Allobetulin and Its Derivatives: Synthesis and Biological Activity
Abstract
:1. Introduction
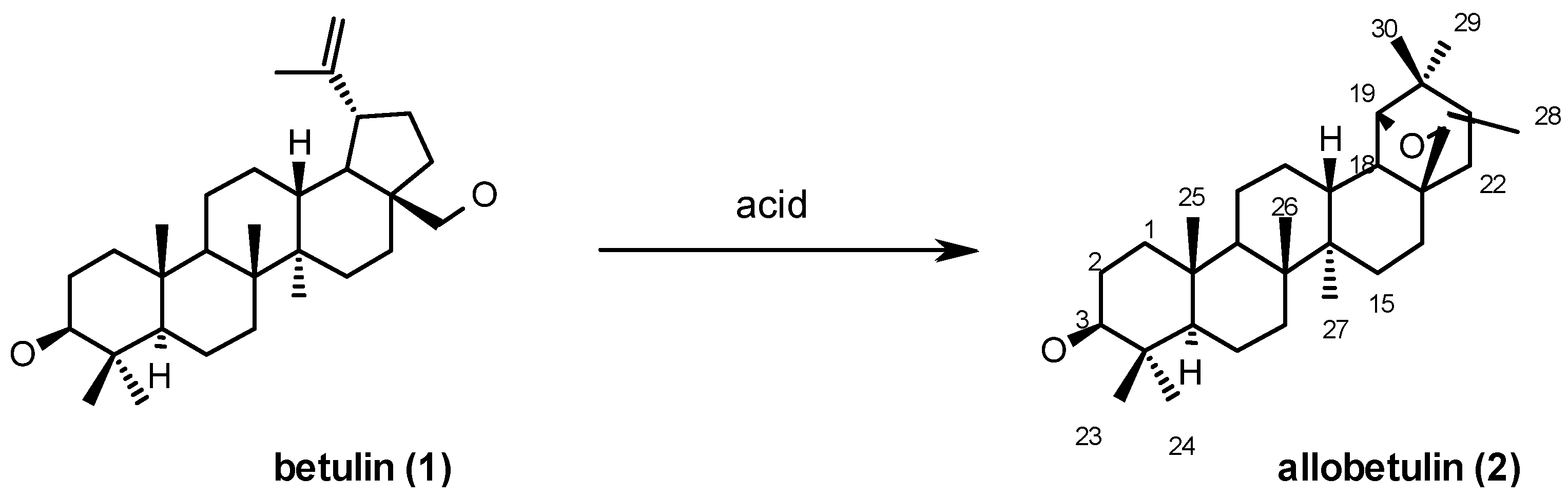
2. Betulin-Allobetulin Rearrangement
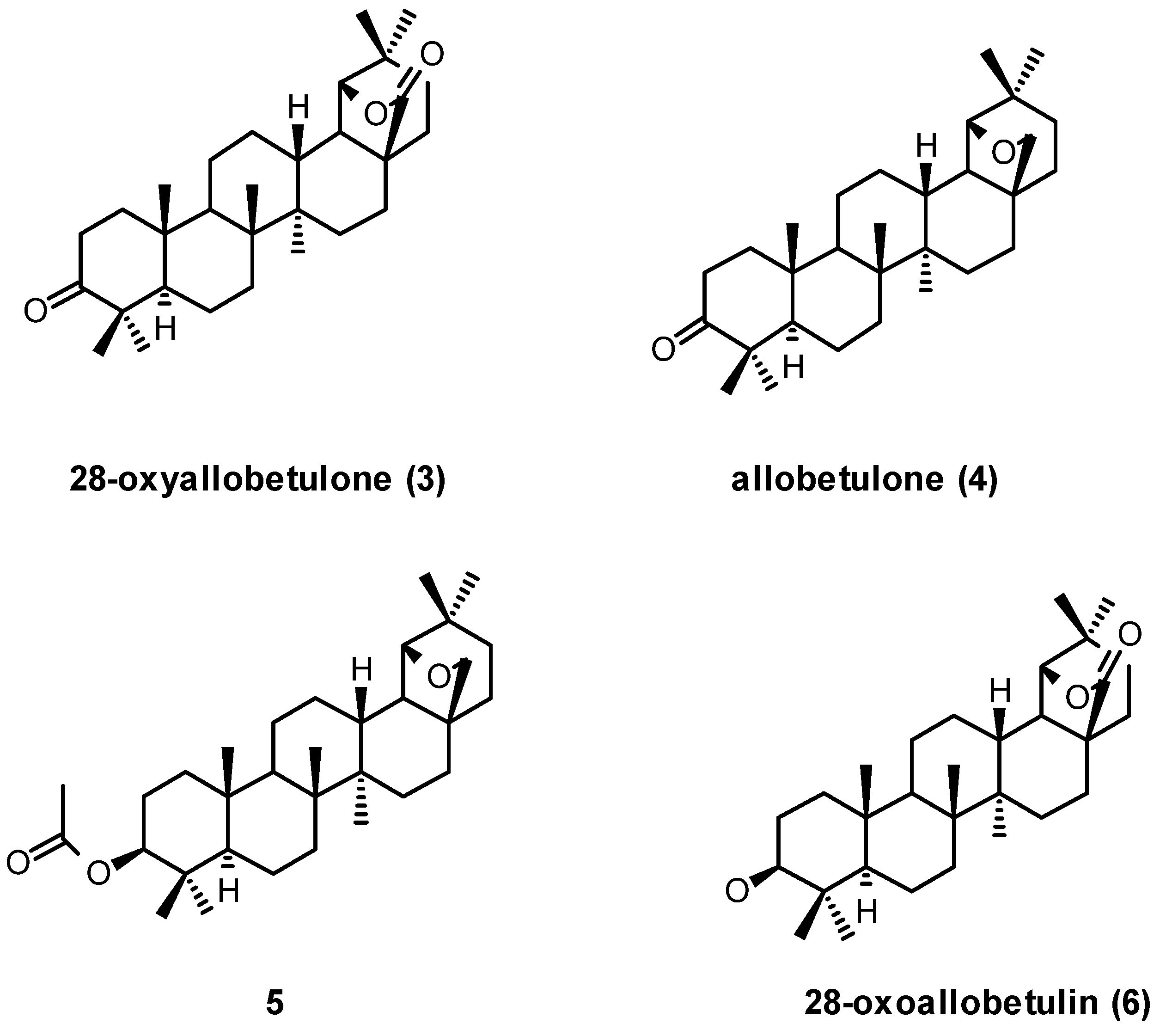
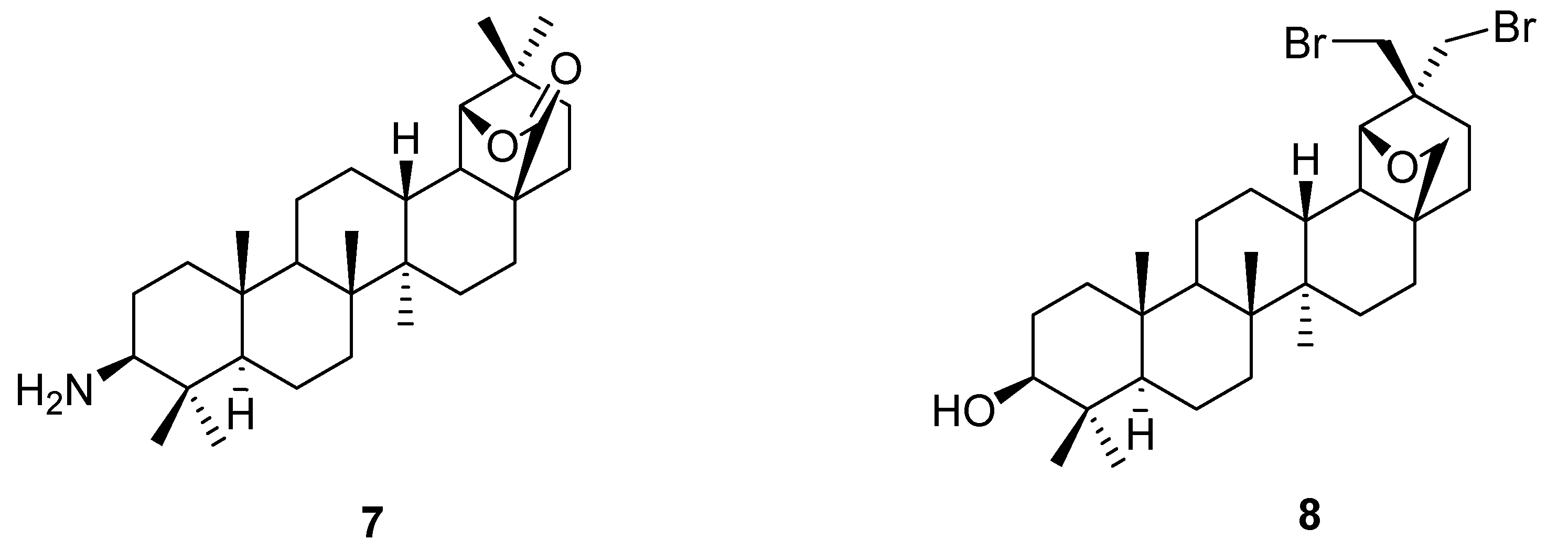
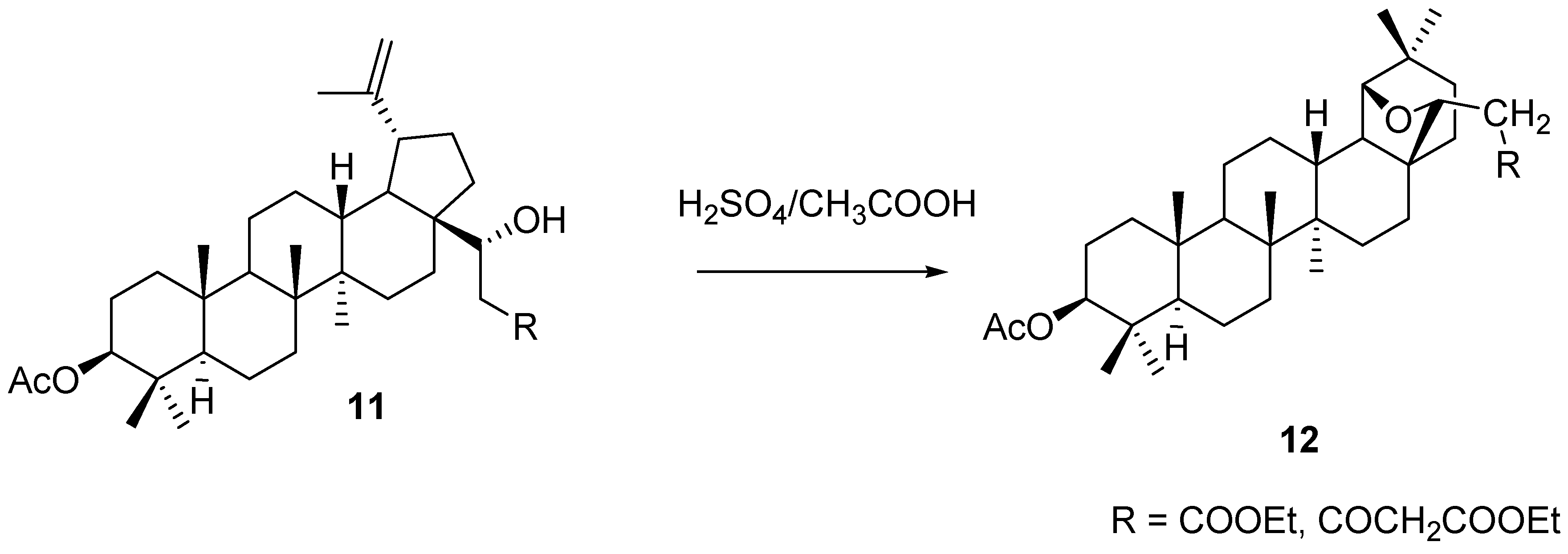
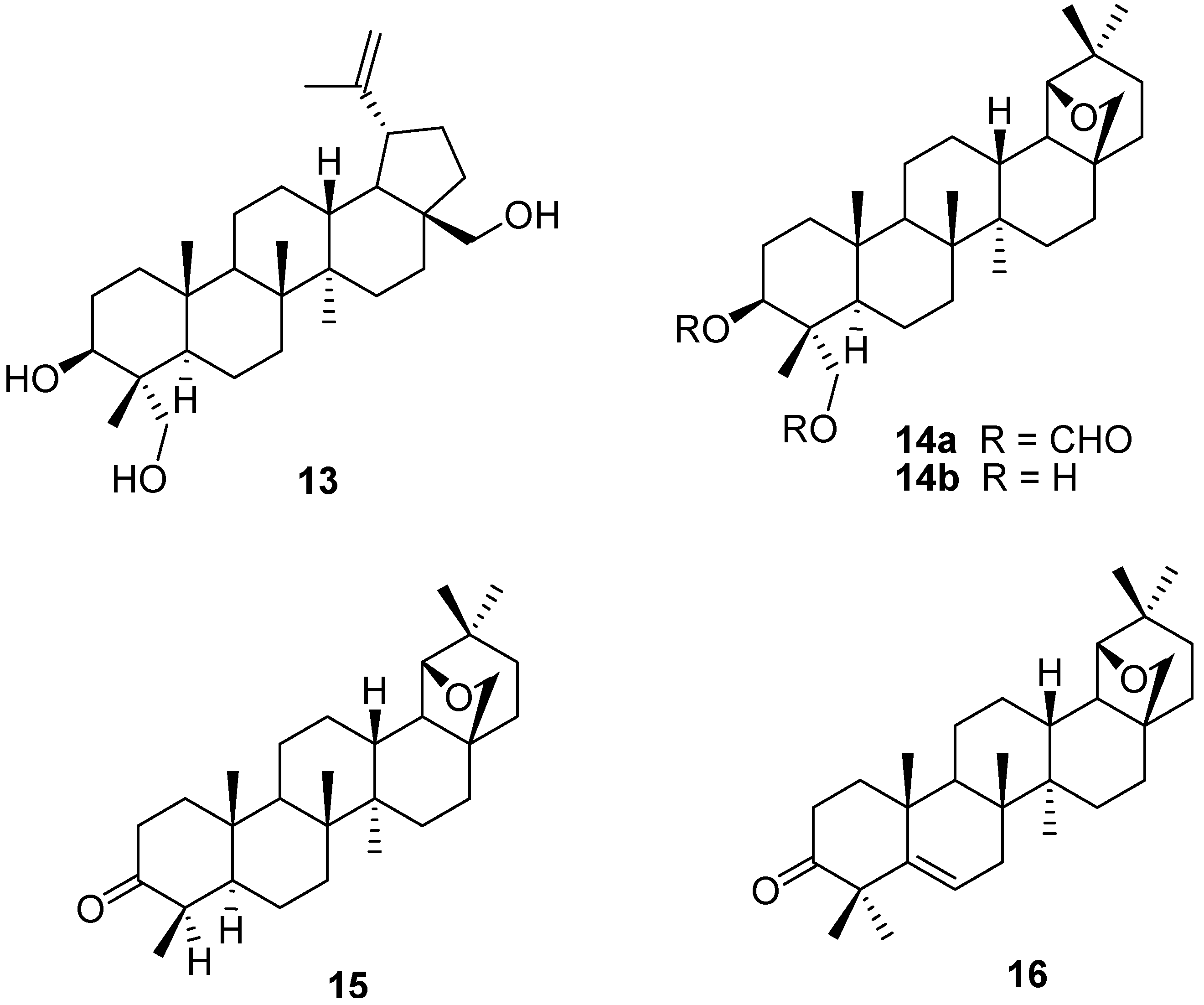
3. Simple Functionalisation Reactions of Allobetulin Analogs
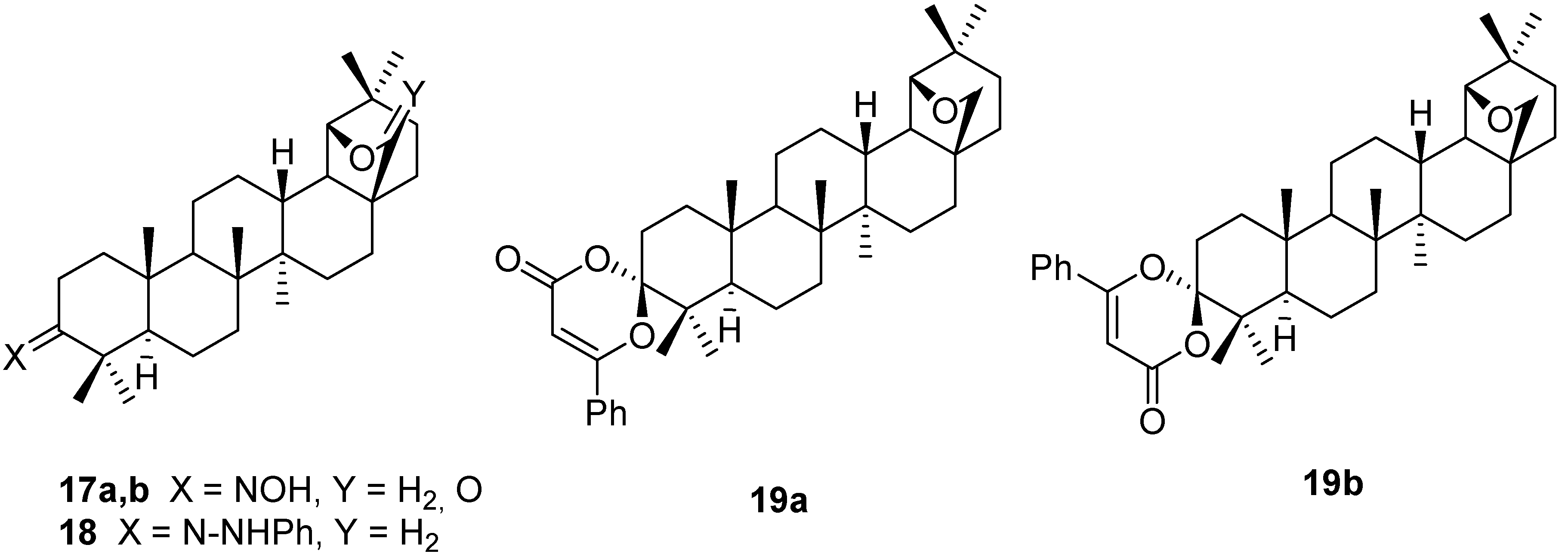
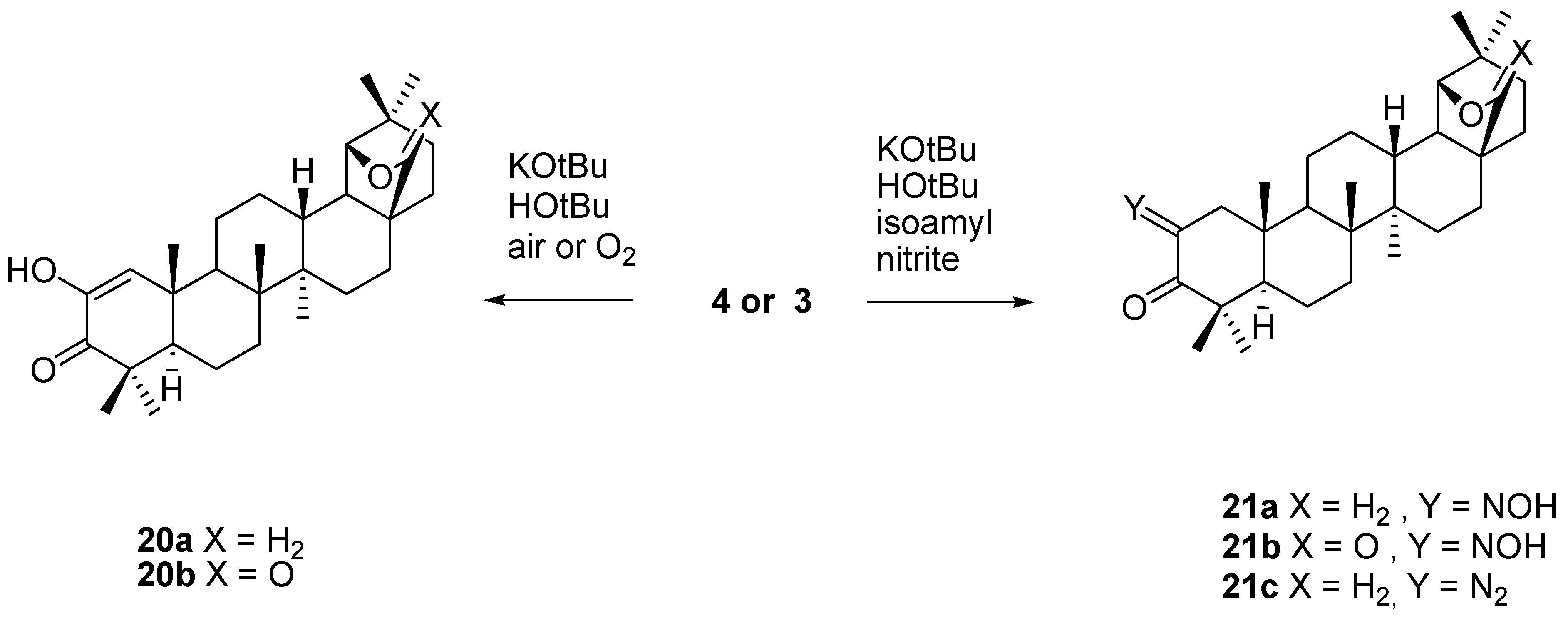
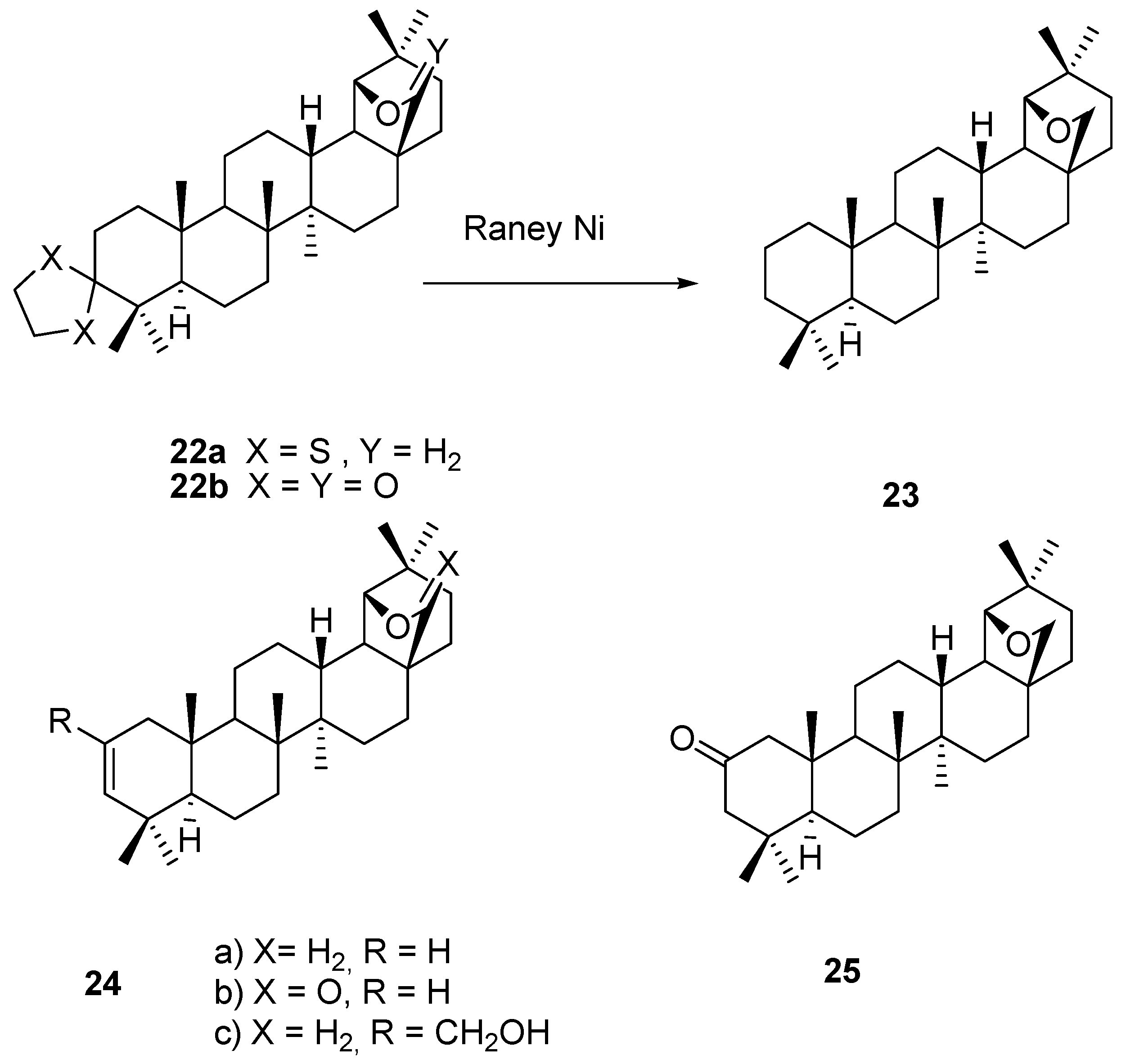
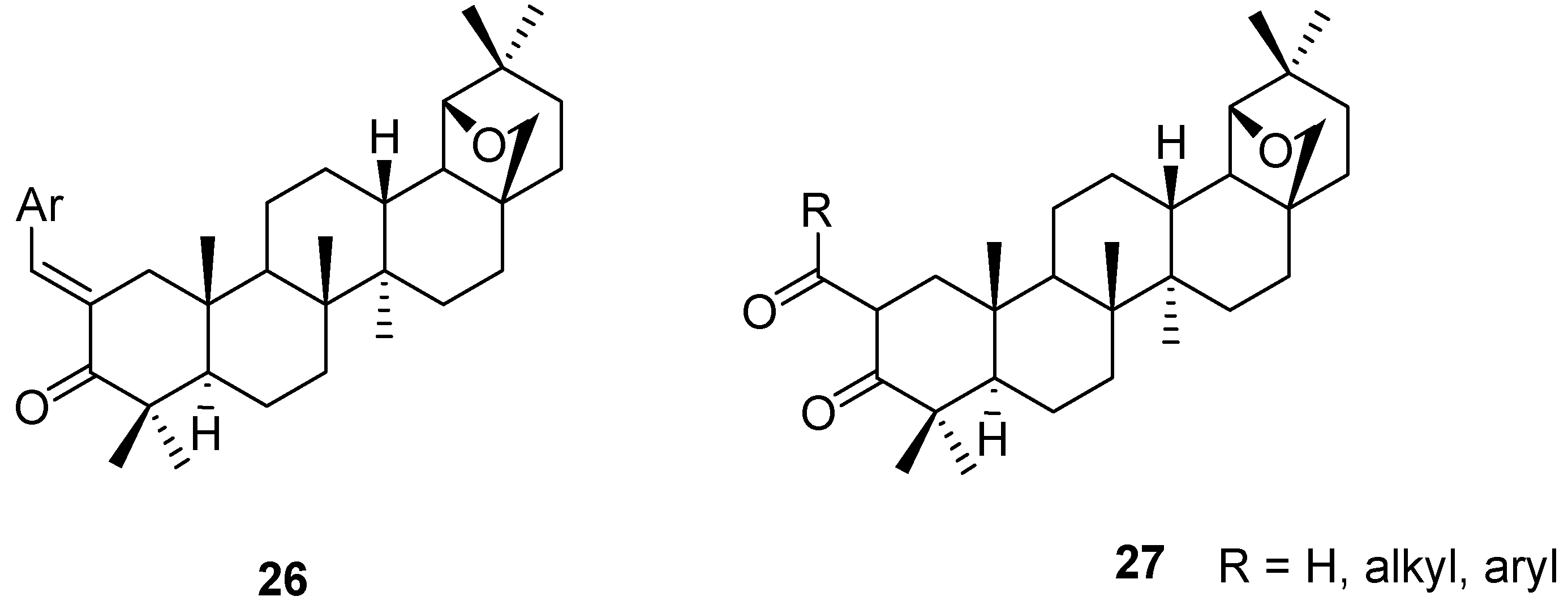
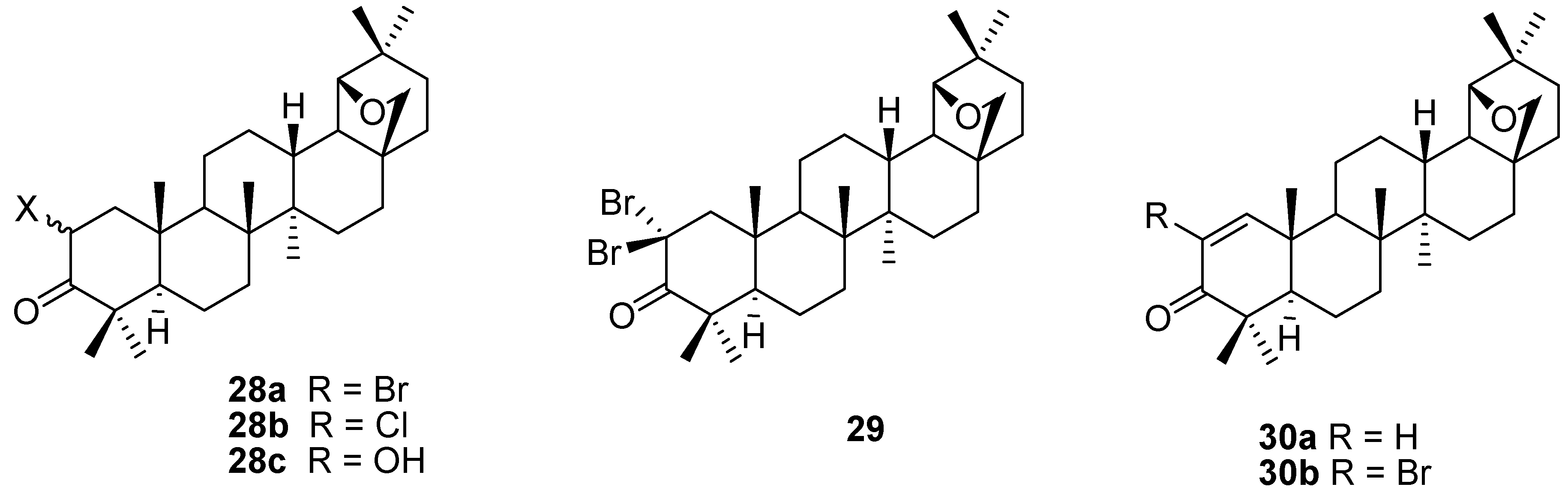
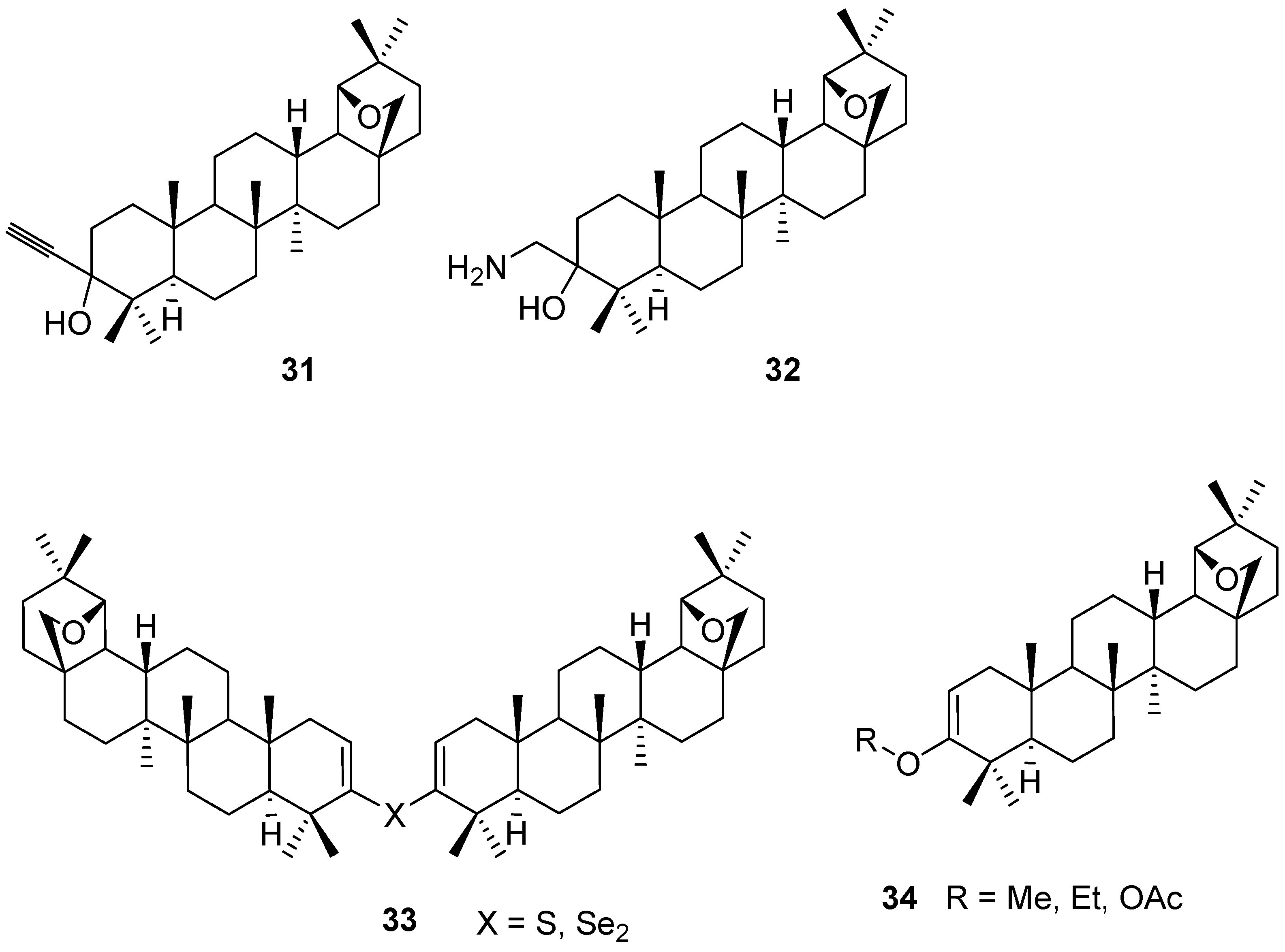
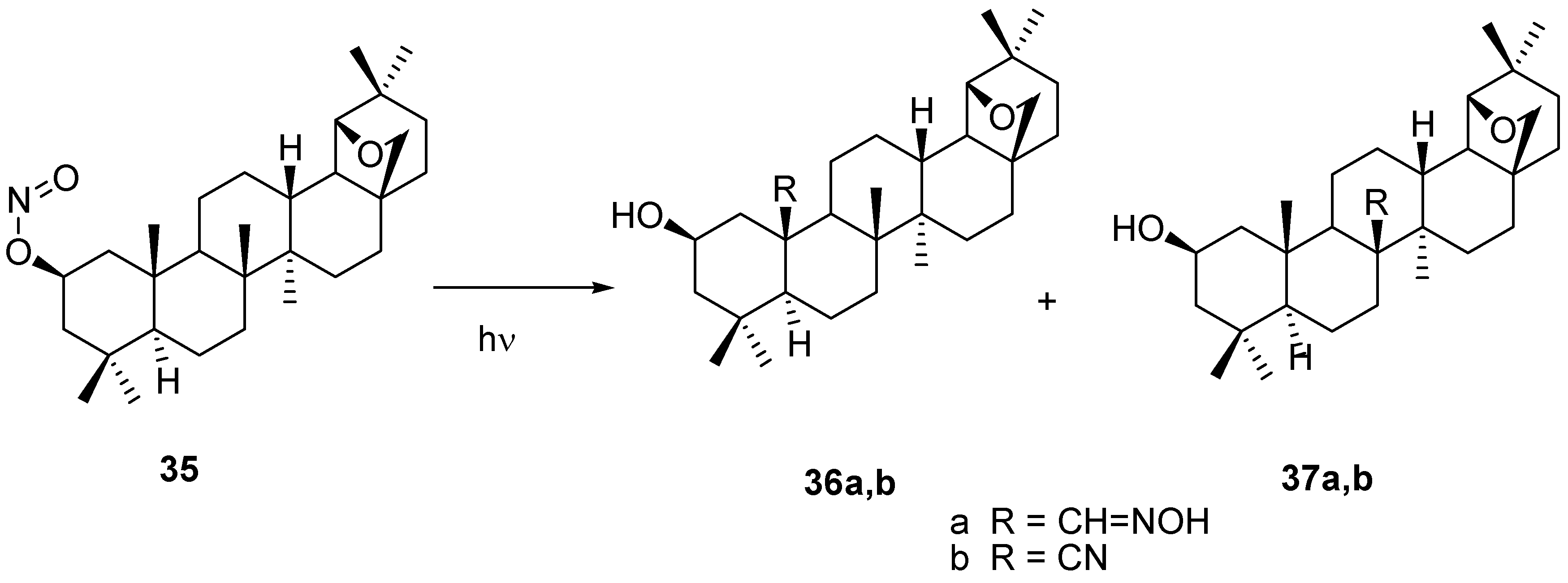
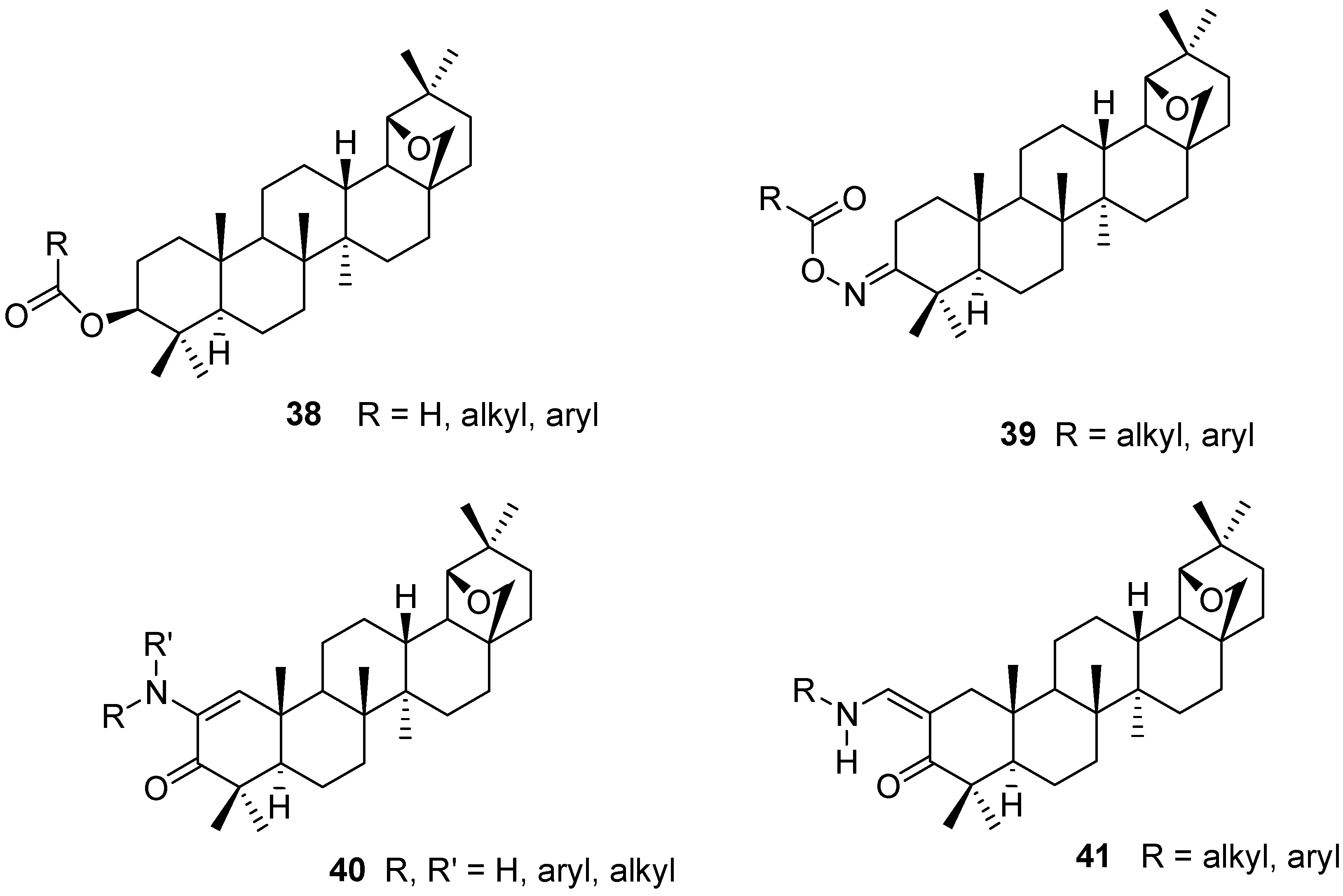
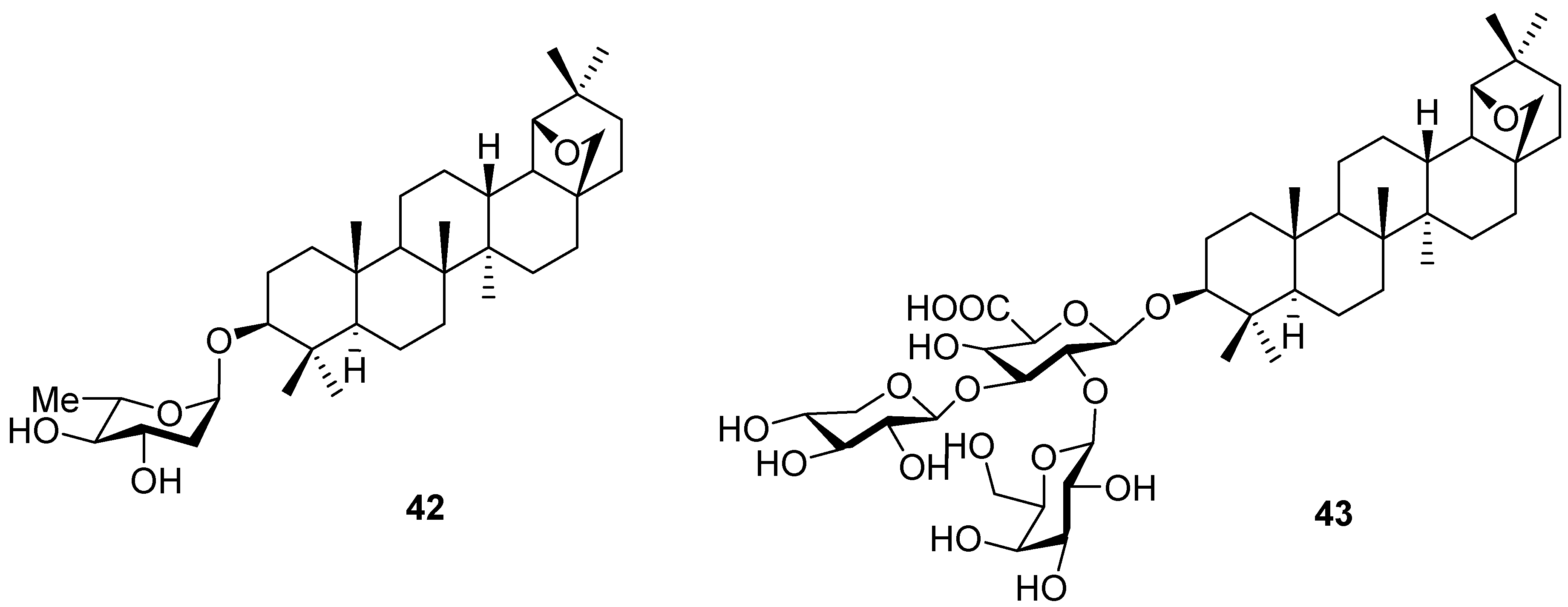
4. Ring Fusion to the A-Ring of Allobetulin
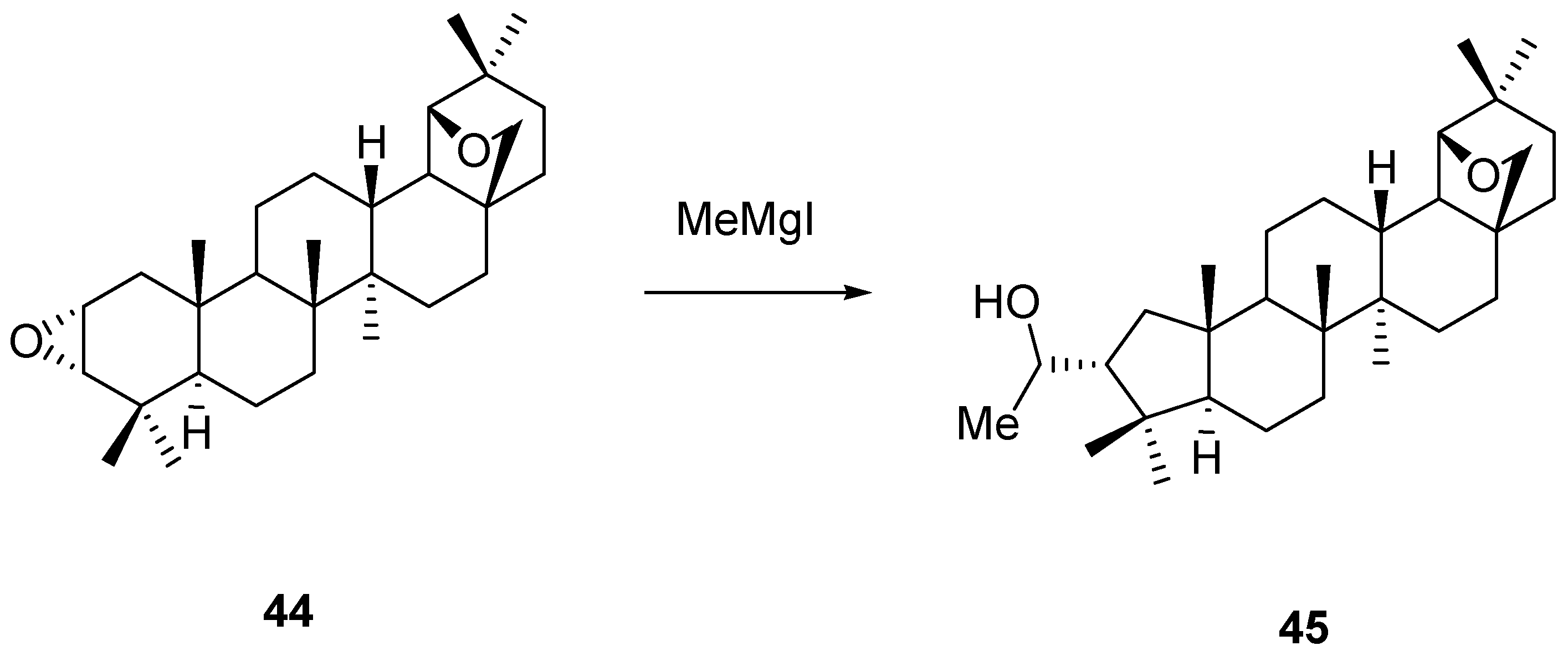
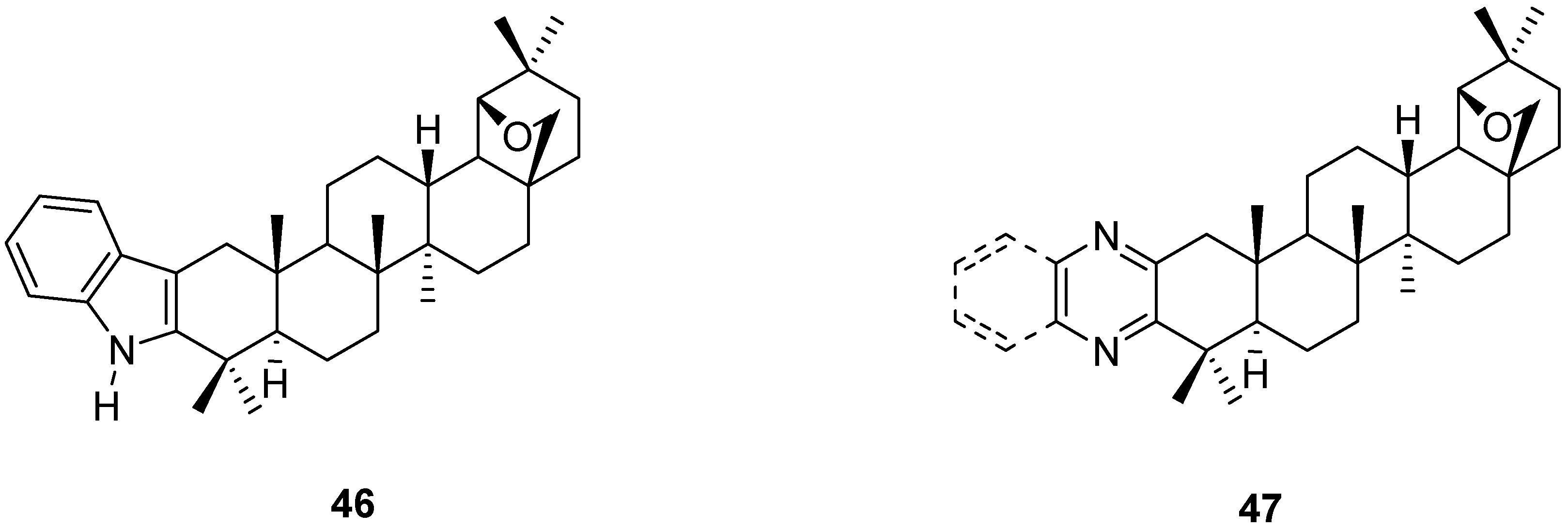
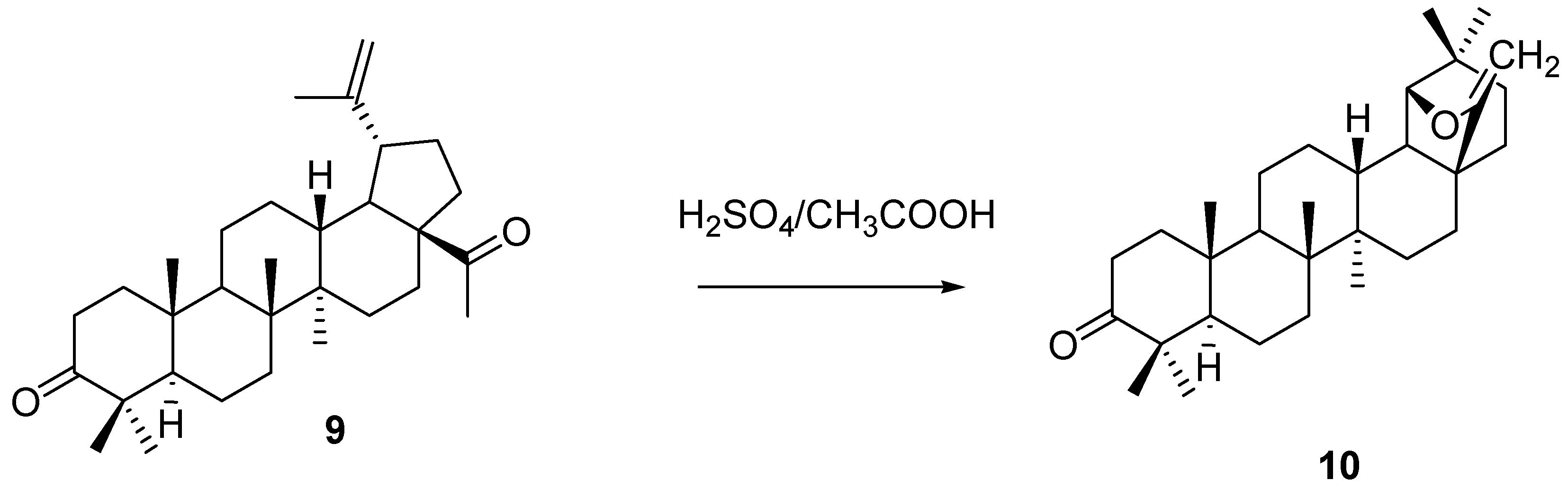
5. Further Rearrangements of Allobetulin, Including Ring Contractions and Ring Expansions
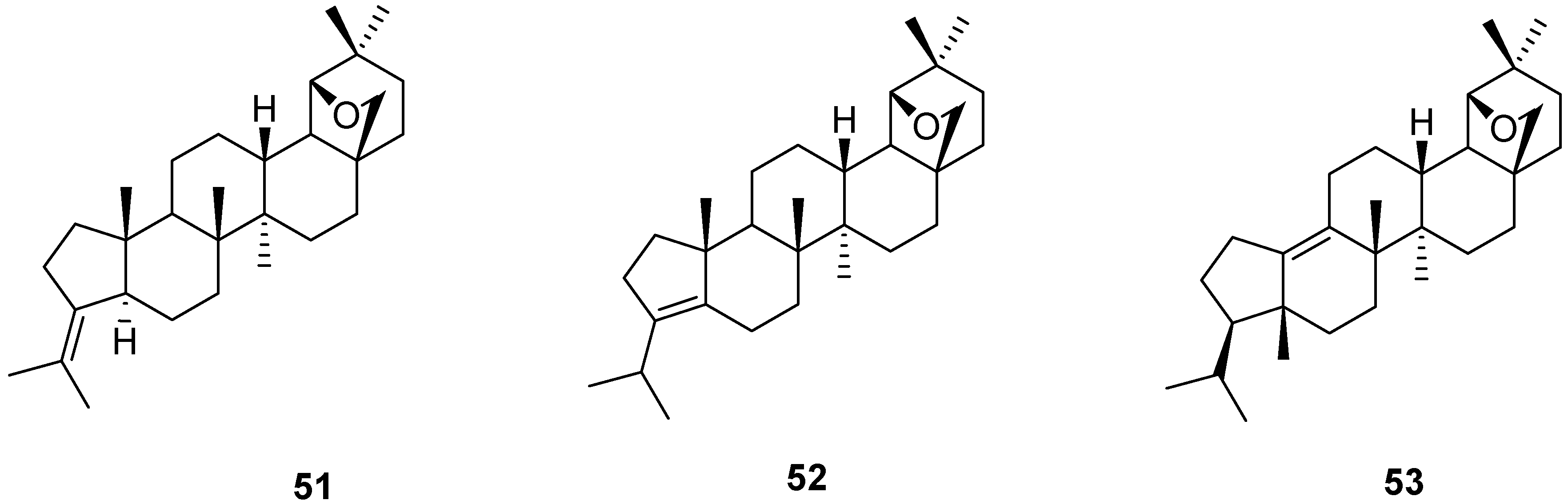
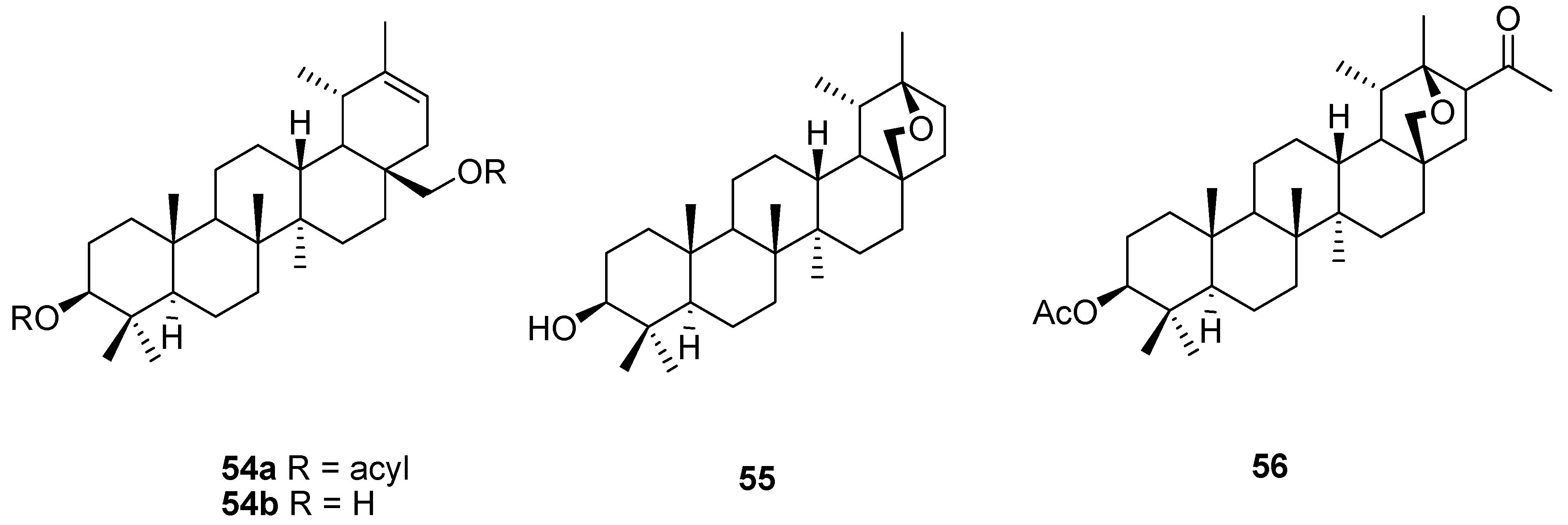
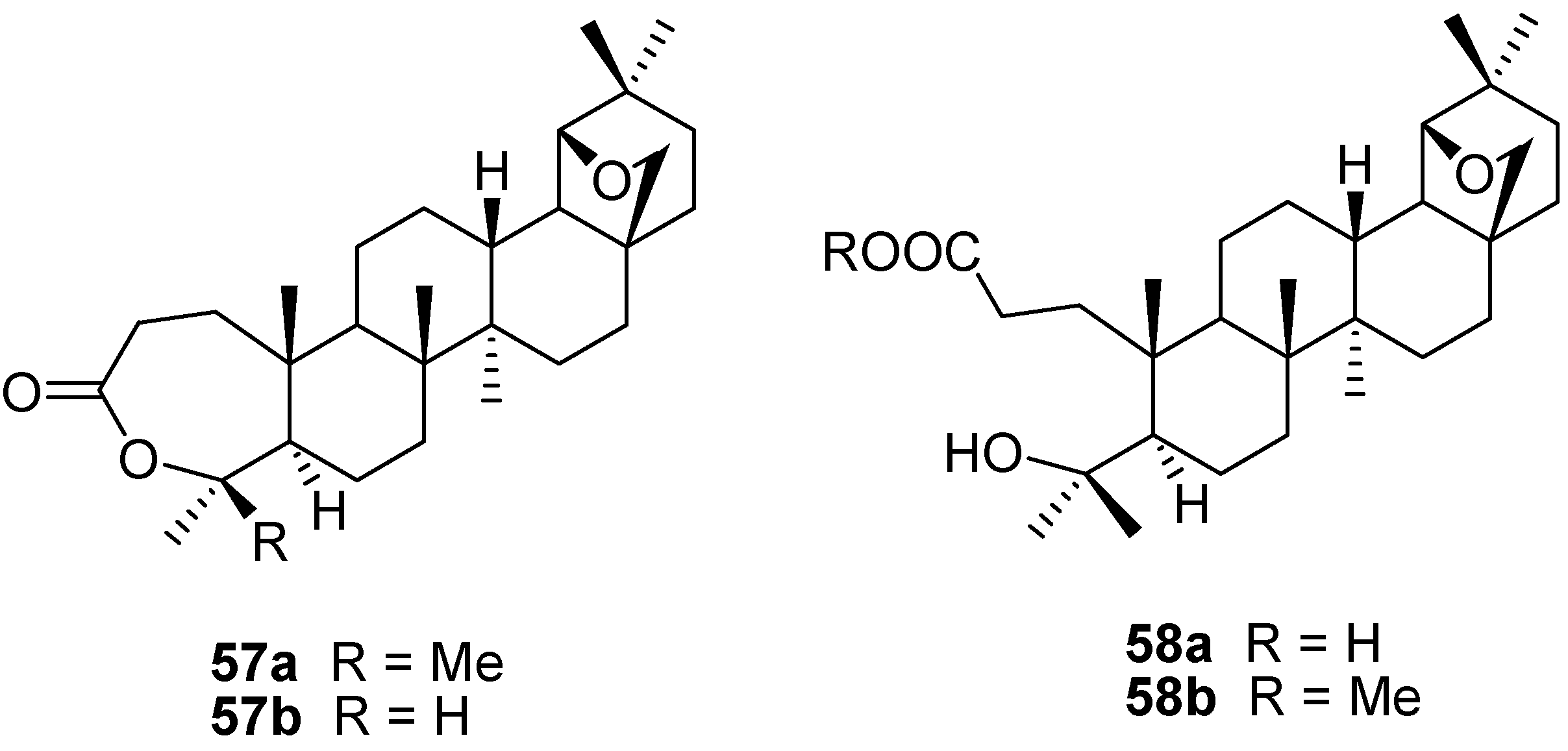
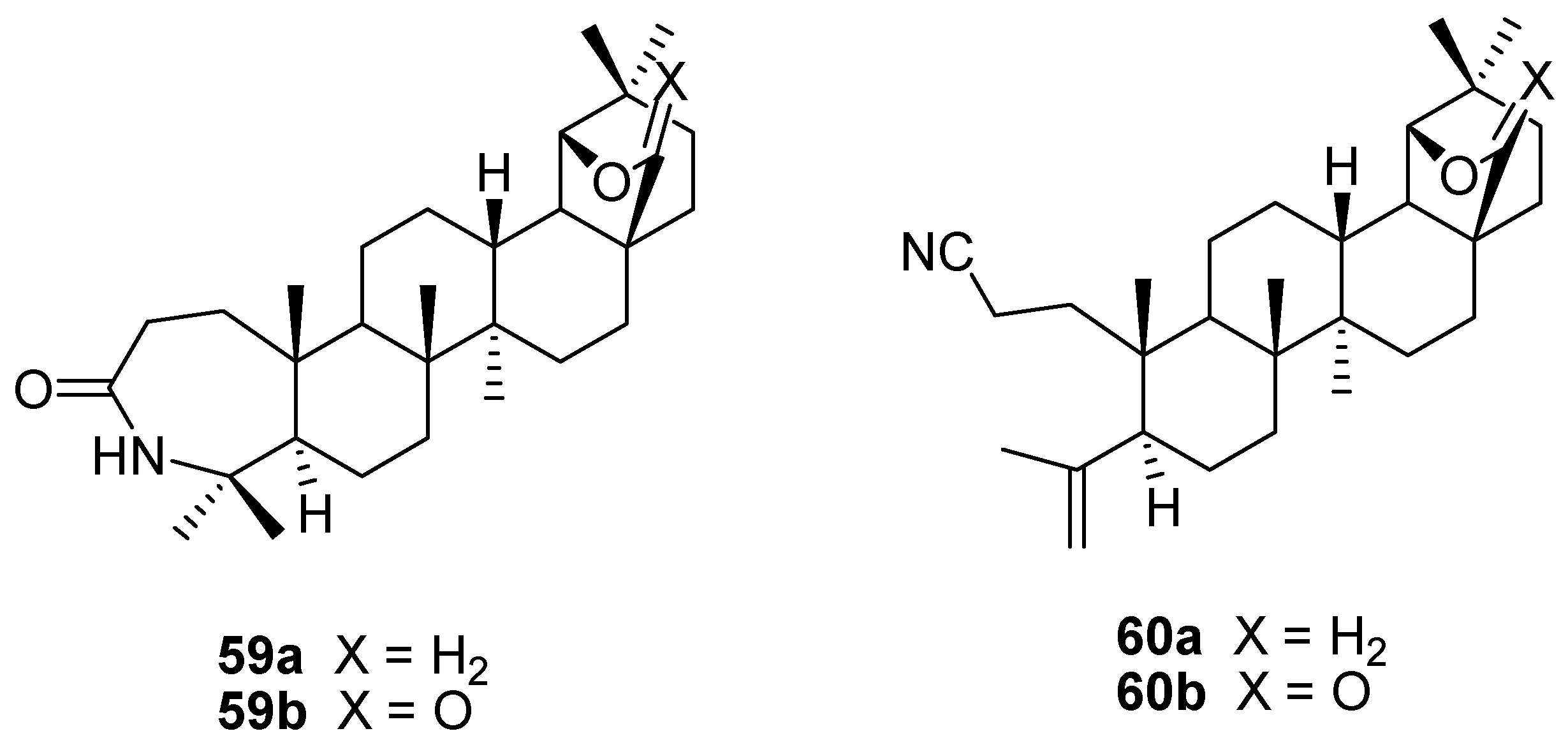
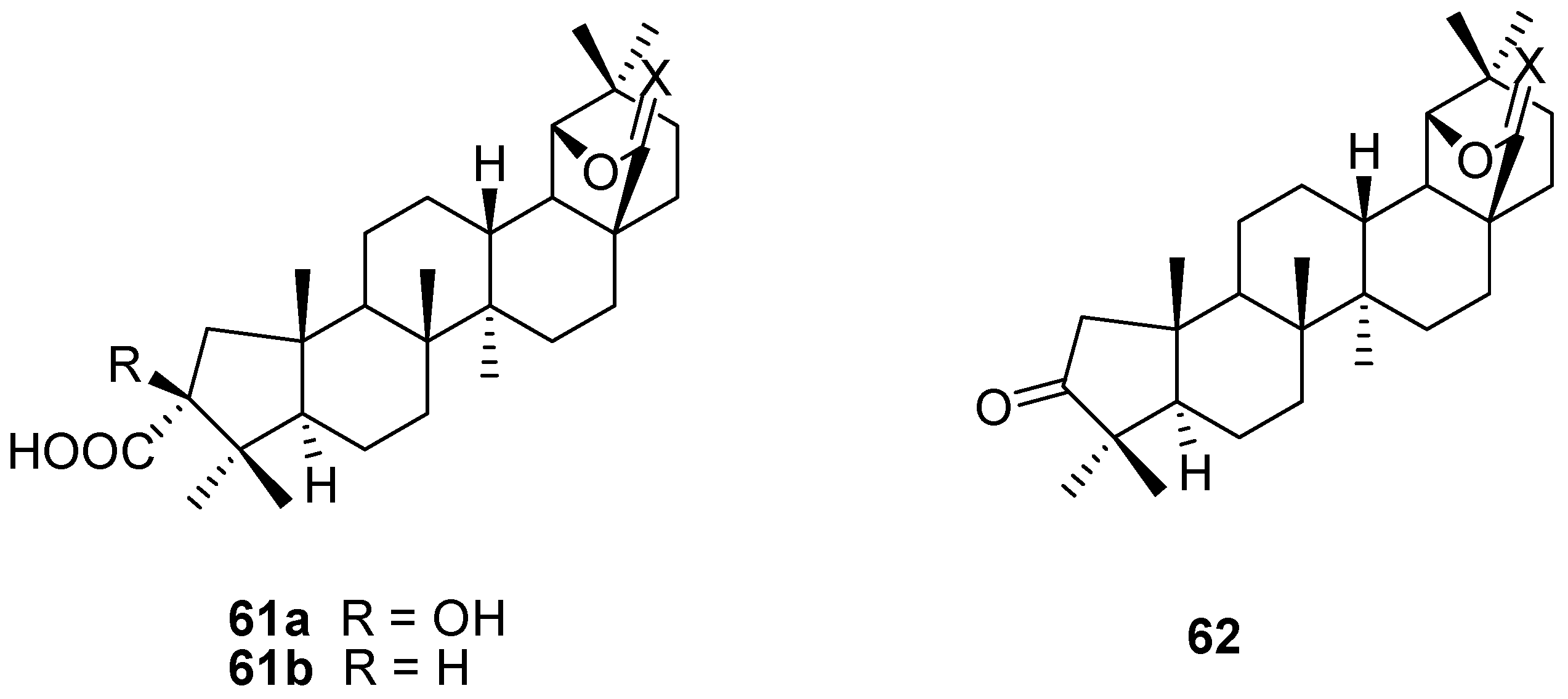
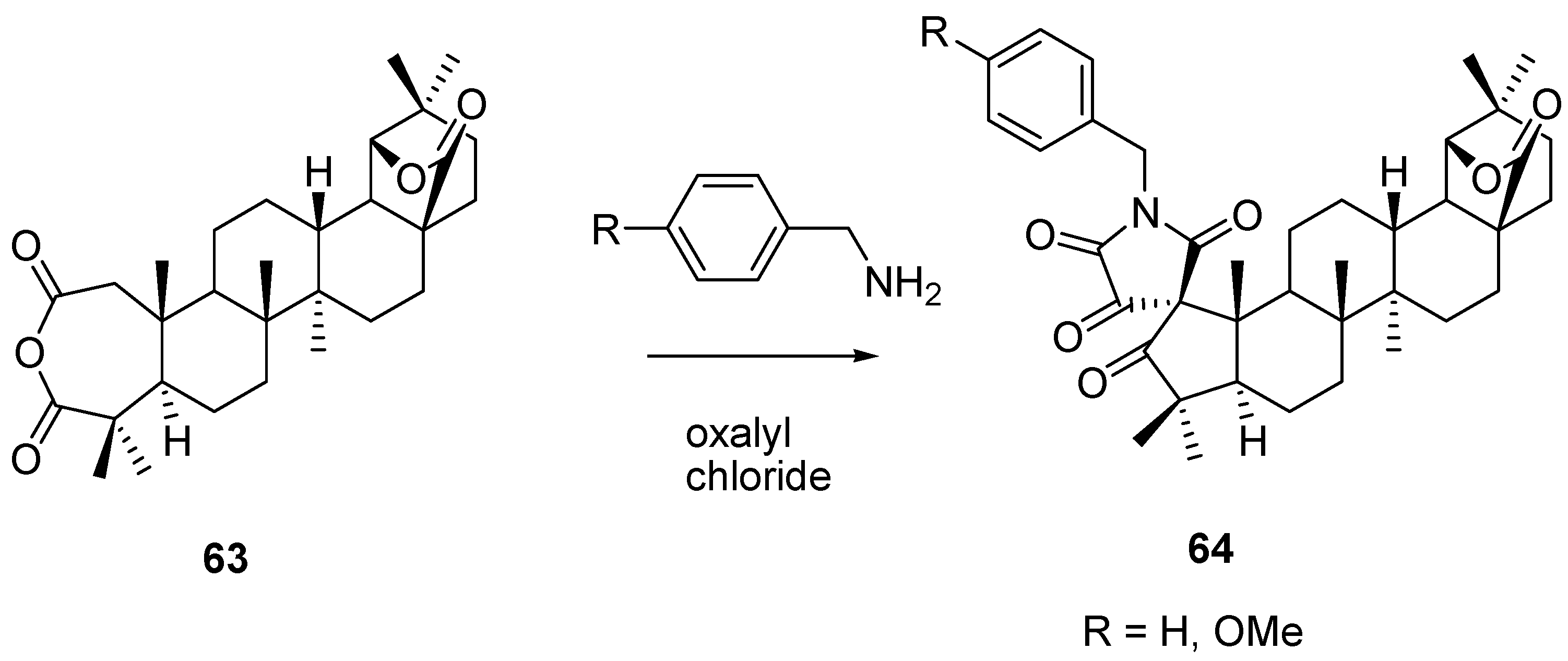
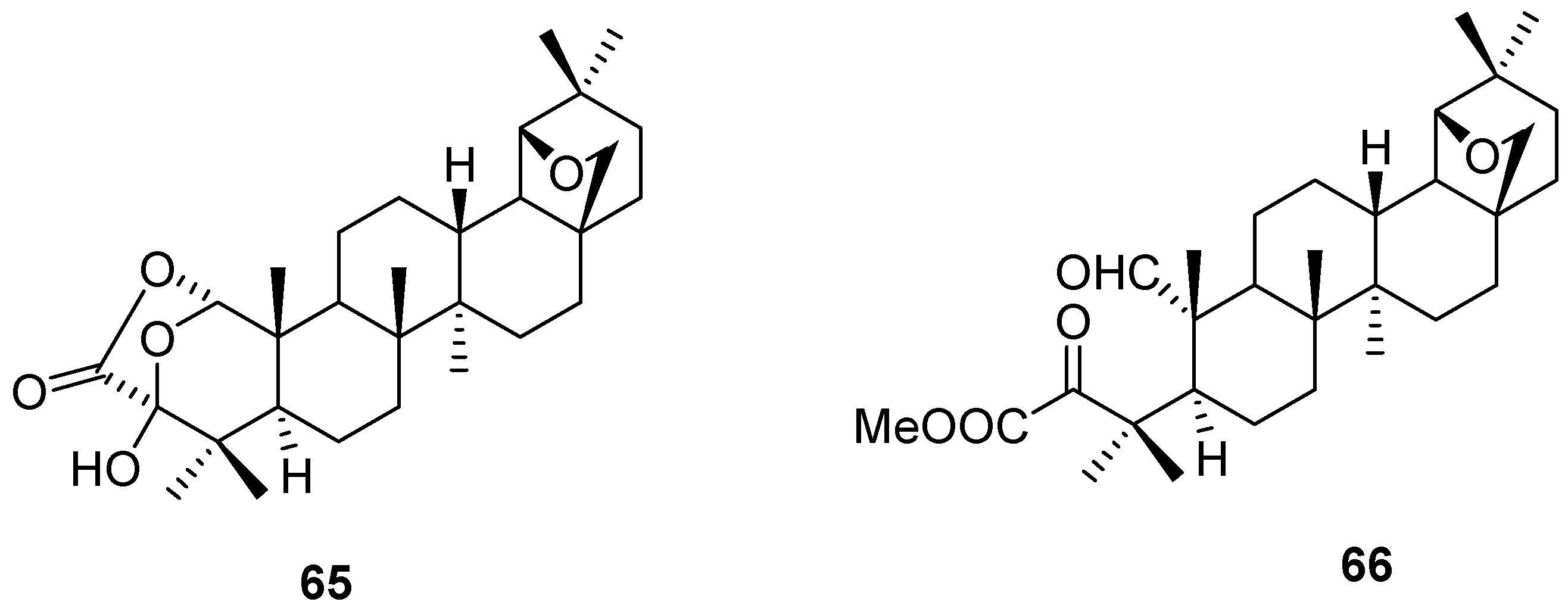
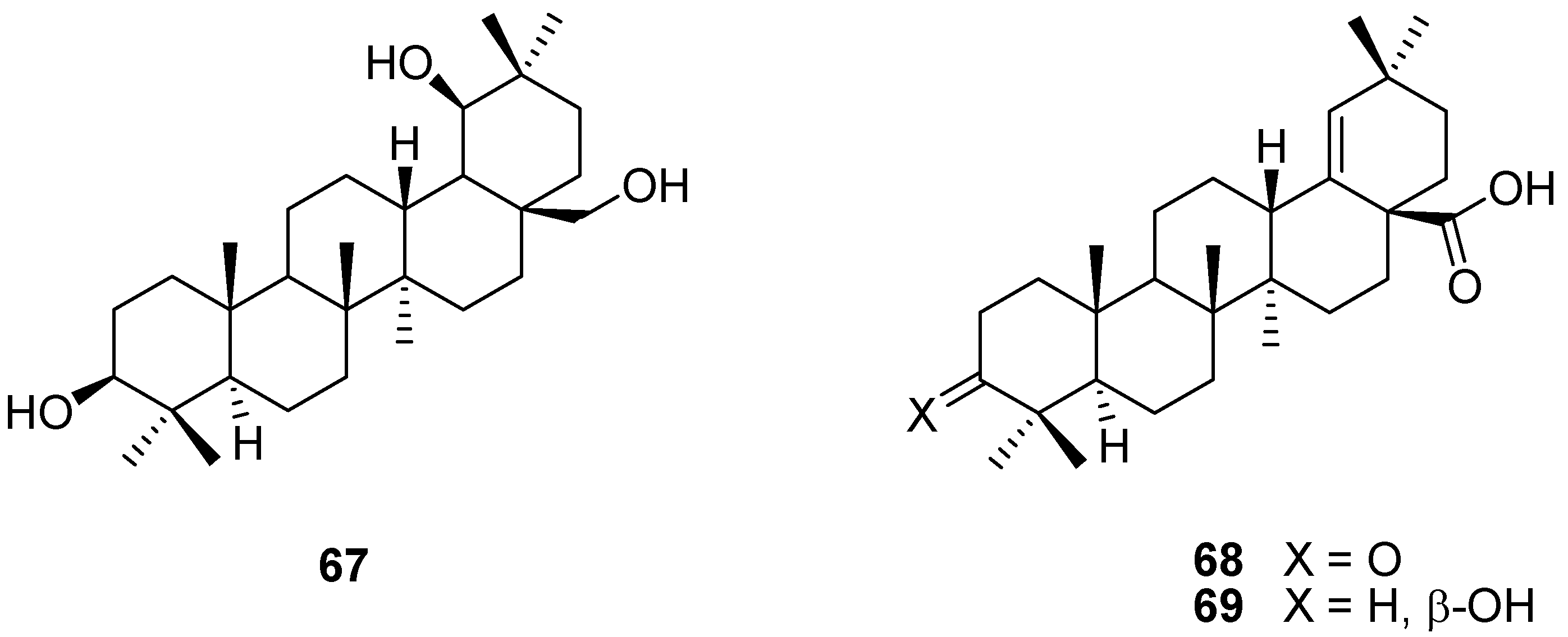
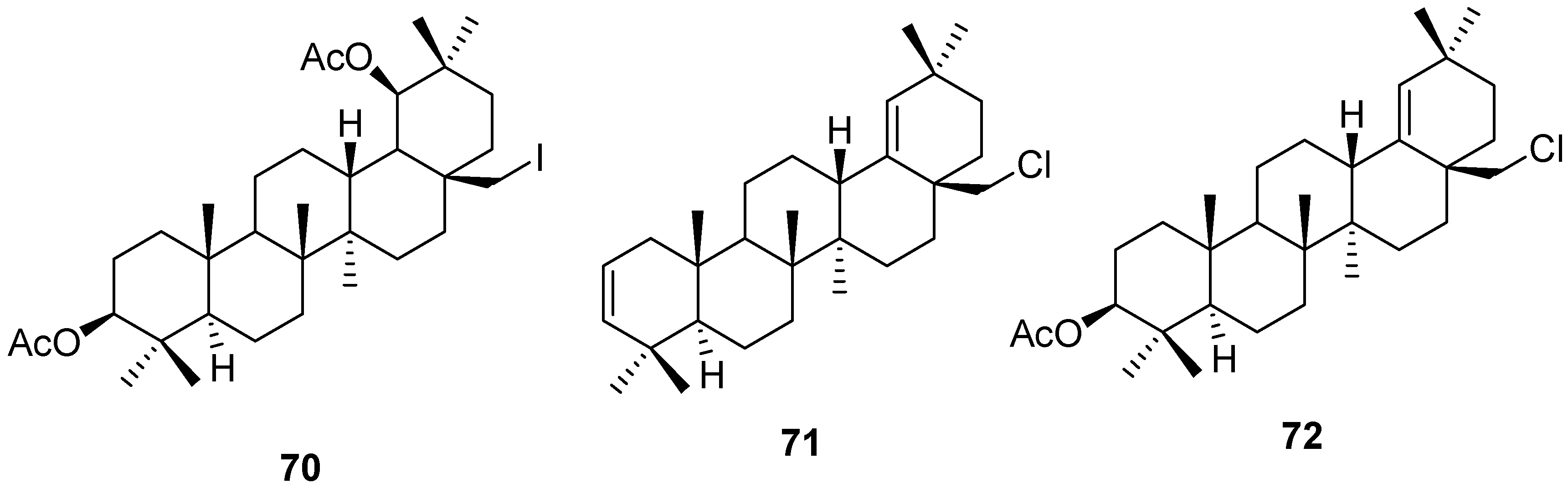
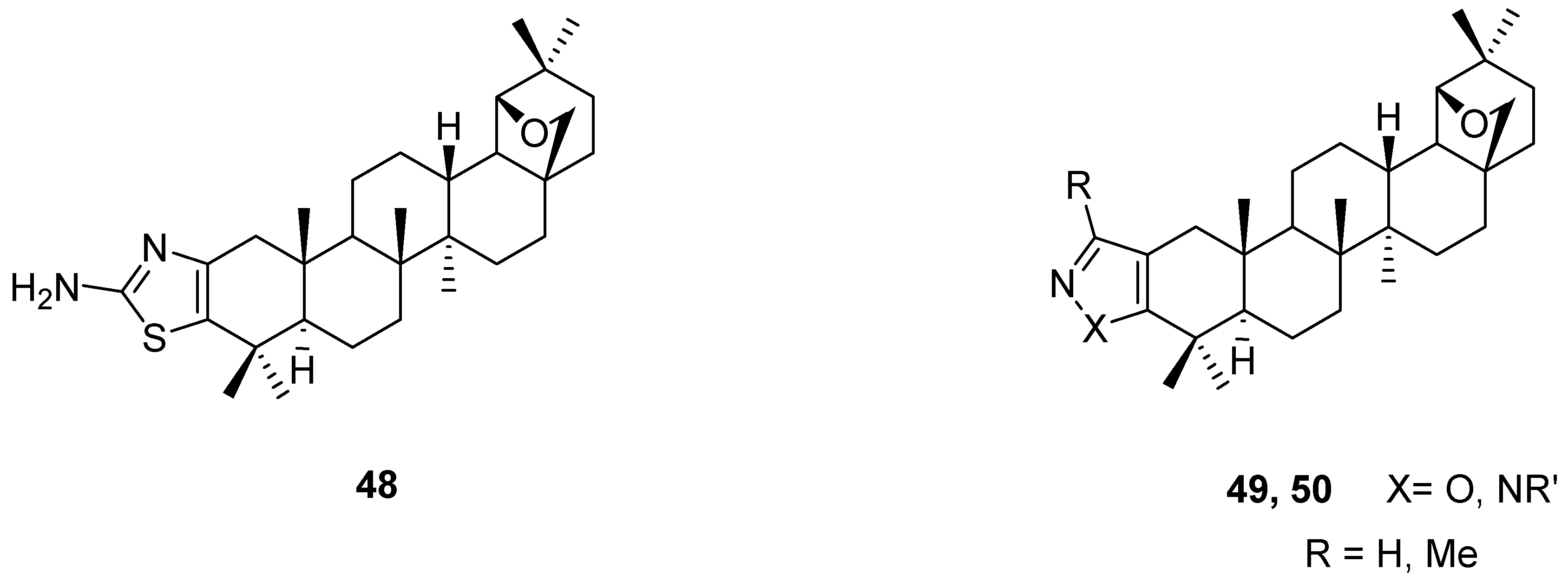
6. Biological Properties of Allobetulin Analogs
6.2. Antifeedant properties
6.3. Immunotropic activities
6.4. Antibacterial and antifungal activities
6.5. Anti-inflammatory and anti-ulcer properties
6.6. Cytotoxicity
6.7. Inhibition of glycogen phosphorylase
7. Conclusions
Acknowledgements
References
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2010, 27, 79–132 and the previous reviews in this series. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Flekhter, O.B.; Shul'ts, E.E.; Baltina, L.A.; Tolstikov, A.G. Betulin and its derivatives. Chemistry and biological activity. Khim. Interes. Ust. Razv. 2005, 13, 1–30. [Google Scholar]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Pathak, A.; Kulshreshtha, D.K.; Maurya, R. Coumaryl triterpene lactone, phenolic and naphthalene glycoside from stem bark of Diospyros angustifolia. Phytochemistry 2004, 65, 2153–2158. [Google Scholar] [CrossRef]
- Schulze, H.; Pieroh, K. Zur Kenntnis des Betulins. Chem. Ber. 1922, 55, 2322–2346. [Google Scholar]
- Dischendorfer, O.; Juvan, H. Untersuchungen auf dem Gebiete der Phytochemie-Über das Allobetulin. Monatsh. Chem. 1930, 52, 272–281. [Google Scholar] [CrossRef]
- Davy, G.S.; Halsall, T.G.; Jones, E.R.H.; Meakins, G.D. The chemistry of the triterpenes. Part X. The structures of some isomerisation products from betulin and betulinic acid. J. Chem. Soc. 1951, 2702–2705. [Google Scholar]
- Santos, R.C.; Pinto, R.M.A.; Beja, A.M.; Salvador, J.A.R.; Paixão, J.A. 19β,28-Epoxy-18α-olean-3β-ol. Acta Cryst. 2009, E65, o2088–o2089. [Google Scholar]
