Role of the Morphology and Polyphosphate in Trichoderma harzianum Related to Cadmium Removal
Abstract
:1. Introduction
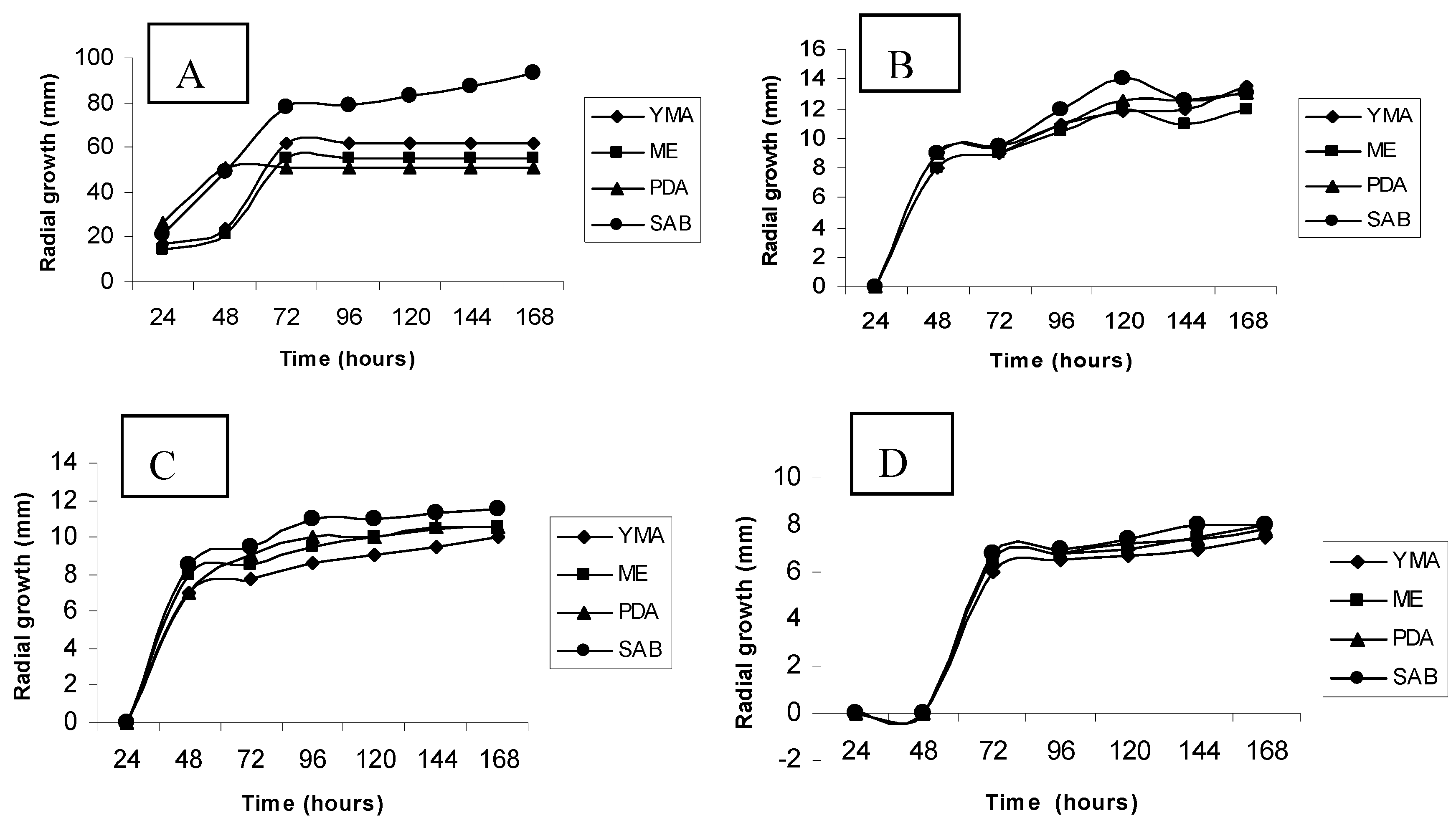
2. Results and Discussion
2.1. Effects of cadmium in the growth, morphology and ultrastructure of Trichoderma harzianum
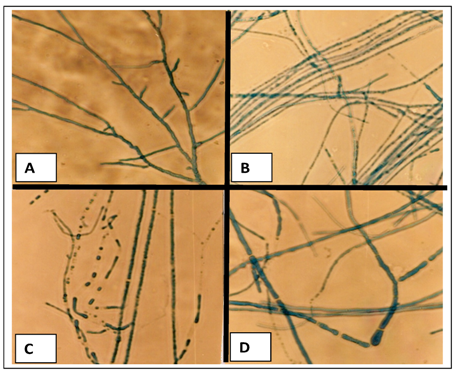
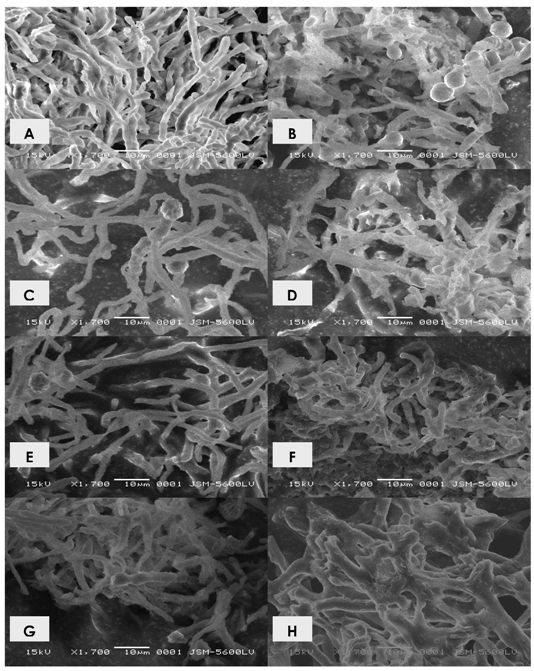
2.2. Polyphosphate behavior of Trichoderma harzianum in the presence of cadmium
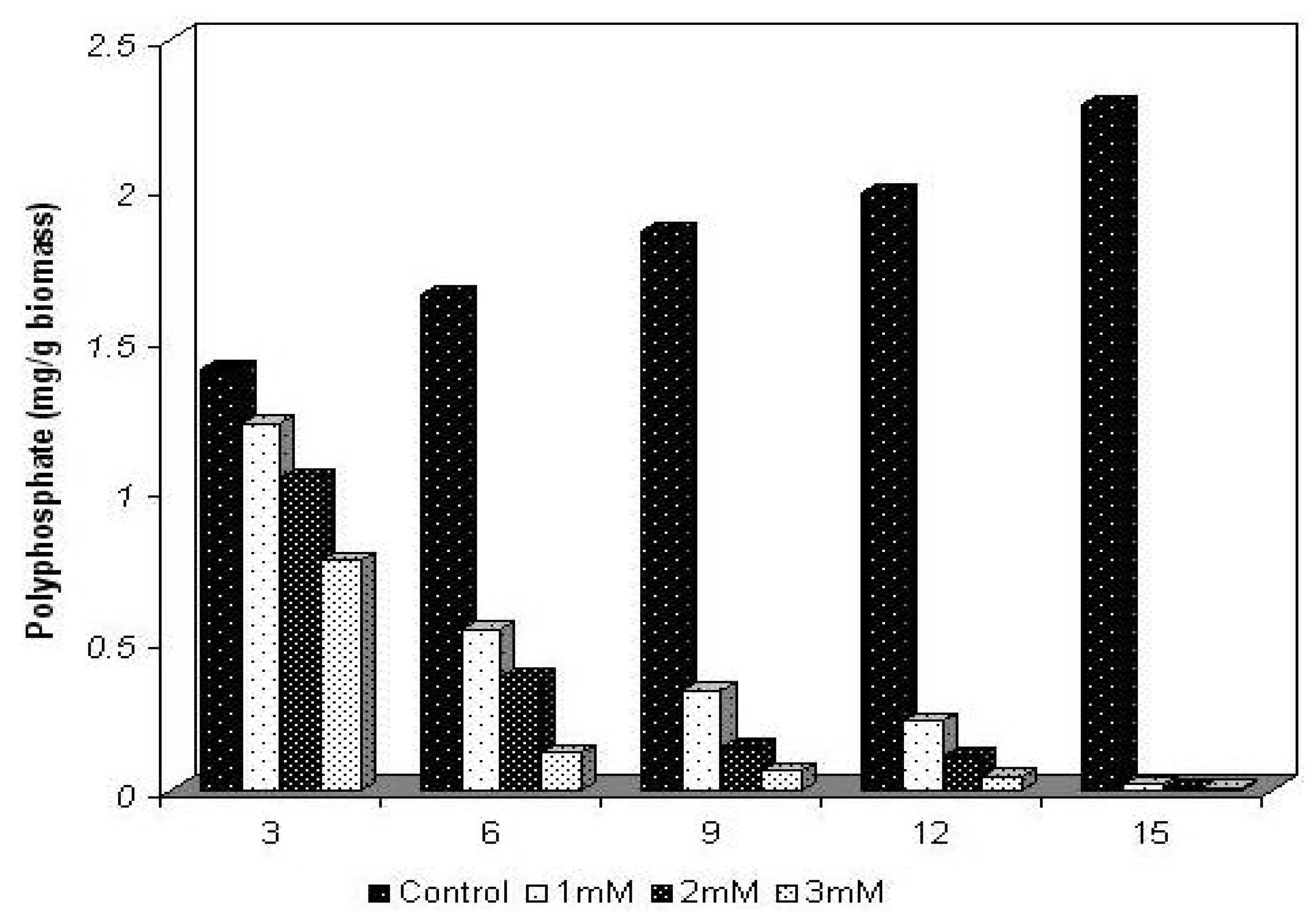
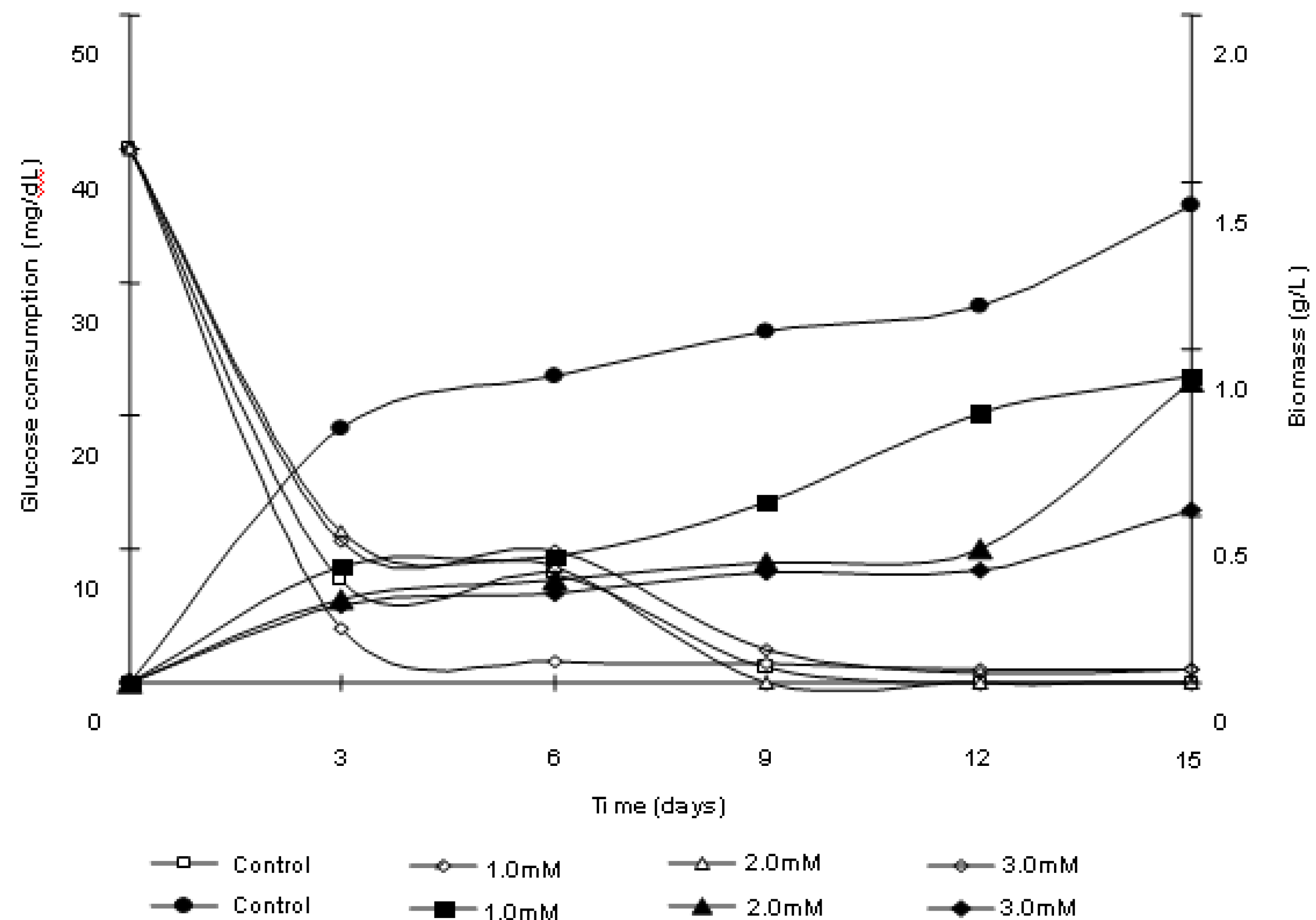
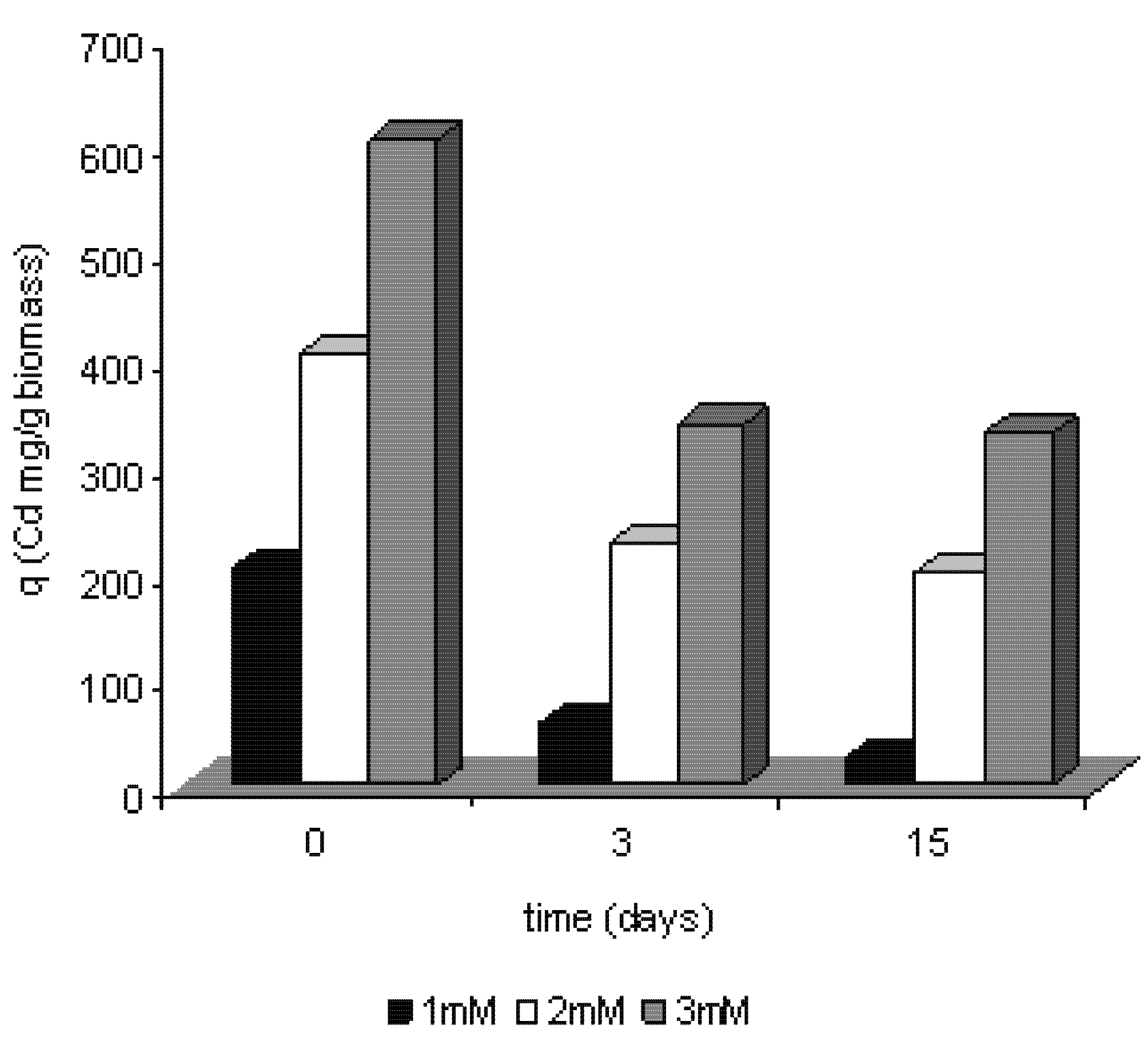
2.3. Removal of cadmium by Trichoderma harzianum

2.4. Efficiency of removal of cadmium by Trichoderma harzianum
2.5. Discussion
3. Experimental
3.1. Microorganism and culture conditions
3.2. Culture media screening by radial growth
3.3. Growth curves
3.4. Effects of cadmium on the cell morphology
3.5. Ultrastructural analysis - scanning electronic microscopy
3.6. Extraction and dosage of total polyphosphate
3.7. Kinetics of cadmium removal
4. Conclusions
Acknowledgments
References
- Gadd, G.M. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr. Opin. Biotechnol. 2000, 11, 271–279. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Rosen, B.P. Transport e detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. 