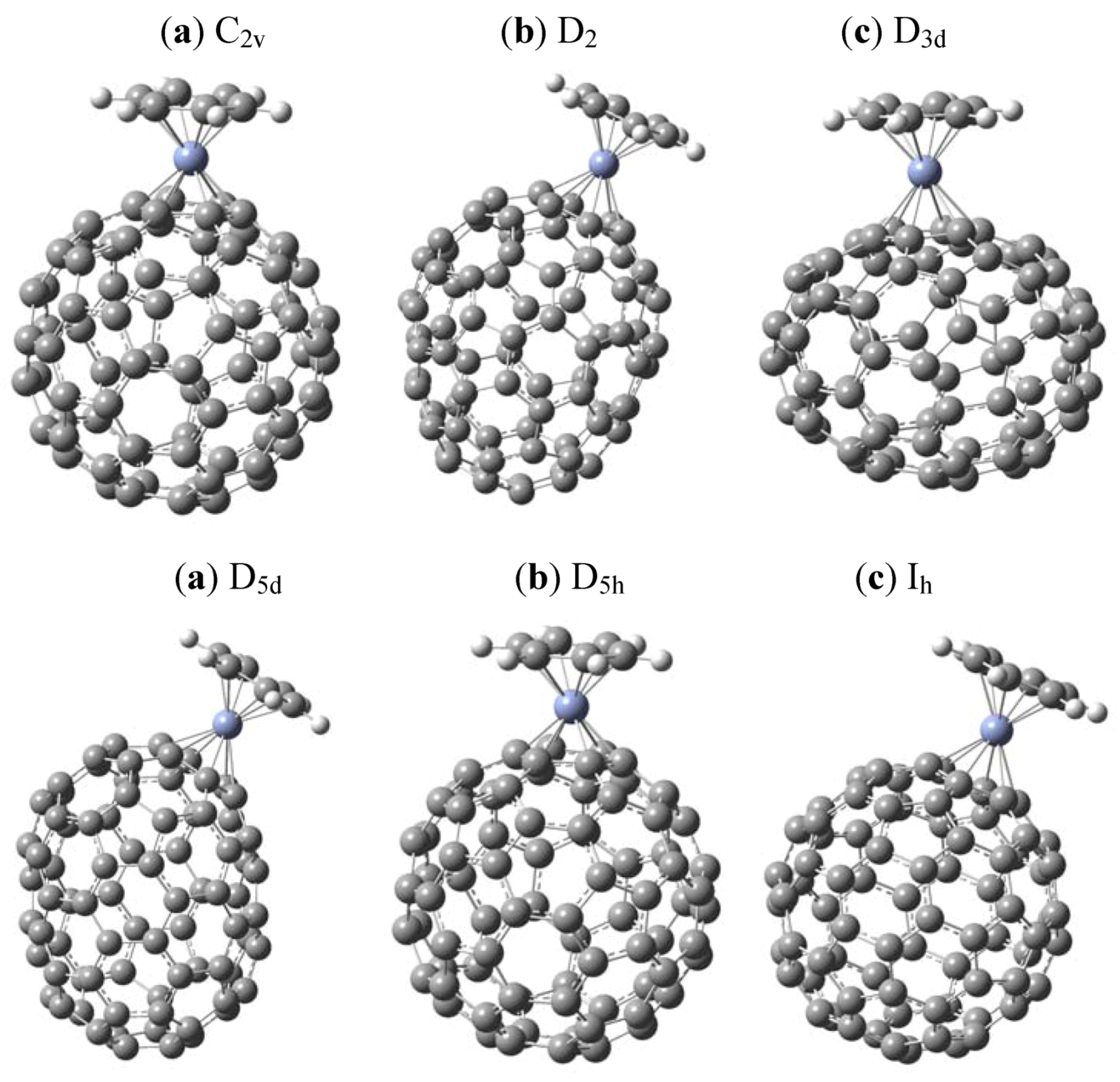
| Isomer | π bond energy (kcal/mol) | Erel (kcal/mol) | Angle (o) |
|---|---|---|---|
| D3d | 25.54 | 2.95 | 20.9 |
| Ih | 27.61 | 18.2 | 28.3 |
| C2v | 28.24 | 0.0 | 32.6 |
| D2 | 33.15 | 1.75 | 33.4 |
| D5d | 37.01 | 2.38 | 33.0 |
| D5h | 29.30 | 23.22 | 28.9 |

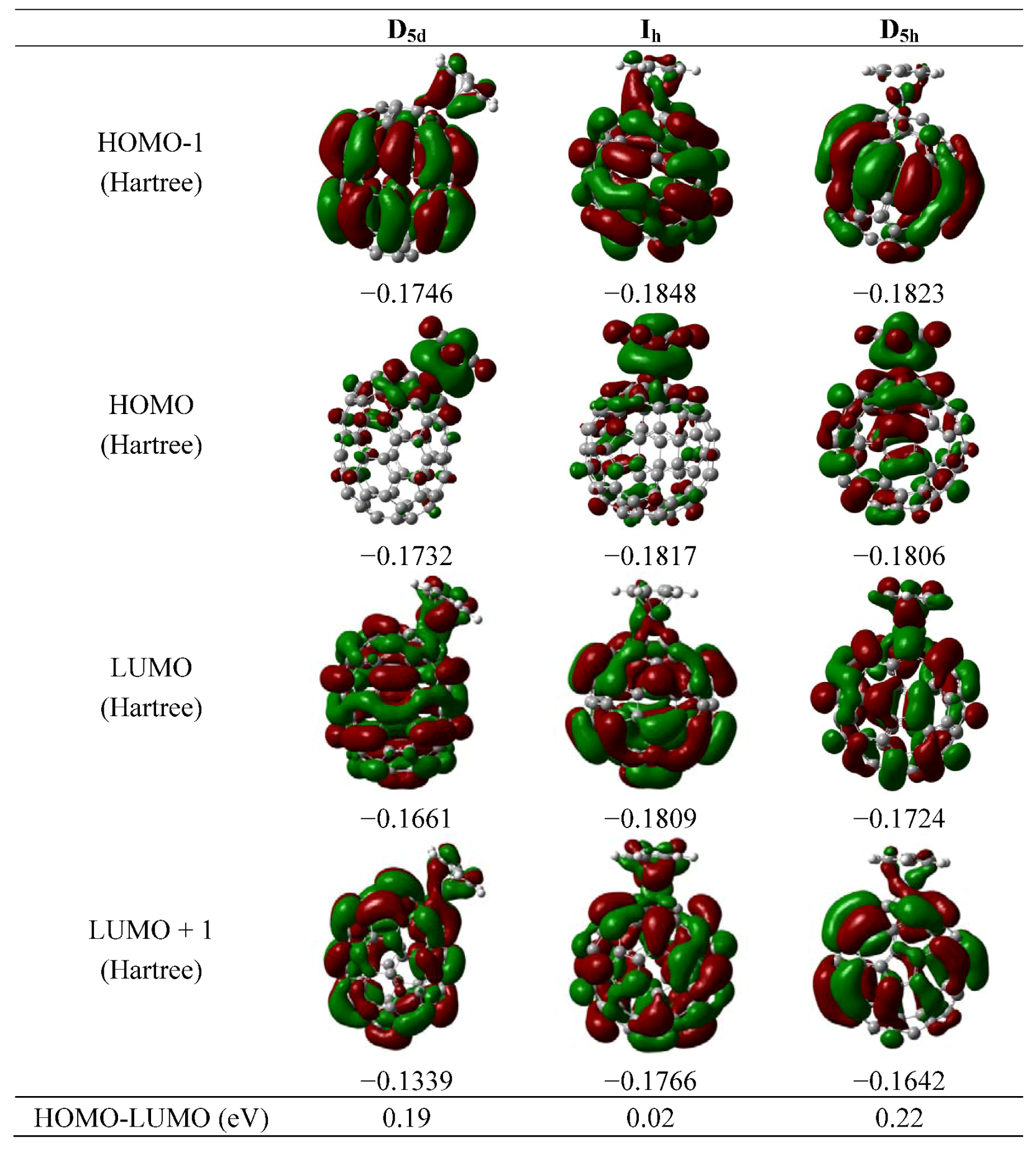
4. Conclusions
Acknowledgements
References
- Prato, M. Fullerene materials. Top. Curr. Chem. 1999, 199, 173–187. [Google Scholar]
- Hirsch, A. Principles of fullerene reactivity. Top. Curr. Chem. 1999, 199, 1–65. [Google Scholar] [CrossRef]
- Balch, A.L.; Olmstead, M.M. Reactions of transition metal complexes with fullerenes (C60, C70, etc.) and related materials. Chem. Rev. 1998, 98, 2123–2165. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Sharma, P.K. A theoretically study of transition metal complexes of C60 and C70 and their ring-opened alternatives. J. Mol. Graph. Model. 2001, 19, 256–265. [Google Scholar] [CrossRef]
- Ross, M.M.; Nelson, H.H.; Callahan, J.H.; McElvany, S.W. Production and characterization of metallofullerenes. J. Phys. Chem. 1992, 96, 5231–5234. [Google Scholar] [CrossRef]
- Fagan, P.J.; Calabrese, J.C.; Malone, B. The chemical nature of buckminsterfullerene (C60) and the characterization of a platinum derivative. Science 1991, 252, 1160–1161. [Google Scholar]
- Matsuo, Y.; Matsuo, K.; Nanao, T.; Marczak, R.; Gayathri, S.S.; Guldi, D.M.; Nakamura, E. A rhutenium bridge in fullerene-ferrocene arrays: Synthesis of [Ru(C60Me5)R(CO)2, (R = C6H4Fc, C≡CFc) and their charge transfer properties. Chem. Asian J. 2008, 3, 841–848. [Google Scholar] [CrossRef]
- Fagan, P.J.; Calabrese, J.C.; Malone, B. Metal complexes of buckminsterfullerene (C60). Acc. Chem. Res. 1992, 25, 134–140. [Google Scholar] [CrossRef]
- Rogers, J.R.; Marynick, D.S. Theoretical estimates of the η6 bonding capability of buckminsterfullerene in transition metal complexes. Chem. Phys. Lett. 1993, 205, 197–200. [Google Scholar] [CrossRef]
