Crystallization Products of Risedronate with Carbohydrates and Their Substituted Derivatives †
Abstract
:1. Introduction
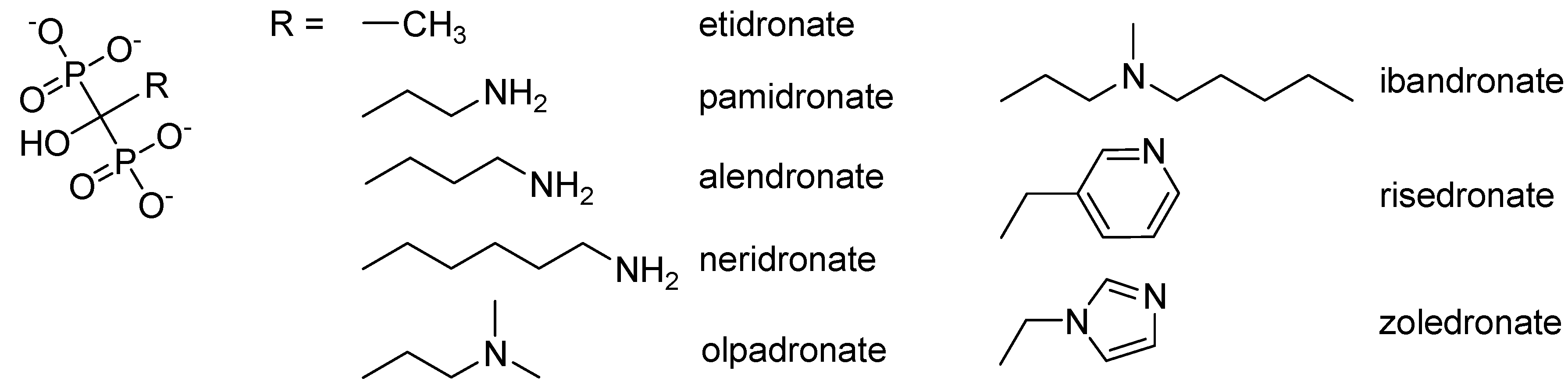
2. Results and Discussion
2.1. Chemistry
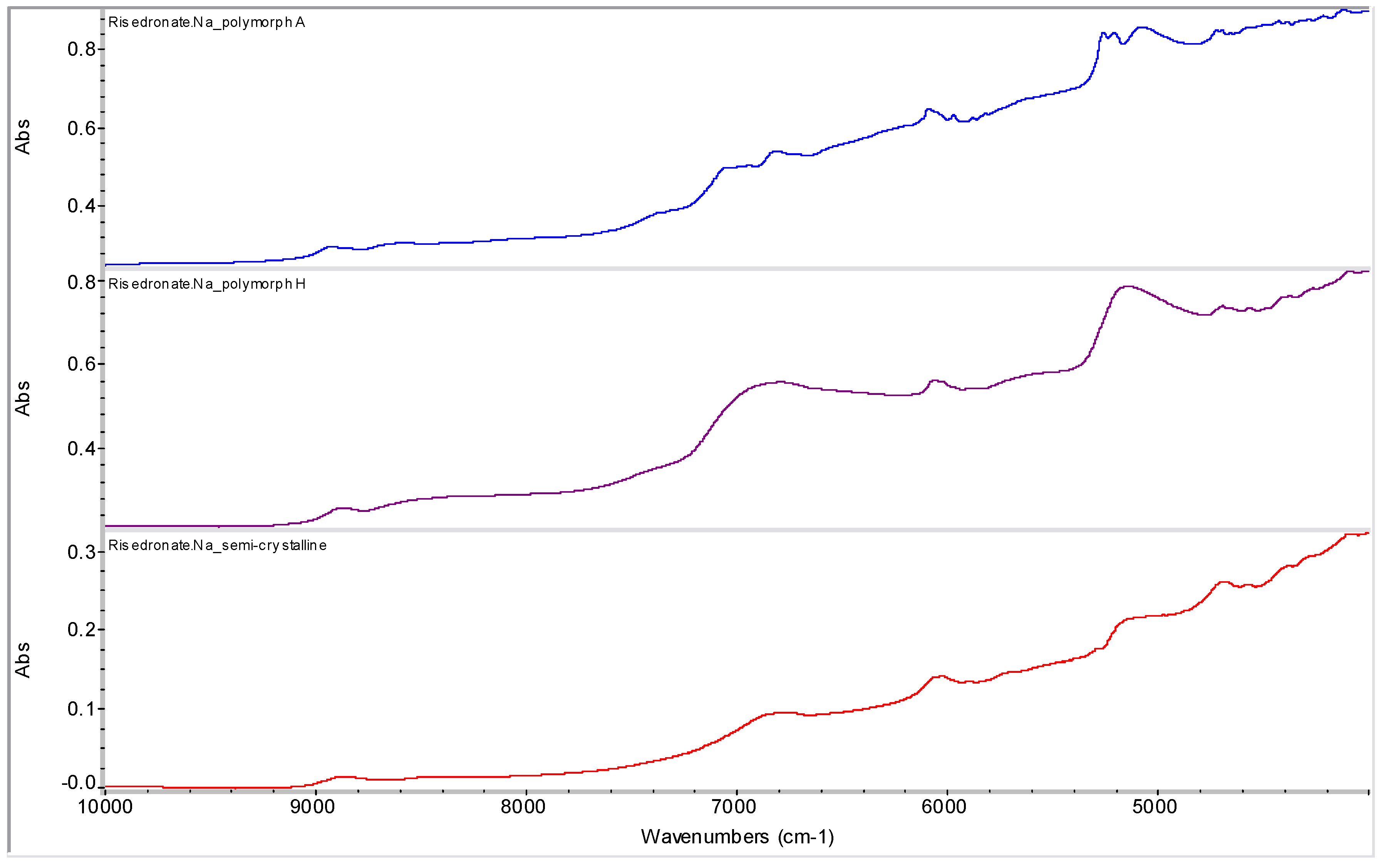