2002, 133, 689–693. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Fungi biosorption - an alternative treatment option for heavy metal bearing wastewaters: A review. Bioresour. Technol. 1995, 53, 195–206. [Google Scholar]
- Leyval, C.; Turnau, K.; Haselweter, K. Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 1997, 7, 139–153. [Google Scholar] [CrossRef]
- Pepper, I.L. Microbial responses to environmentally toxic cadmium. Microb. Ecol. 2000, 38, 358–364. [Google Scholar]
- Bhattacharyya, M.H.; Wilson, A.K.; Rajan, S.S.; Jonah, M. Biochemical pathways in cadmium toxicity. In Molecular Biology and Toxicology of Metals; Zalups, R.K., Koropatnick, E.J., Eds.; Taylor & Francis: London, UK, 2000; pp. 34–74. [Google Scholar]
- Satoh, M.; Koyama, H.; Kaji, T.; Kito, H.; Tohyama, C. Perspectives on cadmium toxicity research. Tohoku J. Exp. Med. 2002, 196, 23–32. [Google Scholar] [CrossRef]
- Deckert, J. Cadmium toxicity in plants: Is there any analogy to its carcinogenic effect in mammalian cells? Biometals 2005, 18, 475–481. [Google Scholar] [CrossRef]
- Järup, L. Cadmium overload and toxicity. Nephrol. Dialysis Transplant. 2002, 17, 35–39. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.E.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Gavrilesca, M. Removal of heavy metals from the environmental by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Vijver, M.G.; Gestel, C.A.M.V.; Lanno, R.P.; Van-Straalen, N.M.; Peijnenburg, W.J.G.M. Internal metal sequestration and its ecotoxicological relevance: A review. Environ. Sci. Technol. 2004, 8, 4705–4712. [Google Scholar]
- Wang, J.L. Microbial cell-surface display and the application in bioremediation of contaminated environment. Chin. Biotechnol. 2005, 25, 112–117. [Google Scholar]
- Mehra, R.K.; Winge, D.R. Metal-ion resistance in fungi: molecular mechanisms and their regulated expression. J. Cell Biochem. 1991, 45, 30–40. [Google Scholar] [CrossRef]
- Keasling, J.D.; Hupf, G.A. Genetic manipulation of polyphosphate metabolism affects cadmium tolerance in Escherichia coli. Appl. Environ. Microbiol. 1996, 87, 743–746. [Google Scholar]
- Keasling, L.D.; Van Dien, S.J.; Trelstad, P.; Renninger, N.; McMahon, K. Application of polyphosphate metabolism to environmental and biotechnological problems. Biochemistry 1999, 65, 324–331. [Google Scholar]
- Aiking, H.; Stijnman, A.; Garderen, C.V.; Heerikhuizen, H.V.; Riet, J.V. Inorganic phosphate accumulation and cadmium detoxification in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl. Environ. Microbiol. 1984, 47, 374–377. [Google Scholar]