- Dischendorfer, O. Phytochemical studies. I. Betulin. Monatsh. Chem. 1923, 44, 123–139. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Holness, N.J.; Triterpenoids, V. Some relative configurations in rings C, D, and E of the β -amyrin and the lupeol group of triterpenoids. J. Chem. Soc. 1952, 78–92. [Google Scholar]
- Lawrie, W.; McLean, J.; Taylor, G.R. Triterpenoids in the bark of mountain ash Sorbus aucuparia. J. Chem. Soc. 1960, 4303–4308. [Google Scholar]
- Errington, S.G.; Ghisalberti, E.L.; Jefferies, P.R. The chemistry of the Euphorbiaceae. XXIV. Lup-20(29)-ene-3β,16β ,28-triol from Beyeria brevifolia var brevifolia. Aust. J. Chem. 1976, 29, 1809–1814. [Google Scholar] [CrossRef]
- Linkowska, E. Triterpenoids. Part XI. Isomerization of betulin and its derivatives. Pol. J. Chem. 1994, 68, 875–876. [Google Scholar]
- Li, T.S.; Wang, J.X.; Zheng, X.J. Simple synthesis of allobetulin, 28-oxyallobetulin and related biomarkers from betulin and betulinic acid catalysed by solid acids. J. Chem. Soc. Perkin Trans. 1 1998, 3957–3965. [Google Scholar]
- Lavoie, S.; Pichette A., A.; Garneau, F.-X.; Girard, M.; Gaudet, D. Synthesis of betulin derivatives with solid supported reagents. Synth. Commun. 2001, 31, 1565–1571. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Khusnutdinova, E.F.; Tolstikov, G.A.; Suponitsky, K.Y. Synthesis of new olean-18-(19)-ene derivatives from allobetulin. Russ. J. Bioorg. Chem. 2010, 36, 512–515. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Flekhter, O.B.; Kukovinets, O.S.; Galin, F.Z.; Tolstikov, G.A.; Baglin, I.; Cavé, C. Synthesis of 19β,28-epoxy-23,24-dinor-A-neo -18α-olean-4-en-3-one from betulin. Russ. Chem. Bull. 2007, 56, 835–837. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Santos, R.C.; Le Roux, C.; Beja, A.M.; Paixão, J.A. Bismuth triflate-catalyzed Wagner-Meerwein rearrangement in terpenes. Application to the synthesis of the 18α-oleanone core and A-neo-18α-oleanene compounds from lupanes. Org. Biomol. Chem. 2009, 7, 508–517. [Google Scholar] [CrossRef]
- Levdanskii, V.A.; Levdanskii, A.V.; Kuznetsov, B.N. Method of producing allobetulin from birch bark via isomerization of betulinol. Russ. Patent RU 2374261, 2009. [CA 2009, 151, 571219]. [Google Scholar]
- Kuznetsova, S.A.; Kuznetsov, B.N.; Red'kina, E.S.; Skvortsova, G.P. Method of allobetulin production. Russ. Patent RU 2334759, 2008. [CA 2008, 149, 378906]. [Google Scholar]
- Green, B.; Bentley, M.D.; Chung, B.Y.; Lynch, N.G.; Jensen, B.L. Isolation of betulin and rearrangement to allobetulin. A biomimetic natural product synthesis. J. Chem. Ed. 2007, 84, 1985–1987. [Google Scholar] [CrossRef]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and structure-activity relationship study of cytotoxic germanicane- and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorg. Med. Chem. 2007, 15, 6144–6157. [Google Scholar]