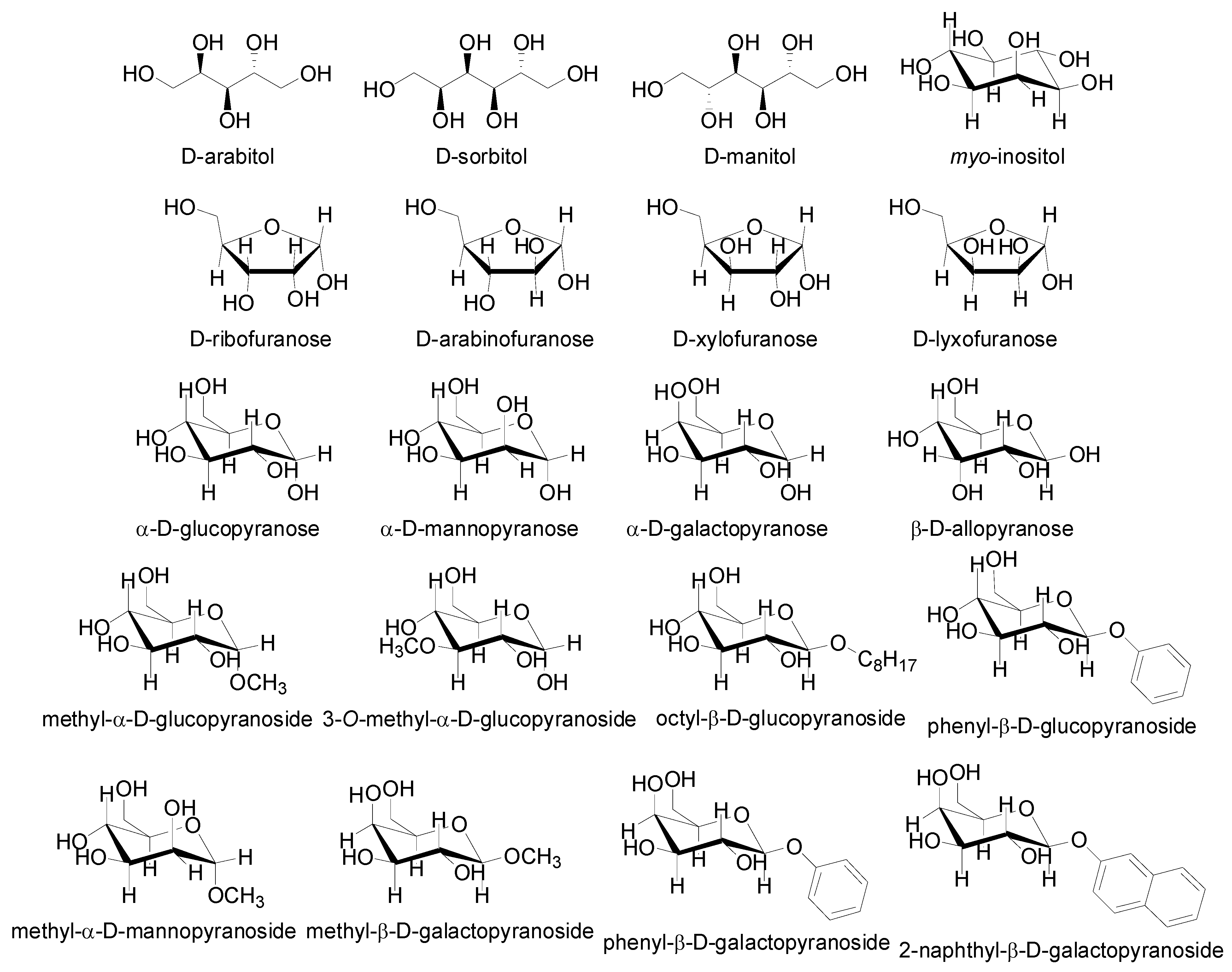
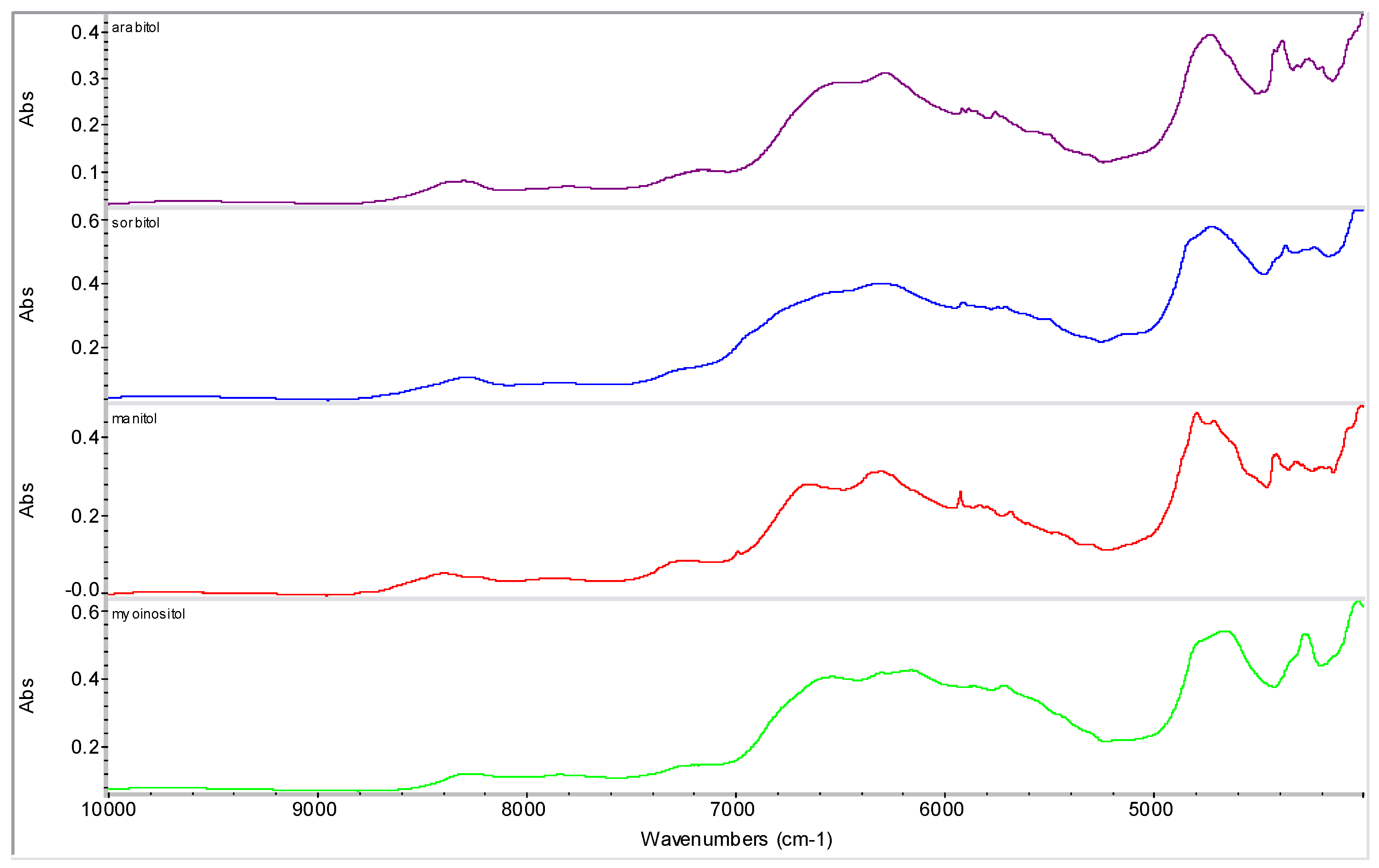
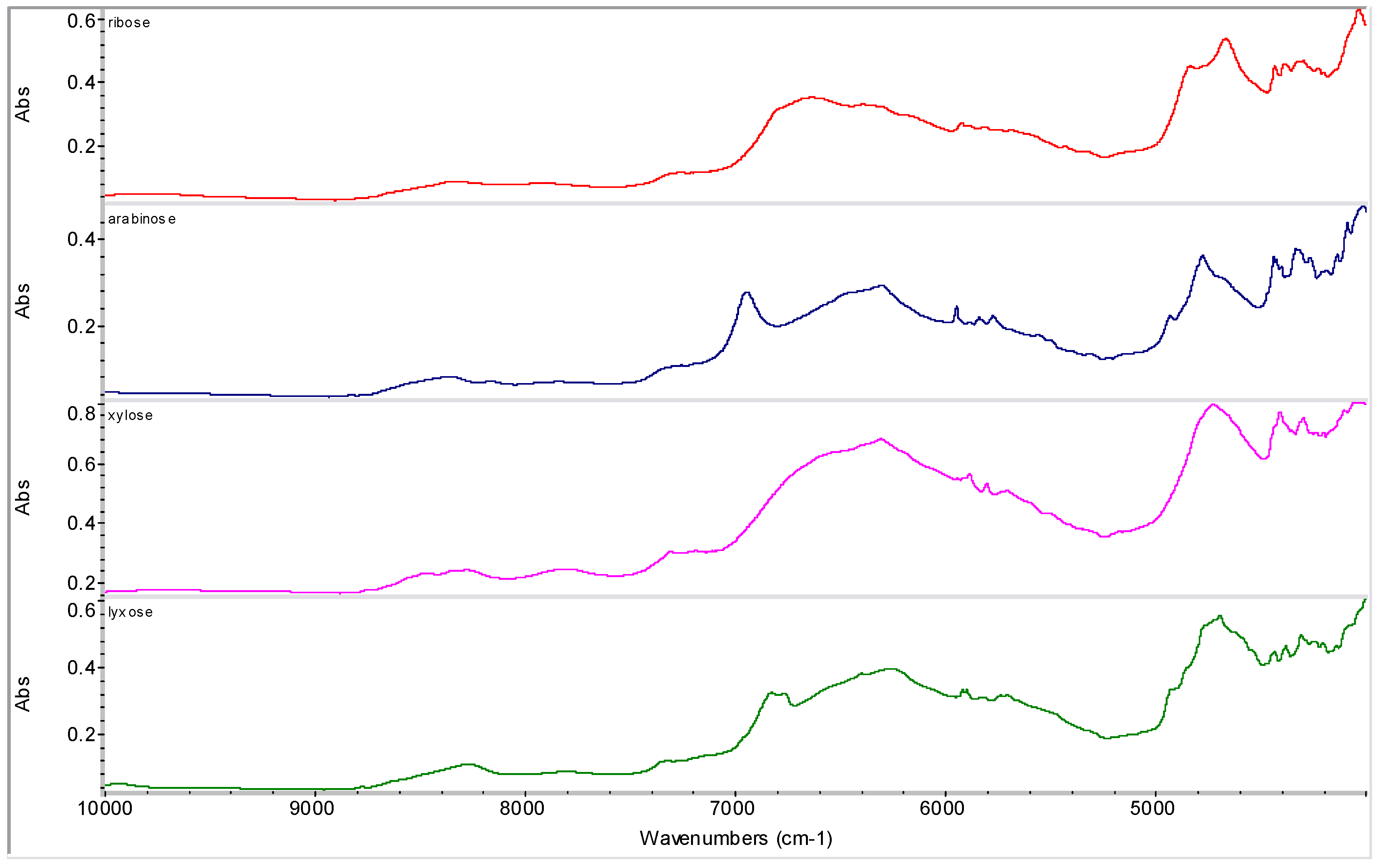
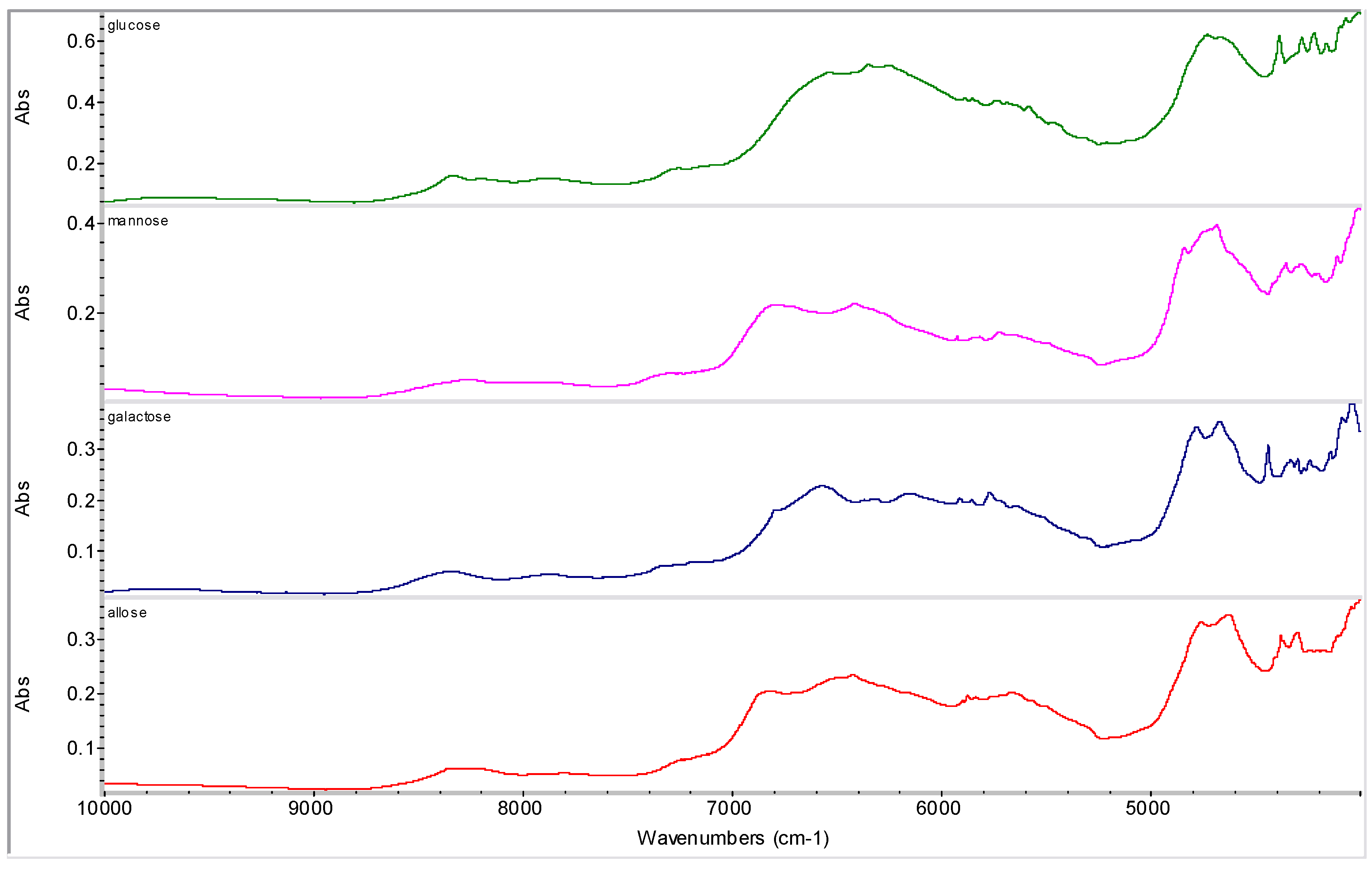

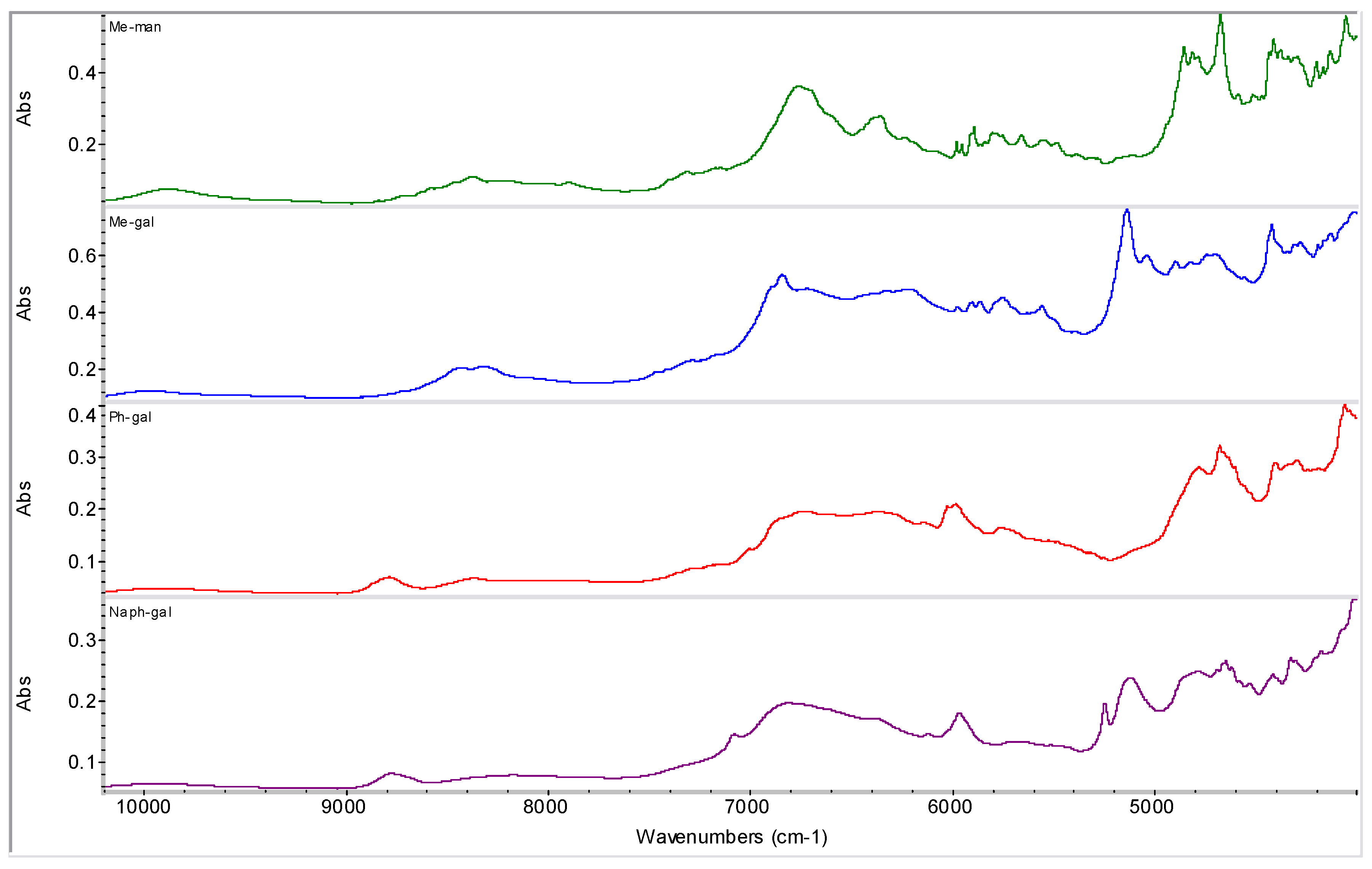
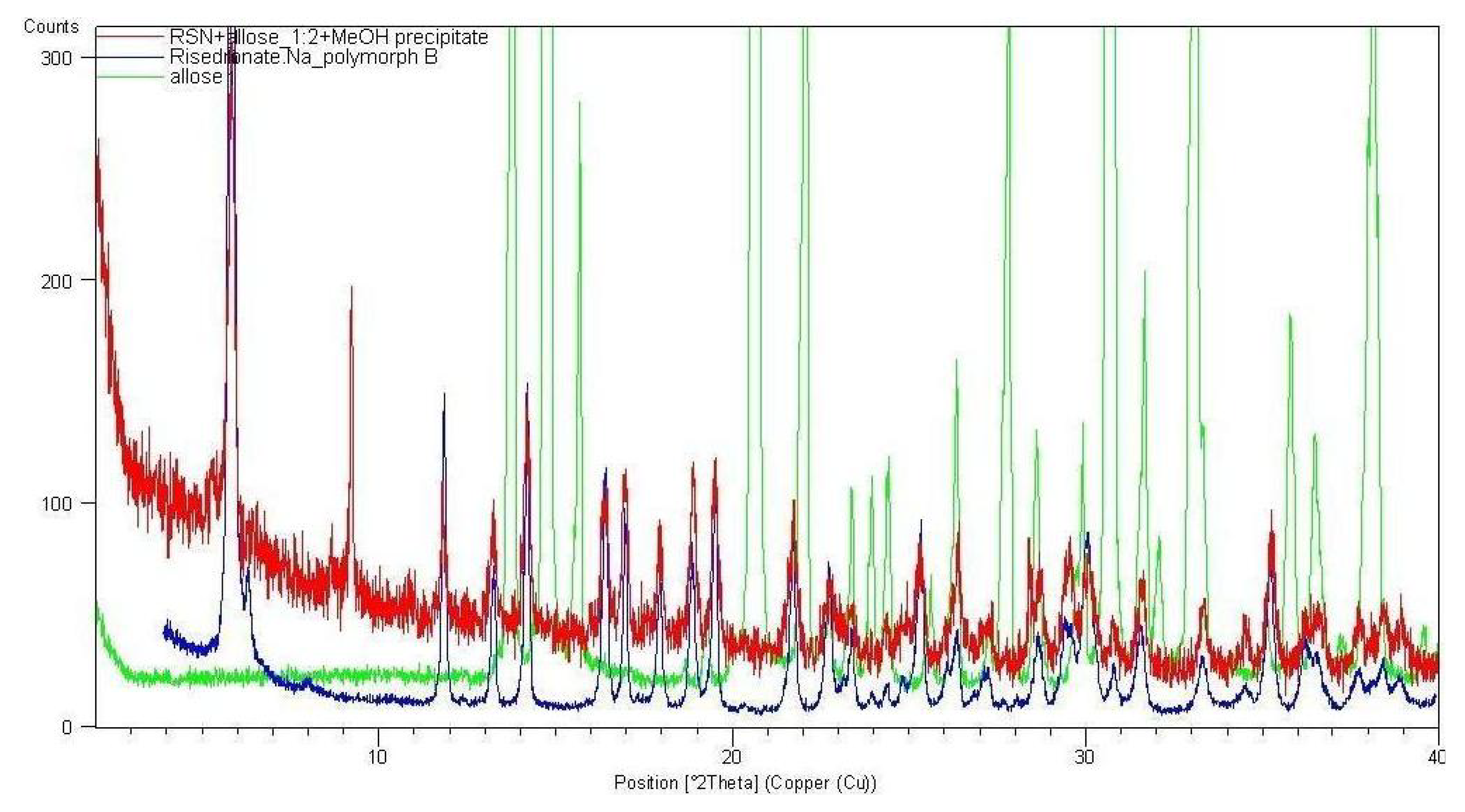