- Torres, M.; Goldberg, J.; Jensen, T.E. Heavy metal uptake by polyphosphate bodies in living and killed cells of Plectonema boryanum (cyanophycae). Microbios 1998, 96, 141–147. [Google Scholar]
- Kulaev, I.S. Biochemistry and biotechnology of inorganic polyphosphates. Biochemistry 2000, 65, 269–270. [Google Scholar]
- Malik, A. Metal bioremediation through growing cells. Environ. Inter. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, H. Characterization of cadmium removal by Rhodotorula sp. Y11. Appl. Microbiol. Biotechnol. 2006, 73, 458–463. [Google Scholar] [CrossRef]
- Sag, Y.; Kutsal, T. The selective biosorption of chromium(VI) and copper(II) ions from binary metal mixtures by Rhizopus arrhizus. Process Biochem. 1995, 31, 561–572. [Google Scholar]
- Lloyd, J.R. Bioremediation of metals; the application of micro-organisms that make and break minerals. Microbiol. Today 2002, 29, 67–69. [Google Scholar]
- Lopez, E.E.; Vazquez, C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere 2003, 50, 137–143. [Google Scholar] [CrossRef]
- Moreno, J.L.; García, C.; Lei, L.; Falchini, L.; Pietramellara, G.; Nannipieri, P. The ecological dose value (ED50) for assessing Cd toxicity on ATP content and dehydrogenase and urease activities of soil. Soil Biol. Biochem. 2001, 33, 483–489. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Gadd, G.M. Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. Biometals 2004, 17, 183–188. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, H.; Hu, X. Cadmium-resistance in growing Rhodotorula sp. Y11. Bioresour. Technol. 2007, 99, 1339–1344. [Google Scholar]
- Hasan, H.A.H. Role of rock phosphate in alleviation of heavy metals stress on Fusarium oxysporum. Plant Soil Environ. 2007, 53, 1–6. [Google Scholar]
- Kim, S.U.; Cheong, Y.H.; Seo, D.C.; Hur, J.S.; Heo, J.S.; Cho, J.S. Characterization of heavy metal tolerance and biosorption capacity of bacterium strain CPB4 (Bacillus spp.). Water Sci. Technol. 2007, 55, 105–111. [Google Scholar] [CrossRef]
- Göksungur, Y.; Üren, S.; Güvenc, U. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef]
- Remonsellez, F.; Orell, A.; Jerez, C.A. Copper tolerance of the thermoacidophilic archaeon Sulfolobus metallicus: possible role of polyphosphate metabolism. Microbiology 2006, 152, 59–66. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.; Smolders, G.J.; Kuba, T.; Heijnen, J.J. Metabolism of microorganisms responsible for enhanced biological phosphorus removal from wastewater. Anton. Leeuwenhoek 1997, 71, 109–116. [Google Scholar] [CrossRef]