- Vystrčil, A.; Stejskalova-Vondraskova, E.; Cerny, J. Identity of gratiolone and betulic acid. Collect. Czech. Chem. Commun. 1959, 24, 3279–3286. [Google Scholar]
- Flekhter, O.B.; Boreko, E.I.; Nigmatullina, L.R.; Pavlova, N.I.; Medvedeva, N.I.; Nikolaeva, S.N.; Ashavina, O.A.; Savinova, O.V.; Baltina, L.A.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A. Synthesis and antiviral activity of lupane triterpenoids and their derivatives. Pharm. Chem. J. 2004, 38, 355–358. [Google Scholar] [CrossRef]
- Giniyatullina, G.V.; Flekhter, O.B.; Baikova, I.P.; Starikova, Z.A.; Tolstikov, G.A. Effective synthesis of methyl 3β-amino-3-deoxybetulinate. Chem. Nat. Comp. 2008, 44, 603–605. [Google Scholar] [CrossRef]
- Lezhneva, M.Y.; Schultz, E.E.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Shakirov, M.M.; Tolstikov, G.A.; Adekenov, S.M. Synthesis and crystal structure of 29,30-dibromoallobetulin. Chem. Nat. Comp. 2006, 42, 186–188. [Google Scholar] [CrossRef]
- Pradhan, B.P.; Chakraborty, D.K.; Roy, A. Novel method for reductive cleavage of triterpenoid lactones with lithium in ethylenediamine. Ind. J. Chem. B Org. Chem. Med. Chem. 1993, 32B, 721–725. [Google Scholar]
- Pradhan, B.P.; Chakraborty, D.K.; Dutta, S.; Ghosh, R.; Roy, A. Studies on the reactions of N-bromosuccinimide in dimethyl sulfoxide: Part IV. Action on lupenyl acetate. Ind. J. Chem. B Org. Chem. Med. Chem. 1991, 30B, 32–37. [Google Scholar]
- Pradhan, B.P.; Mukherjee, M.M.; Chakrabarti, D.K.; Shoolery, J.N. Action of N-bromosuccinimide on triterpene acids and esters in dimethyl sulfoxide. Ind. J. Chem. B Org. Chem. Med. Chem. 1983, 22B, 12–16. [Google Scholar]
- Davy, G.S.; Halsall, T.G.; Jones, E.R.H. The chemistry of the triterpenes. Part IX. Elucidation of the Betulin-Oleanolic acid relationship. J. Chem. Soc. 1951, 2696–2702. [Google Scholar]
- Csuk, R.; Barthel, A.; Kluge, R.; Kommera, H. Synthesis and biological evaluation of antitumour-active betulin derivatives. Bioorg. Med. Chem. 2010, 18, 1344–1355. [Google Scholar]
- Dračínský, M.; Richtr, V.; Křeček, V.; Sejbal, J.; Klinot, J.; Buděšínský, M. Preparation and conformational study of 19β,28-epoxy-18α-olean-5-ene derivatives. Collect. Czech. Chem. Commun. 2006, 71, 387–410. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Nazarov, A.V.; Maiorova, O.A.; Grishko, V.V. Synthesis of lupane and 19β,28-epoxy-18α-oleane 2,3-seco-derivatives based on betulin. Chem. Nat. Comp. 2008, 44, 606–611. [Google Scholar] [CrossRef]
- Carlson, R.M.; Gibson, D.J. Anti-fungal formulation of triterpene and essential oil. PCT Int. Appl. WO 2004089357, 2004. [CA 2004, 141, 370537]. [Google Scholar]
- Carlson, R.M. Preparation of allobetulin triterpenoids for therapeutic use in the treatment of herpes virus infection. U.S. Patent US 6369101, 2002. [CA 2002, 136, 279583]. [Google Scholar]