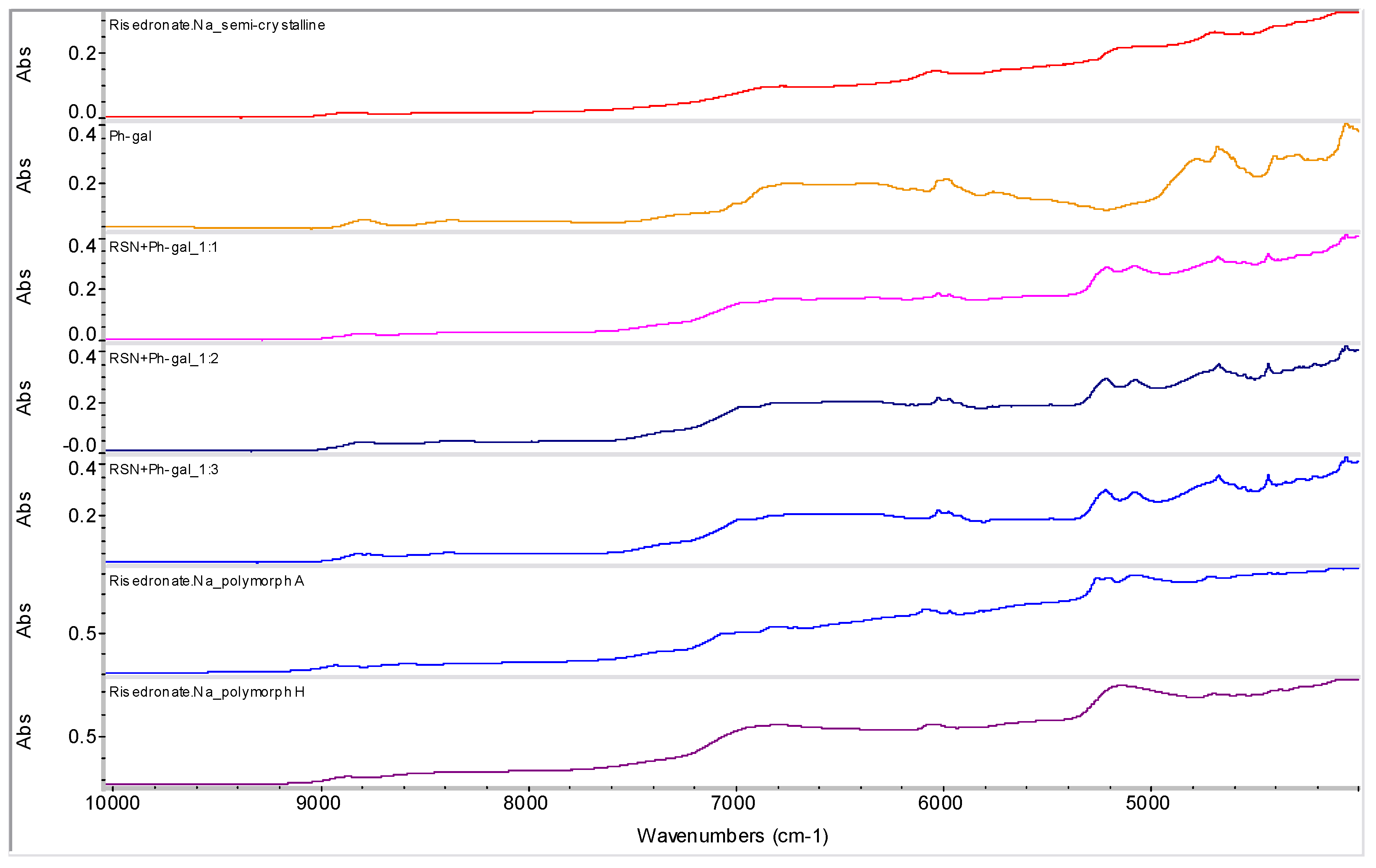
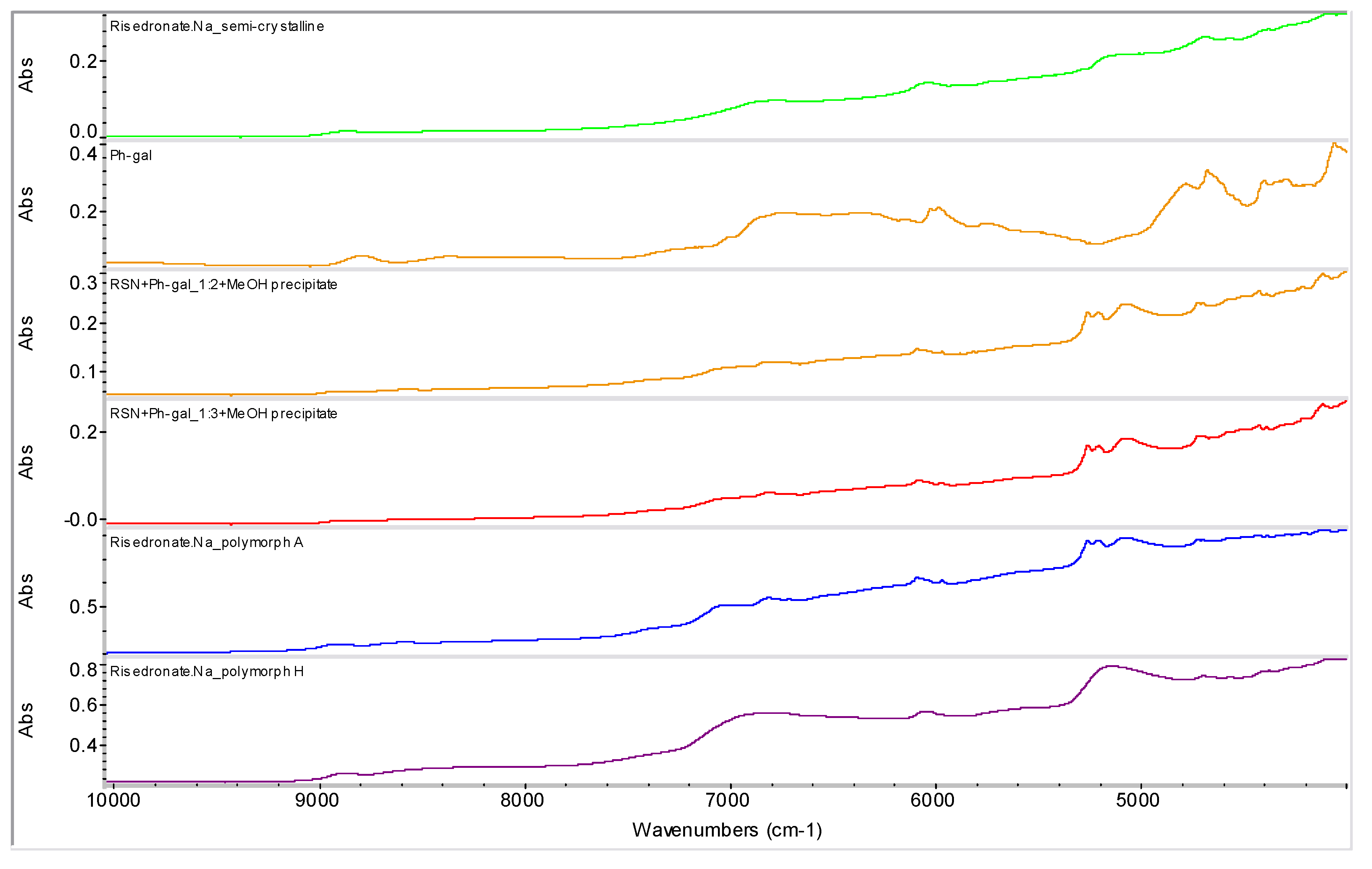
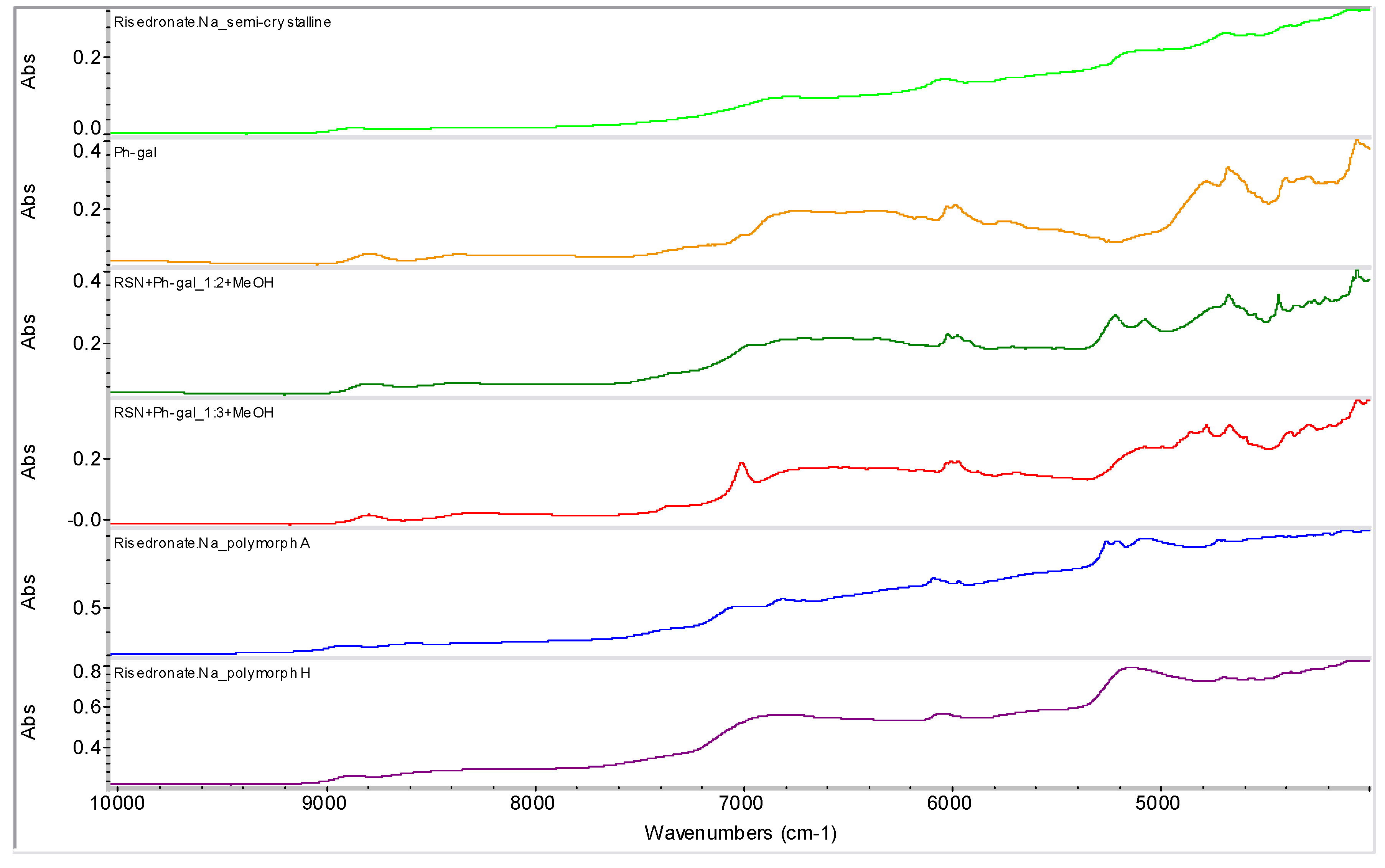
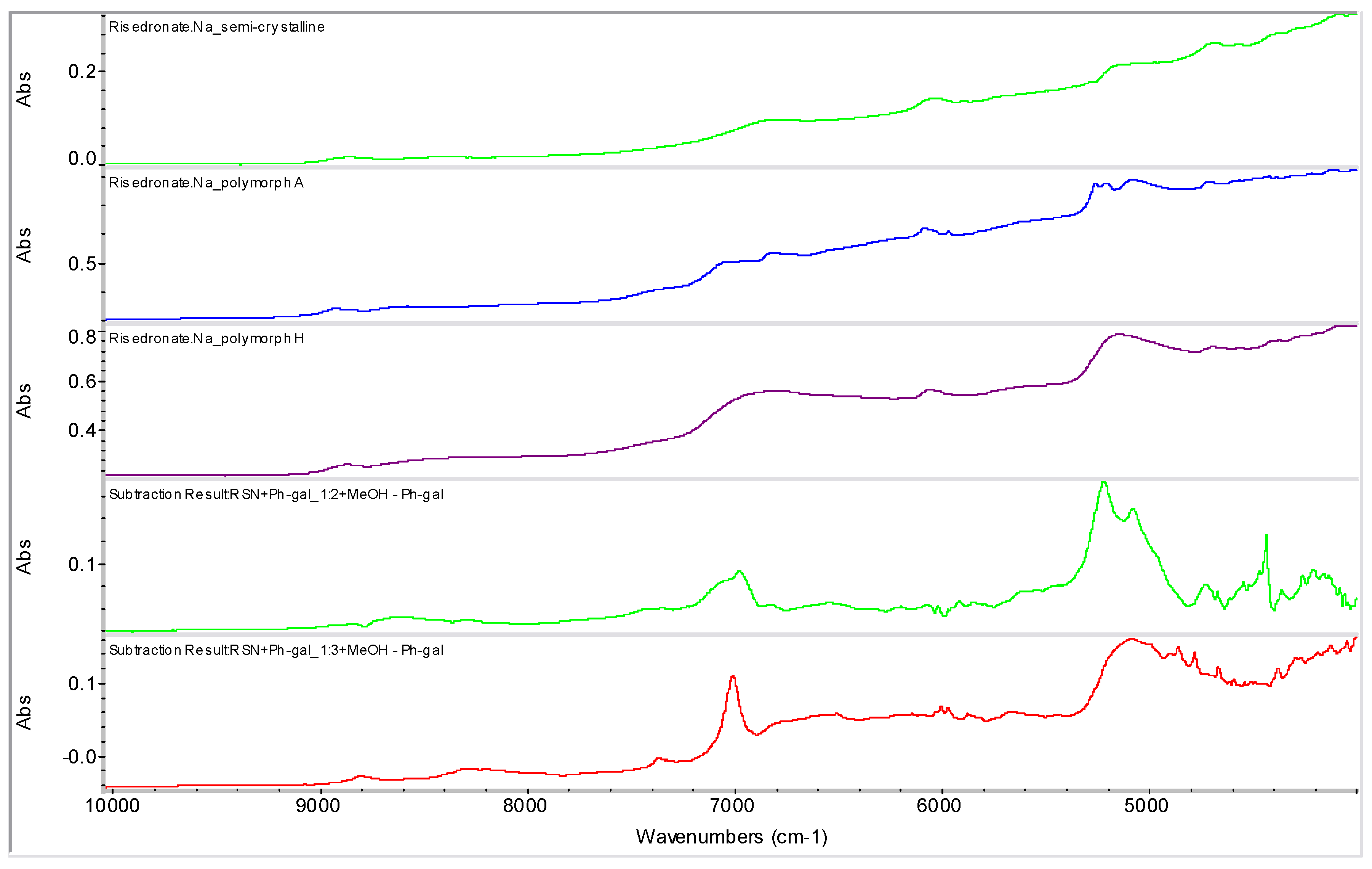
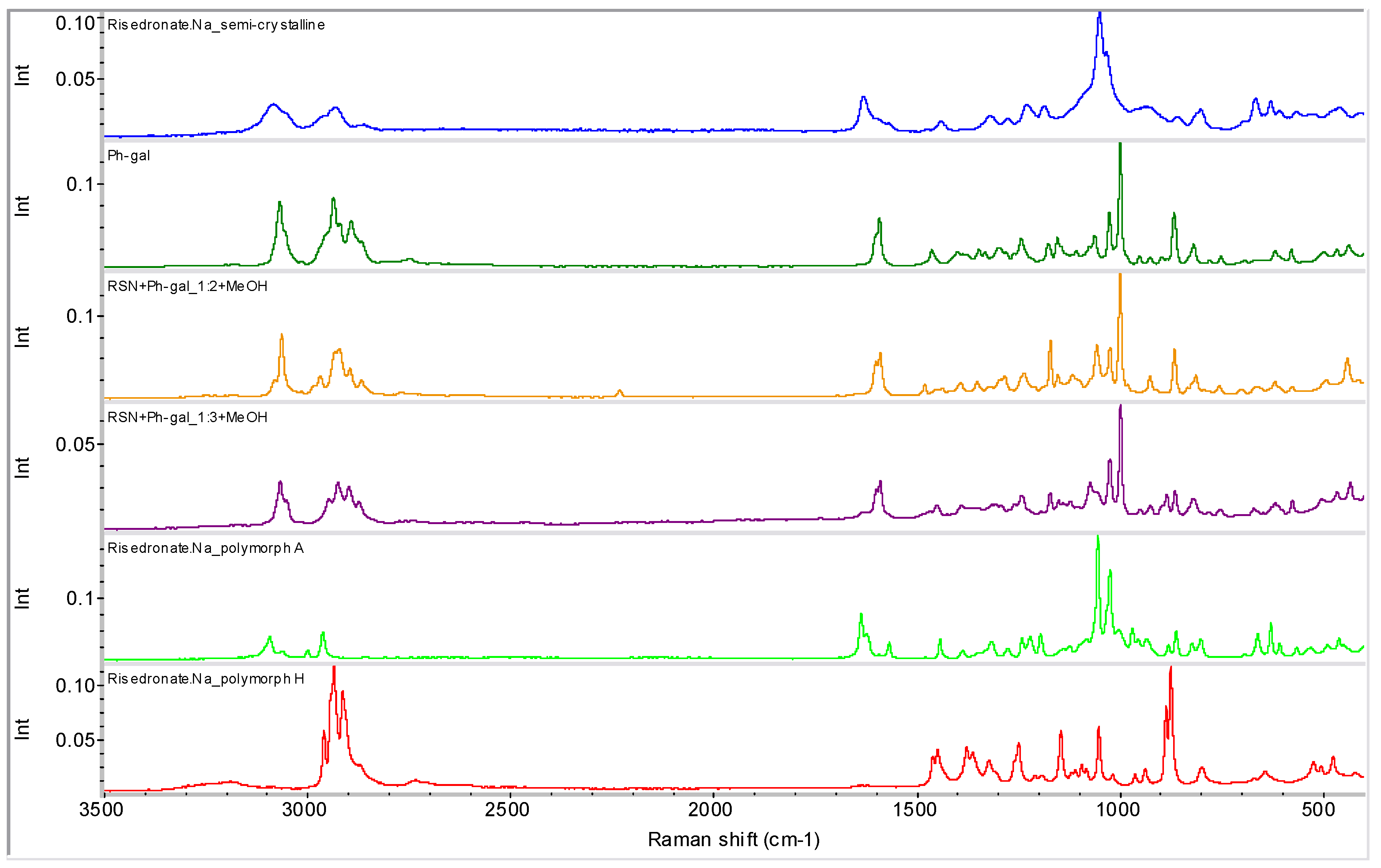
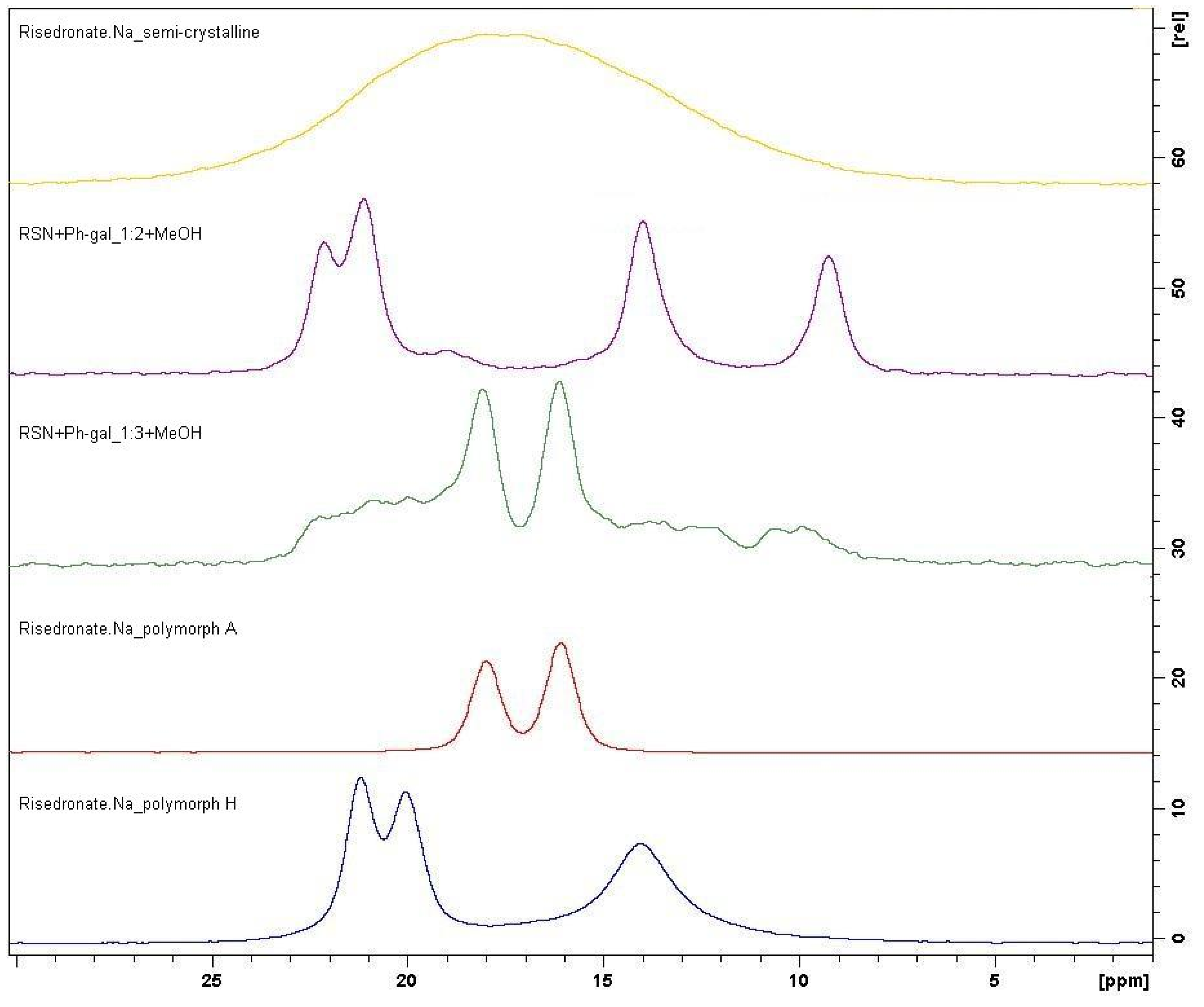
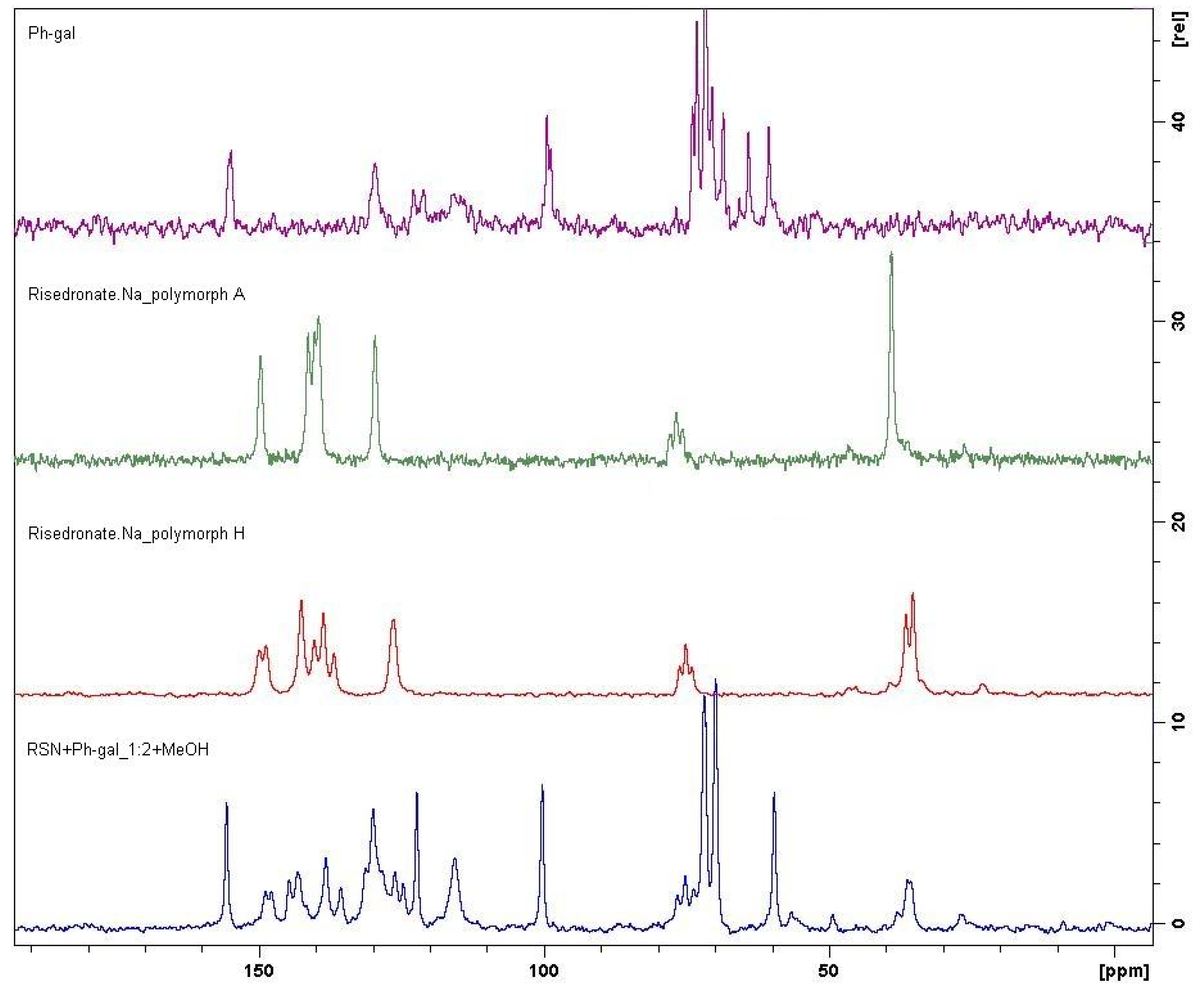