- Lichtenberger, D.L.; Wrigth, L.L.; Gruhn, N.E.; Rempe, M.E. Electronic structure and bonding of C60 to metals. Synth. Met. 1993, 59, 353–367. [Google Scholar] [CrossRef]
- Lichtenberger, D.L.; Wrigth, L.L.; Gruhn, N.E.; Rempe, M.E. Electronic structure of exohedral interactions of C60 and transition metals. J. Organomet. Chem. 1994, 478, 213–221. [Google Scholar]
- Chistyakov, A.L.; Stankevich, I.V. Complexes eta5-pi-C60H5XCp (X=Fe, Si): Ab initio estimates of stability and electronic structure. Mol. Mater. 2000, 13, 311–314. [Google Scholar]
- Sokolov, V.I.; Gasanov, R.G.; Goh, L.Y.; Weng, Z.; Chistyakov, A.L.; Stankevich, I.V. Cyclopentadienyl) Chromiumtricarbonyl dimers as a source of metal-centered free radicals to form stable eta2-bonded spin-adducts with fullerene. J.Organomet.Chem. 2005, 690, 2370–2375. [Google Scholar]
- Nikawa, H.; Yamada, T.; Cao, B.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Akasaka, T.; Yoza, K.; Nagase, S. Missing metallofullerene with C80 cage. J. Am. Chem. Soc. 2009, 131, 10950–10954. [Google Scholar]
- Nakao, Y.; Kurita, N.; Fujita, M. Ab initio molecular-orbital calculations of C70 and 7 isomers of C80. Phys. Rev. B 1994, 49, 11415–11420. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, C.; Li, S.; Mi, W.; Jin, P. Which configuration is more stable for La2@C80, D3d or D2h? Recomputation with ZORA methods whitin ADF. J. Phys. Chem. C 2007, 111, 7862–7867. [Google Scholar]
- Purcell, K.F.; Kotz, J.C. Inorganic Chemistry; W.B. Sanders Company: New York, NY, USA, 1977. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseira, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian03; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Shanina, Z.; Zhao, X.; Deota, P.; Osawa, E. Calculations of higher fullerenes and quasi-fullerenes. In Fullerenes: Chemistry, Physics and Technology; Kadish, K.M., Rufo, R.S., Eds.