- Flekhter, O.B.; Ashavina, O.Y.; Smirnova, I.E.; Baltina, L.A.; Galin, F.Z.; Kabal’nova, N.N.; Tolstikov, G.A. Selective oxidation of triterpene alcohols by sodium hypochlorite. Chem. Nat. Comp. 2004, 40, 141–143. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Boreko, E.I.; Nigmatullina, L.R.; Pavlova, N.I.; Medvedeva, N.I.; Nikolaeva, S.N.; Tret’yakova, E.V.; Savinova, O.V.; Baltina, L.A.; Karachurina, L.T.; Galin, F.Z.; Zarudii, F. S; Tolstikov, G.A. Synthesis and pharmacological activity of acylated betulonic acid oximes and 28-oxo-allobetulone. Pharm. Chem. J. 2004, 38, 148–152. [Google Scholar] [CrossRef]
- Rybalova, T.V.; Galitov, Y.V.; Nekrasov, D.D.; Rubtsov, A.E.; Tolstikov, A.G. Synthesis of spiro[(6-phenyl-3,4-dihydro-2H-1,3-dioxine)-2R(S), 3’-(19’,28’-oxidooleanan)]-4-ones and X-ray diffraction analysis of their configuration. J. Struct. Chem. 2005, 46, 1126–1130. [Google Scholar] [CrossRef]
- Nekrasov, D.D.; Obukhova, A.S.; Lisovenko, N.Y.; Roubtsov, A.E. Effect of substituents in the cumulene and aryl fragments of aroylketenes on the stereoselectivity of Diels-Alder heteroreaction with mono-, bi, and polycyclic terpenoids containing a carbonyl group. Chem. Het. Comp. 2010, 46, 413–418. [Google Scholar]
- Lugemwa, F.N.; Huang, F.-Y.; Bentley, M.D.; Mendel, M.J.; Alford, A.R. A heliothis-zea antifeedant from the abundant birchbark triterpene betulin. J. Agric. Food Chem. 1990, 38, 493–496. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, J.; Liu, J.; Zhang, L.; Sun, H. Efficient synthesis of morolic acid and relared triterpenes starting from betulin. Tetrahedron 2009, 65, 4304–4309. [Google Scholar] [CrossRef]
- Huneck, S. Triterpenes. XV. Preparation and Forster reaction of 19β, 28-epoxy-3-oxo-2-anti-oximino-lα -cyano-18α H-oleanane and the bromination of 19β,28-epoxy-3-oxo-1α-cyano-18αH-oleanane. Chem. Ber. 1965, 98, 3204–3209. [Google Scholar] [CrossRef]
- Korovin, A.V.; Tkachev, A.V. Synthesis of quinoxalines fused with triterpenes, ursolic acid and betulin derivatives. Russ. Chem. Bull. 2001, 50, 304–310. [Google Scholar] [CrossRef]
- Protiva, J.; Ocadlik, R.; Klinotova, E.; Klinot, J.; Vystrčil, A. Triterpenes. Part LXXXI. Ethylene dithioketals in ring A of 19β, 28-epoxy-18α -oleanane; mass spectra and reduction with deuterated Raney nickel. Coll. Czech Chem. Commun. 1987, 52, 501–507. [Google Scholar] [CrossRef]
- Huneck, S. Triterpenes. X. Photochemical transformations. 2. Preparation of 19β, 28-epoxy-3-oxo-2-diazo-18α H-oleanane and its photochemical conversion to A-nor compounds. Chem. Ber. 1965, 98, 1837–1857. [Google Scholar] [CrossRef]
- Ruzicka, L.; Brungger, H.; Gustus, E.L. Polyterpenes and polyterpenoids. LXVIII. Betulin. Helv. Chim. Acta 1932, 15, 634–648. [Google Scholar] [CrossRef]
- Klinot, J.; Vystrčil, A. Side-products in the conversion of allobetulin to heterobetulin. Collect. Czech. Chem. Commun. 1964, 29, 516–530. [Google Scholar]
- Rybina, A.V.; Shepelevich, I.S.; Talipov, R.F.; Galin, F.Z.; Spirikhin, L.V. Transformation of 19β,28-epoxy-18α-olean-2-ene by the Prins reaction. Chem. Nat. Comp. 2006, 42, 740–741. [Google Scholar] [CrossRef]