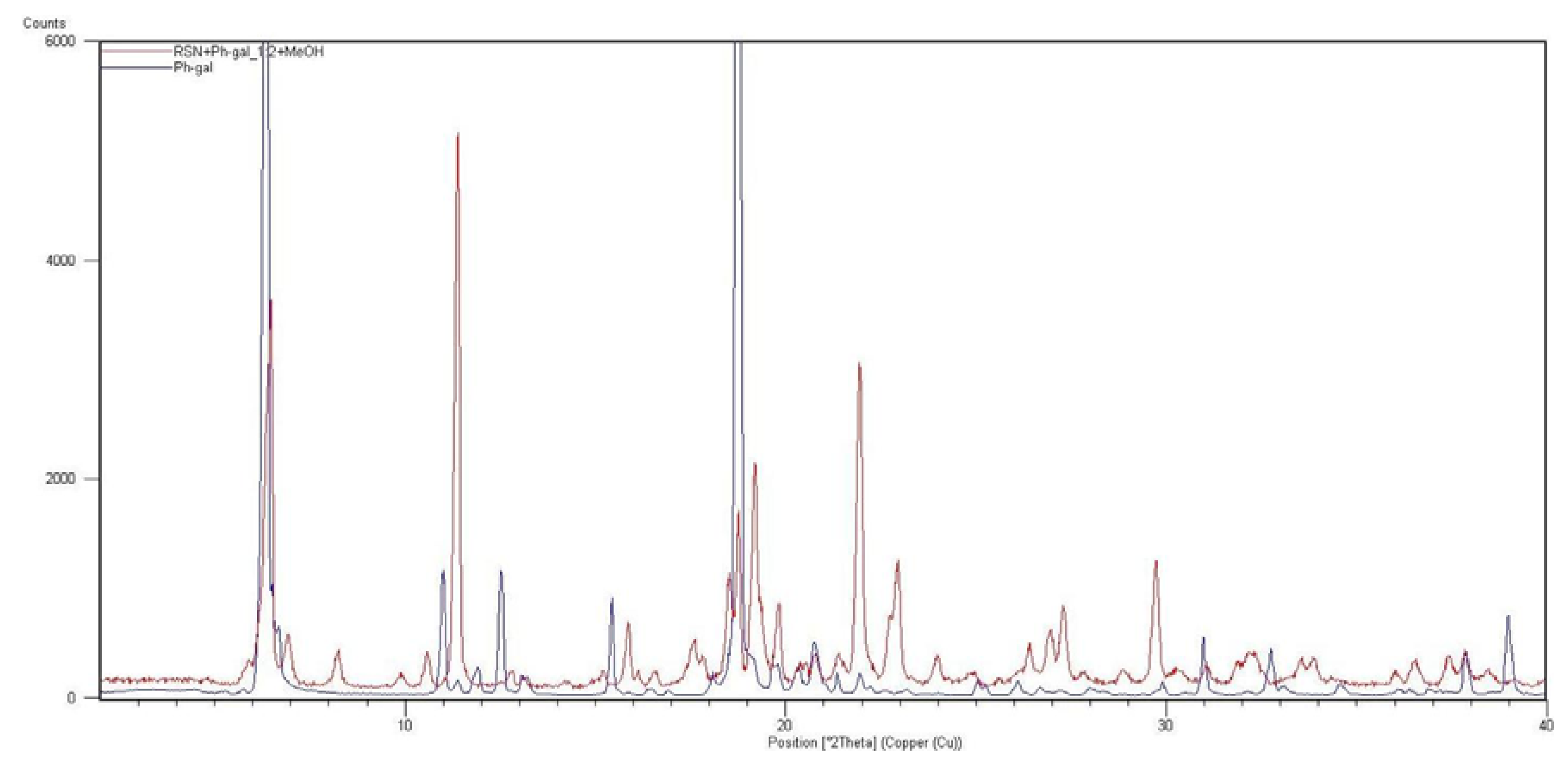
2.2. Molecular Modelling

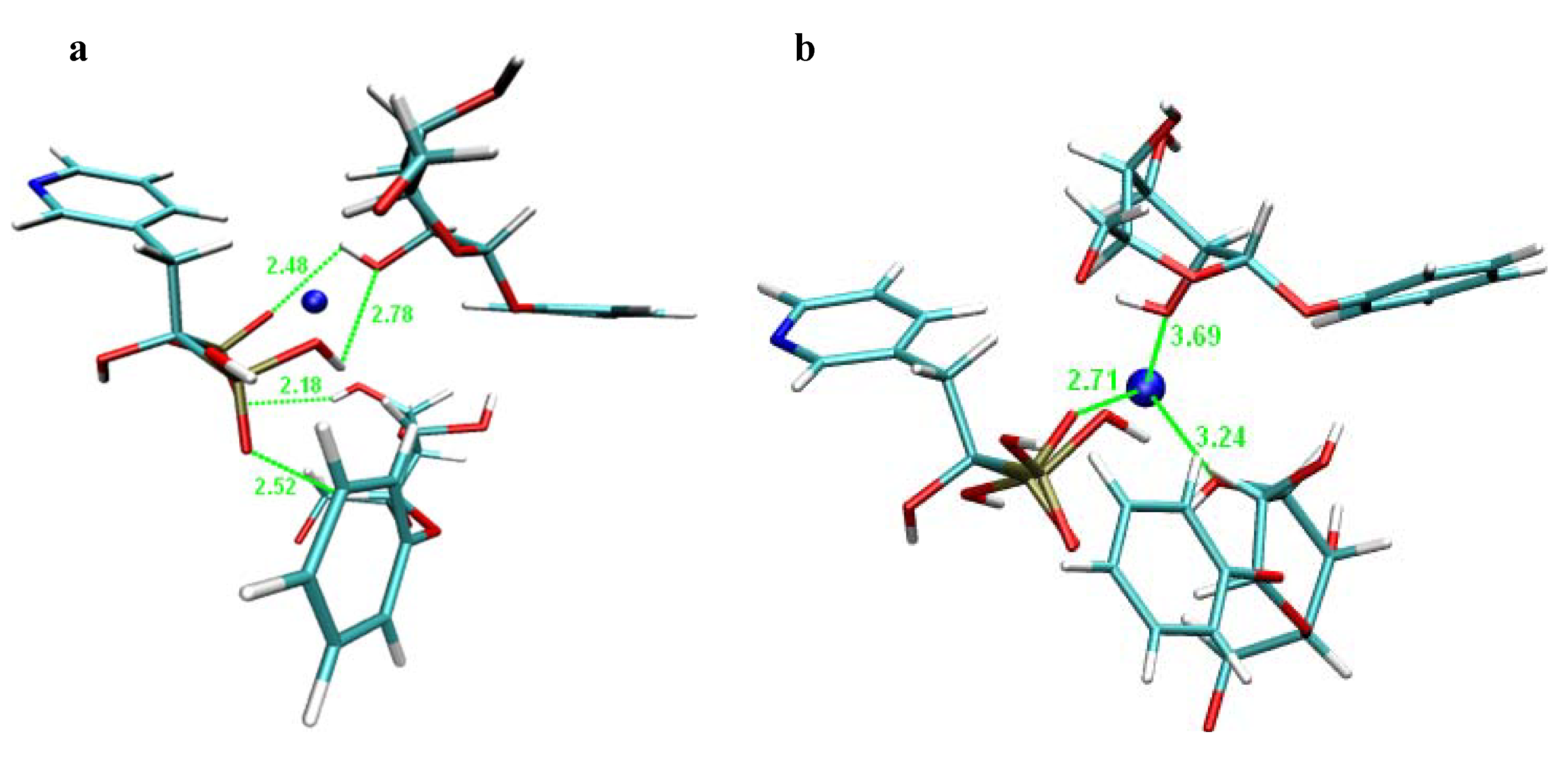
2.3. In vitro Screening of Absorption (PAMPA experiments)
3. Experimental
3.1. General
3.2. Generation of Sampless
| Comp. | 1:1 | 1:2 | 1:3 | |||
|---|---|---|---|---|---|---|
| Amount [g] | Water [mL] | Amount [g] | Water [mL] | Amount [g] | Water [mL] | |
| RSN | 0.6017 | 10 | 0.6010 | 10 | 0.6007 | 10 |
| Phe-gal | 0.5054 | 2.0 | 1.0095 | 3.0 | 1.5136 | 3.5 |
| Comp. | 1:2 | 1:3 | ||||
|---|---|---|---|---|---|---|
| Amount [g] | Water [mL] | MeOH [mL] | Amount [g] | Water [mL] | MeOH [mL] | |
| RSN | 0.6015 | 10 | 5.0 | 0.6012 | 10 | 5.0 |
| Phe-gal | 1.0104 | 3.0 | 5.0 | 1.5149 | 3.5 | 5.0 |
3.3. Molecular Modelling
4. Conclusions
Acknowledgements
References
- Pharmaterials Ltd. Available online: http://www.pharmaterials.co.uk/co-crystals.html (accessed on 11 January 2011).
- Frontiers in Crystal Engineering; Tiekink, E.R.T.; Vittal, J. (Eds.) Wiley-VCH: Wienheim, Germany, 2005.