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Yamada, M.; Mizorogi, N.; Tsuchiya, T.; Akasaka, T.; Nagase, S. Synthesis and characterization of the D5h isomer of the endohedral dimetallofullerene Ce2@C80: Two-dimensional circulation of encapsulated metal atoms inside a fullerene cage. Chem. Eur. J. 2009, 15, 9486–9493. [Google Scholar] [CrossRef]
- Loboda, O. A DFT study on exohedral metallofullerenes: Structural and electronic properties. Fuller. Nanotub. Car. N. 2009, 17, 457–475. [Google Scholar] [CrossRef]
- Hedberg, K.; Hedberg, L.; Bethune, D.; Brown, C.; Dorn, H.; Johnson, R.; de Vries, M. Bond lengths in free molecules of buckminsterfullerene, C60, from gas-phase electron-diffraction. Science 1991, 254, 410–412. [Google Scholar]
- Delgado, J.L.; Bouit, P.A.; Filippone, S.; Herranz, M.A.; Martín, N. Organic photovoltaics: A chemical approach. Chem. Commun. 2010, 46, 4853–4865. [Google Scholar]
- Salcedo, R. Fullerenocene. Polyhedron 2009, 28, 431–436. [Google Scholar] [CrossRef]
- Kuc, A.; Heine, T.; Seifert, G. Structural and electronic properties of graphene nanoflakes. Phys. Rev. B 2010. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Molina, B.; Pérez-Manriquez, L.; Salcedo, R. On the π Coordination of Organometallic Fullerene Complexes. Molecules 2011, 16, 4652-4659. https://doi.org/10.3390/molecules16064652
Molina B, Pérez-Manriquez L, Salcedo R. On the π Coordination of Organometallic Fullerene Complexes. Molecules. 2011; 16(6):4652-4659. https://doi.org/10.3390/molecules16064652
Chicago/Turabian StyleMolina, Bertha, Liliana Pérez-Manriquez, and Roberto Salcedo. 2011. "On the π Coordination of Organometallic Fullerene Complexes" Molecules 16, no. 6: 4652-4659. https://doi.org/10.3390/molecules16064652
APA StyleMolina, B., Pérez-Manriquez, L., & Salcedo, R. (2011). On the π Coordination of Organometallic Fullerene Complexes. Molecules, 16(6), 4652-4659. https://doi.org/10.3390/molecules16064652