- Sejbal, J.; Klinot, J.; Protiva, J.; Vystrčil, A. Triterpenes. Part. LXXIII. Reactions of triterpenoid ketones with sulfur and morpholine under Willgerodt-Kindler reaction conditions. Collect. Czech. Chem. Commun. 1986, 51, 118–127. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Head, A.J.; May, P.J. Long-range effects in alicyclic systems. II. Rates of condensation of some triterpenoid ketones with benzaldehyde. J. Chem. Soc. 1957, 935–944. [Google Scholar]
- Kim, H.O.; Tolstikov, G.A.; Bazalitskaya, V.S. Triterpenoids. XXI. Condensation of 3-oxotriterpenes with esters and synthesis of C-substituted triterpenoid azoles. Zh. Obsh. Khim. 1970, 40, 492–497. [Google Scholar]
- Klinot, J.; Světlŷ, J.; Kudláčková, D.; Buděšínskŷ, M.; Vystrčil, A. Triterpenes.59 Preparation of 2-methyl-3-oxotriterpenoids of 18-α-oleanane series and the conformation of ring A. Collect. Czech. Chem. Commun. 1979, 44, 211–225. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Anikina, L.V.; Vikharev, Y.B.; Shelepen’kina, L.N.; Grishko, V.V.; Tolstikov, G.A. Synthesis and immunotropic activity of 2-alkylaminomethylene-19β,28-epoxyolean-3-ones. Russ. J. Bioorg. Chem. 2008, 34, 125–129. [Google Scholar] [CrossRef]
- Biedermann, D.; Sarek, J.; Klinot, J.; Hajduch, M.; Dzubak, P. Fluorination of betulinines and other triterpenoids with DAST. Synthesis 2005, 1157–1163. [Google Scholar]
- Klinot, J.; Vystrčil, A. Conformation of epimeric 2-bromoallobetulones. Chem. Ind. 1960, 1360–1361. [Google Scholar]
- Hanna, R.; Ourisson, G. Studies of cyclic ketones. VIII. Preparation and properties of polycyclic α-diketones. Bull. Soc. Chim. Fr. 1961, 1945–1951. [Google Scholar]
- Green, G.F.H.; Long, A.G. Compounds related to the steroid hormones. II. Action of hydrogen bromide on 2-bromo-3-oxo-Δ1-5α -steroids. J. Chem. Soc. 1961, 2532–2543. [Google Scholar]
- Lehn, J.M.; Ourisson, G. Cyclic ketones. XIV. Conformation of 2-bromo- and 2,2-dibromo-3-oxotriterpenes. Bull. Soc. Chim. Fr. 1963, 1113–1121. [Google Scholar]
- Klinot, J.; Vystrčil, A. Triterpenes. 7. Stereochemistry of 2-bromo derivatives of allobetulin and alloheterobetulin. Collect. Czech. Chem. Commun. 1966, 31, 1079–1091. [Google Scholar]
- Hajduch, M.; Sarek, J. Preparation of betulin type triterpenoid derivatives as antitumor agents. PCT Int. Appl. WO 2001090096, 2001. [CA 2001, 136, 6180]. [Google Scholar]
- Klinot, J.; Richtr, V.; Vystrčil, A. Triterpenes. XLIII. Conformation of ring A of 2,3-disubstituted triterpenes with chlorine or an alkoxy group in position 2. Collect. Czech. Chem. Commun. 1975, 40, 1758–1767. [Google Scholar] [CrossRef]
- Novotny, J.; Podlaha, J.; Klinot, J. Triterpenes. CII. Crystal structure and conformation of ring A of 2α-bromo-19β,28-epoxy-18α-oleanan-3-one. Collect. Czech. Chem. Commun. 1993, 58, 2737–2744. [Google Scholar] [CrossRef]
- Tolstikova, L.F.; Tolstikov, G.A. Triterpenoids. XXIII. Synthesis and reactions of acetylenic derivatives of triterpenes. Izv. Akad. Nauk Kazakh. SSR, Ser. Khim. 1971, 21, 65–71. [Google Scholar]