- Making crystals by Design: Methods, Techniques and Applications; Braga, D.; Grepioni, F. (Eds.) Wiley-VCH: Wienheim, Germany, 2006.
- Ebetino, F.H.; Francis, M.D.; Rogers, M.J.; Russell, R.G.G. Mechanisms of action of etidronate and other bisphosphonates. Rev. Contemp. Pharmacother. 1998, 9, 233–243. [Google Scholar]
- Sato, M.; Grasser, W.; Endo, N.; Akins, R.; Simmons, H.; Thompson, D.D.; Golub, E.; Rodan, G.A. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J. Clin. Invest. 1991, 88, 2095–2105. [Google Scholar] [CrossRef]
- Carano, A.; Teitelbaum, S.L.; Konsek, J.D.; Schlesinger, P.H.; Blair, H.C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J. Clin. Invest. 1990, 85, 456–461. [Google Scholar] [CrossRef]
- Hughes, D.E.; Wright, K.R.; Uy, H.L.; Sasaki, A.; Yoneda, T.; Roodman, G.D.; Mundy, G.R.; Boyce, B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995, 10, 1478–1487. [Google Scholar]
- Selander, K.S.; Monkkonen, J.; Karhukorpi, E.K.; Harkonen, P.; Hannuniemi, R.; Vaananen, H.K. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol. Pharmacol. 1996, 50, 1127–1138. [Google Scholar]
- Ito, M.; Amizuka, N.; Nakajima, T.; Ozawa, H. Ultrastructural and cytochemical studies on cell death of osteoclasts induced by bisphosphonate treatment. Bone 1999, 25, 447–452. [Google Scholar] [CrossRef]
- Reszka, A.A.; Halasy-Nagy, J.M.; Masarachia, P.J.; Rodan, G.A. Bisphosphonates Act Directly on the Osteoclast to Induce Caspase Cleavage of Mst1 Kinase during Apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J. Biol. Chem. 1999, 274, 34967–34975. [Google Scholar]
- Rogers, M.J.; Xiong, X.; Brown, R.J.; Watts, D.J.; Russell, R.G.; Bayless, A.V.; Ebetino, F.H. Structure-activity relationships of new heterocycle-containing bisphosphonates as inhibitors of bone resorption and as inhibitors of growth of Dictyostelium discoideum amoebae. Mol. Pharmacol. 1995, 47, 398–402. [Google Scholar]
- Rogers, M.J.; Gordon, S.; Benford, H.L.; Coxon, F.P.; Luckman, S.P.; Monkkonen, J.; Frith, J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000, 88, 2961–2978. [Google Scholar] [CrossRef]
- van Beek, E.R.; Lowik, C.W.; Ebetino, F.H.; Papapoulos, S.E. Binding and antiresorptive properties of heterocycle-containing bisphosphonate analogs: Structure-activity relationships. Bone 1998, 23, 437–442. [Google Scholar] [CrossRef]
- MedicineNet. Available online: http://www.medicinenet.com/risedronate/article.htm (accessed on 11 January 2011).
- eMedTV – Health Information Brought to Life™. Available online: http://osteoporosis.emedtv.com/ (accessed on 11 January 2011).
- Ezra, A.; Golomb, G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv. Drug Del. Rev. 2000, 42, 175–195. [Google Scholar] [CrossRef]
- Kerns, E.H.; Li, D. Drug-like Properties: Concept, Structure Design and Methods; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Luypaert, J.; Massart, D.L.; Vander-Heyden, Y. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta 2007, 72, 865–883. [Google Scholar] [CrossRef]
- Jampilek, J.; Oktabec, Z.; Pekarek, T.; Havlicek, J.; Dohnal, J.; Kral, V. Characterization of polymorphs and API-excipient co-crystals using vibration spectroscopy and solid-state NMR. In Proceedings of the 11th International Workshop on Physical Characterization of Pharmaceutical Solids (IWPCPS® 11), Stamford, CT, USA, 14-19 June 2009.
- Jampilek, J.; Oktabec, Z.; Rezacova, A.; Placek, L.; Kos, J.; Havelkova, L.; Dohnal, J.; Kral, V. Preparation and properties of new co-crystals of ibandronate. In Proceedings of the 13th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-13), 1-30 November 2009.
- Jampilek, J.; Kos, J.; Oktabec, Z.; Mandelova, Z.; Pekarek, T.; Tkadlecova, M.; Havlicek, J.; Dohnal, J.; Kral, V. Co-crystal screening study of risedronate and unsubstituted hexoses. In proceedings of the 14th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-14), 1-30 November 2010.
- Oktabec, Z.; Kos, J.; Havelkova, L.; Mandelova, Z.; Pekarek, T.; Rezacova, A.; Placek, L.; Tkadlecova, M.; Havlicek, J.; Dohnal, J.; Jampilek, J. Preparation and properties of new co-crystals of ibandronate with gluco- or galactopyranoside derivatives. Molecules 2010, 15, 8973–8987. [Google Scholar] [CrossRef]
- Richter, J.; Jirman, J. Crystalline form of the sodium salt of 3-pyridyl-1-hydroxyethylidene-1,1-bisphosphonic acid. Zentiva, a.s. U.S. Patent 7,276,604, 2 October 2007. [Google Scholar]
- Cazer, F.D.; Perry, G.E.; Billings, D.M.; Redman-Furey, N.L. Selective crystallization of 3-pyridyl-1-hydroxy-ethylidene-1,1-bisphosphonic acid sodium as the hemipentahydrate or monohydrate. EP1252170 A2, 1 February 2001. [Google Scholar]
- Aronhime, J.; Lifshitz-Liron, R.; Kovalevski-Ishai, E.; Lidor-Hadas, R. Novel polymorphs and pseudopoly-morphs of risedronate sodium. CA2480764 A1, 23 October 2003. [Google Scholar]
- Aronhime, J.; Lifshitz-Liron, R.; Kovalevski-Ishai, E.; Lidor-Hadas, R. Novel polymorphs and pseudopolymorphs of risedronate sodium. WO 03/086355 A1, 23 October 2003. [Google Scholar]
- Redman-Furey, N.L.; Ficka, M.; Bigalow-Kern, A.; Cambron, R.T.; Lubey, G.; Lester, C.; Vaughn, D. Structural and analytical characterization of three hydrates and an anhydrate form. J. Pharm. Sci. 2005, 94, 893–911. [Google Scholar] [CrossRef]
- Gossman, W.L.; Wilson, S.R.; Oldfield, E. Three hydrates of the isphosphonates risedronate, consisting of one molecular and two ionic structures. Acta Cryst. C 2003, 59, m33–m36. [Google Scholar] [CrossRef]
- Bruning, J.; Petereit, A.C.; Alig, E.; Bolte, M.; Dressman, J.B.; Schmidt, M.U. Characterization of a new solvate of risedronate. J. Pharm. Sci. 2011, 100, 863–873. [Google Scholar] [CrossRef]
- Avogadro: An Open-source Molecular Builder and Visualization Tool, Version 1.0.1. Available online: http://avogadro.openmolecules.net/ (accessed on 28 April 2010).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Merz, K.M.; Pearlman, D.A.; Crowley, M.; Walker, R.C.; Zhang, W.; Wang, B.; Hayik, S.; Roitberg, A.; Seabra, G.; Wong, K.F.; Paesani, F.; Wu, X.; Brozell, S.; Tsui, V.; Gohlke, H.; Yang, L.; Tan, C.; Mongan, J.; Hornak, V.; Cui, G.; Beroza, P.; Mathews, D.H.; Schafmeister, C.; Ross, W.S.; Kollman, P.A. AMBER 9; University of California: San Francisco, CA, USA, 2006. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kos, J.; Pentakova, M.; Oktabec, Z.; Krejcik, L.; Mandelova, Z.; Harokova, P.; Hruskova, J.; Pekarek, T.; Dammer, O.; Tkadlecova, M.; et al. Crystallization Products of Risedronate with Carbohydrates and Their Substituted Derivatives. Molecules 2011, 16, 3740-3760. https://doi.org/10.3390/molecules16053740
Kos J, Pentakova M, Oktabec Z, Krejcik L, Mandelova Z, Harokova P, Hruskova J, Pekarek T, Dammer O, Tkadlecova M, et al. Crystallization Products of Risedronate with Carbohydrates and Their Substituted Derivatives. Molecules. 2011; 16(5):3740-3760. https://doi.org/10.3390/molecules16053740
Chicago/Turabian StyleKos, Jiri, Monika Pentakova, Zbynek Oktabec, Lukas Krejcik, Zuzana Mandelova, Pavla Harokova, Jana Hruskova, Tomas Pekarek, Ondrej Dammer, Marcela Tkadlecova, and et al. 2011. "Crystallization Products of Risedronate with Carbohydrates and Their Substituted Derivatives" Molecules 16, no. 5: 3740-3760. https://doi.org/10.3390/molecules16053740
APA StyleKos, J., Pentakova, M., Oktabec, Z., Krejcik, L., Mandelova, Z., Harokova, P., Hruskova, J., Pekarek, T., Dammer, O., Tkadlecova, M., Havlicek, J., Vinsova, J., Kral, V., Dohnal, J., & Jampílek, J. (2011). Crystallization Products of Risedronate with Carbohydrates and Their Substituted Derivatives. Molecules, 16(5), 3740-3760. https://doi.org/10.3390/molecules16053740