- Alvarez, S.; Jerez, C.A. Copper ions stimulate polyphosphate degradation and phosphate efflux in Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2004, 70, 5177–5182. [Google Scholar] [CrossRef]
- Acosta, I.; Moctezuma-Zárate, M.G.; Cárdenas, J.F.; Gutiérrez, C. Biosorption of cadmium (ii) in aqueous solutions by fungal biomass. Rev. Inform. Technol. 2007, 18, 9–14. [Google Scholar]
- Massaccesi, G.; Romero, M.; Cazau, M.; Bucsinszky, A. Cadmium removal capacities of filamentous soil fungi isolated from industrially polluted sediments, in La Plata (Argentina). World J. Microbiol. Biotechnol. 2002, 18, 817–820. [Google Scholar] [CrossRef]
- McGrath, J.W.; Quinn, J.P. Intracellular accumulation of polyphosphate by the Candida humicola G-1 in response to acid pH. Appl. Environ. Microbiol. 2000, 66, 4068–4073. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar]
- Sample Availability: Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Freitas Lima, A.; Ferreira de Moura, G.; Barbosa de Lima, M.A.; Mendes de Souza, P.; Alves da Silva, C.A.; De Campos Takaki, G.M.; Do Nascimento, A.E. Role of the Morphology and Polyphosphate in Trichoderma harzianum Related to Cadmium Removal. Molecules 2011, 16, 2486-2500. https://doi.org/10.3390/molecules16032486
De Freitas Lima A, Ferreira de Moura G, Barbosa de Lima MA, Mendes de Souza P, Alves da Silva CA, De Campos Takaki GM, Do Nascimento AE. Role of the Morphology and Polyphosphate in Trichoderma harzianum Related to Cadmium Removal. Molecules. 2011; 16(3):2486-2500. https://doi.org/10.3390/molecules16032486
Chicago/Turabian StyleDe Freitas Lima, Adriana, Gabrielle Ferreira de Moura, Marcos Antonio Barbosa de Lima, Patrícia Mendes de Souza, Carlos Alberto Alves da Silva, Galba Maria De Campos Takaki, and Aline Elesbão Do Nascimento. 2011. "Role of the Morphology and Polyphosphate in Trichoderma harzianum Related to Cadmium Removal" Molecules 16, no. 3: 2486-2500. https://doi.org/10.3390/molecules16032486
APA StyleDe Freitas Lima, A., Ferreira de Moura, G., Barbosa de Lima, M. A., Mendes de Souza, P., Alves da Silva, C. A., De Campos Takaki, G. M., & Do Nascimento, A. E. (2011). Role of the Morphology and Polyphosphate in Trichoderma harzianum Related to Cadmium Removal. Molecules, 16(3), 2486-2500. https://doi.org/10.3390/molecules16032486