- Kvasnica, M.; Rudovska, I.; Cisarova, I.; Sarek, J. Reaction of lupane and oleane triterpenoids with Lawesson’s reagent. Tetrahedron 2008, 64, 3736–3743. [Google Scholar] [CrossRef]
- Sejbal, J.; Klinot, J.; Vystrčil, A. Triterpenes. Part LXXXIII. Reaction of 3-keto and 2-ketotriterpenoids with 3-chloroperoxybenzoic acid in aliphatic alcohols. A new method of preparation of α-hydroxy ketones. Collect. Czech. Chem. Commun. 1987, 52, 1052–1061. [Google Scholar] [CrossRef]
- Shelepen’kina, L.N.; Shashkov, A.S.; Grishko, V.V.; Glushkov, V.A.; Tolstikov, G.A. Reaction of 3-acetoxy-(2,3),(19β,28)diepoxyoleanane with cyclic and linear amines. Chem. Nat. Comp. 2007, 43, 153–158. [Google Scholar] [CrossRef]
- Sejbal, J.; Klinot, J.; Vystrčil, A. Triterpenes. LXXXV. Photolysis of 19β, 28-epoxy-18α -oleanan-2β -ol nitrites: functionalization of 10β - and 8β -methyl groups. Collect. Czech. Chem. Commun. 1988, 53, 118–131. [Google Scholar] [CrossRef]
- Sejbal, J.; Klinot, J.; Budesinsky, M. Triterpenes. XCIV. Photolyses and pyrolyses of triterpenoid nitrites. Collect. Czech. Chem. Commun. 1991, 56, 1732–1743. [Google Scholar] [CrossRef]
- Sejbal, J.; Klinot, J.; Budesinsky, M. Triterpenes. Part CV. Oleanane triterpenoids functionalized at C-25 and C-26. Collect. Czech. Chem. Commun. 1996, 61, 1360–1370. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Medvedeva, N.I.; Karachurina, L.T.; Baltina, L.A.; Zarudi, F.S.; Galin, F.Z.; Kabal’nova, N.N.; Tolstikov, G.A. Synthesis and anti-inflammatory activity of new acylated betulin derivatives. Pharm. Chem. J. 2002, 36, 488–491. [Google Scholar] [CrossRef]
- Krasutsky, P.A.; Carlson, R.M. Preparation of triterpene derivatives having fungicidal activity against yeast. PCT Int. Appl. WO 2002026761, 2002. [CA 2002, 136, 294955]. [Google Scholar]
- Krasutsky, P.A.; Avilov, D.V. Preparation of triterpene quaternary salts having antibacterial, antifungal, and surfactant properties. PCT Int. Appl. WO 2003062260, 2003. [CA 2003, 139, 133696]. [Google Scholar]
- Nekrasov, D.D.; Obukhova, A.S. Benzoylacetylation of allobetulin and allobetulone oxime by benzoylketene generated in situ during thermolysis of 5-phenyl-2,3-dihydrofuran-2,3-dione. Chem. Het. Comp. 2005, 41, 1426–1427. [Google Scholar]
- Odinokova, L.E.; Denisenko, M.V.; Denisenko, V.A.; Uvarova, N.I. Glycosylation of triterpene alcohols of the lupane series. Khim. Prirod. Soedin. 1988, 212–217. [Google Scholar]
- Baltina, L.A.; Flekhter, O.B.; Vasil'eva, E.V.; Tolstikov, G.A. Stereoselective synthesis of 2-deoxy-α -D-arabino-hexopyranosides of triterpenic alcohols. Izv. Akad. Nauk, Ser. Khim. 1996, 2340–2346. [Google Scholar]
- Baltina, L.A.; Flekhter, O.B.; Vasiljieva, E.V. Stereoselective synthesis of triterpene 3-O-2-deoxy-α -glycosides. Mendeleev Commun. 1996, 63–64. [Google Scholar]
- Baltina, L.A.; Flekhter, O.B.; Vasil'eva, E.V.; Davydova, V.A.; Ismagilova, A.F.; Zarudii, F.S.; Tolstikov, G.A. The synthesis of triterpene 2-deoxy-α -D-hexopyranosides from glycals and their effect on reparative processes. Bioorg. Khim. 1997, 23, 512–518. [Google Scholar]
- Flekhter, O.B.; Medvedeva, N.I.; Tret’yakova, E.V.; Galin, F.Z.; Tolstikov, G.A. Synthesis of methyl esters of betulinic acid 2-deoxy-α-glycosides and 28-oxo-19,28-epoxyoleanane. Chem. Nat. Comp. 2006, 42, 706–709. [Google Scholar] [CrossRef]
- Eleutério, M.I. P.; Schimmel, J.; Ritter, G.; Costa, M.D.; Schmidt, R.R. Synthesis of saponins with allobetulin and glycyrrhetic acid as aglycones. Eur. J. Org. Chem. 2006, 5293–5304. [Google Scholar]
- Gauthier, C.; Legault, J.; Piochon, M.; Lavoie, S.; Tremblay, S.; Pichette, A. Synthesis, cytotoxicity and haemolytic activity of chacotrioside lupane-type neosaponins and their germanicane-type rearrangement products. Bioorg. Med. Chem. Lett. 2009, 19, 2310–2314. [Google Scholar] [CrossRef]
- Pichette, A.; Legault, J.; Gauthier, C. Synthesis of triterpene derivatives as antitumor or anti-inflammatory agents. Can. Pat. Appl. CA 2586614, 2008. [CA 2008, 148, 538411]. [Google Scholar]
- Klinot, J.; Waisser, K.; Streinz, L.; Vystrčil, A. Triterpenes. XX. Participation of dimethylformamide in the addition of halogens to the Δ2-double bond-a route for the preparation of β-epoxides. Collect. Czech. Chem. Commun. 1970, 35, 3610–3617. [Google Scholar] [CrossRef]
- Klinot, J.; Krumpolc, M.; Vystrčil, A. Triterpenes. IX. Reaction of isomeric 2,3-epoxides with a Grignard reagent. Collect. Czech. Chem. Commun. 1996, 31, 3174–3181. [Google Scholar]
- Tolstikov, G.A.; Kim, H.-O.; Goryaev, M.I. Synthesis of triterpenoid indoles. Zh. Obsh. Khim. 1967, 37, 1690. [Google Scholar]
- Kim, H.-O.; Tolstikov, G.A.; Shumov, I.P.; Nasonova, A.M. Triterpenoids. XX. Synthesis of triterpenoid indoles. Zh. Org. Khim. 1969, 5, 1987–1991. [Google Scholar]
- Urban, M.; Sarek, J.; Kvasnica, M.; Tislerova, I.; Hajduch, M. Triterpenoid Pyrazines and Benzopyrazines with Cytotoxic Activity. J. Nat. Prod. 2007, 70, 526–532. [Google Scholar] [CrossRef]
- Kim, H.-O.; Tolstikov, G.A.; Goryaev, M.I. Triterpenoids. XXII. Heterocyclic derivatives of glycyrrhetic acid. Izv. Akad. Nauk Kazakh. SSR Ser. Khim. 1969, 19, 46–48. [Google Scholar]
- Kim, H.-O.; Tolstikov, G.A.; Goryaev, M.I. Triterpenoids. XXIV. Isomerism of triterpene azoles. Izv. Akad. Nauk Kazakh. SSR Ser. Khim. 1970, 20, 49–54. [Google Scholar]
- Pettit, G.R.; Green, B.W.; Bowyer, W.J. Steroids and related natural products. VI. The structure of α-apoallobetulin. J. Org. Chem. 1961, 26, 2879–2883. [Google Scholar] [CrossRef]
- Dischendorfer, O.; Crillmayer, H. Phytochemistry. III. Betulin. 3. Monatsh. Chem. 1926, 47, 419–425. [Google Scholar] [CrossRef]
- Bradbury, B.J.; Huang, M. Substituted taraxastanes useful for treating viral infections. U.S. Pat. Appl. US 20070197646, 2007. [CA 2007, 147, 269186]. [Google Scholar]
- Vystrčil, A.; Klinot, J. Structure of alloheterobetulin. Collect. Czech. Chem. Commun. 1959, 24, 3273–3278. [Google Scholar]
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef]
- Hase, T. Exhaustive Baeyer-Villiger oxidation of the allobetulone triterpenoid. J. Chem. Soc. Chem. Commun. 1972, 755–756. [Google Scholar] [CrossRef]
- Sejbal, J.; Klinot, J.; Hrncirova, D.; Vystrčil, A. Triterpenes. Part LXXI. Oxidation of 19β, 28-epoxy-18α -oleanan-3-one and -1-one with peracids. Collect. Czech. Chem. Commun. 1985, 50, 2753–2759. [Google Scholar] [CrossRef]
- Klinot, J.; Vystrčil, A. Beckmann rearrangement of triterpene 3-ketoximes. Collect. Czech. Chem. Commun. 1962, 27, 377–386. [Google Scholar]
- Hase, T. Dehydration of triterpenoid ring A ε -lactams. Acta Chem. Scand. 1970, 24, 364–365. [Google Scholar] [CrossRef]
- Rao, K.L.; Ramraj, S.K.; Sundararamaiah, T. Aza triterpenes. II. A-Aza triterpenes of the lactones of methyl oleanonate and methyl betulonate. J. Ind. Chem. Soc. 1980, 57, 833–834. [Google Scholar]
- Tolmacheva, I.A.; Galaiko, N.V.; Grishko, V.V. Synthesis of acylhydrazones from lupane and 19β ,28-epoxy-18α-oleanane 2,3-seco-aldehydonitriles. Chem. Nat. Comp. 2010, 46, 39–43. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Igosheva, E.V.; Grishko, V.V.; Zhukova, O.S.; Gerasimova, G.K. The synthesis of triterpenic amides on the basis of 2,3-seco-1-cyano-19β ,28-epoxy-18α -olean-3-oic acid. Russ. J. Bioorg. Chem. 2010, 36, 377–382. [Google Scholar] [CrossRef]
- Klinot, J.; Rozen, J.; Klinotova, E.; Vystrčil, A. Triterpenes. Part LXXXII. A-nor-derivatives of 19β,28-epoxy-18α-oleanane: preparation and stereochemistry. Collect. Czech. Chem. Commun. 1987, 52, 493–500. [Google Scholar] [CrossRef]
- Dischendorfer, O.; Polak, O. Phytochemistry. V. Allobetulin. Monatsh. Chem. 1929, 51, 43–58. [Google Scholar] [CrossRef]
- Ruzicka, L.; Isler, O. Polyterpenes, and polyterpenoids. CVI. Oxidation of dihydrobetulinol and dihydrobetulonic acid with nitric acid. Helv. Chim. Acta 1936, 19, 506–519. [Google Scholar] [CrossRef]
- Shernyukov, A.V.; Mainagashev, I.Y.; Korchagina, D.V.; Gatilov, Y.V.; Salakhutdinov, N.F.; Tolstikov, G.A. Spirocyclization of 2,3-seco-19β,28-epoxy-28-oxo-18α-olean-2,3-dicarboxylic anhydride with benzylamines. Dokl. Chem. 2009, 429, 286–289. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Khusnutdinova, E.F.; Kukovinets, O.S.; Zvereva, T.I.; Tolstikov, G.A. Effective synthesis of 2,3-seco-2,3-dicarboxyplatanic acid. Chem. Nat. Comp. 2010, 46, 393–396. [Google Scholar] [CrossRef]
- Ruzicka, L.; Govaert, F.; Goldberg, M.W.; Lamberton, A.H. Polyterpenes and polyterpenoids. CXXIII. Degradation of allobetulin and hydroxymethyleneallobetulone with chromium trioxide. Helv. Chim. Acta 1938, 21, 73–83. [Google Scholar] [CrossRef]
- Sejbal, J.; Homolova, M.; Tislerova, I.; Krecek, V. Preparation and conformational analysis of 1,2-seco derivatives of 19β,28-epoxy-18α-oleanane. Collect. Czech. Chem. Commun. 2000, 65, 1339–1356. [Google Scholar] [CrossRef]
- Thibeault, D.; Legault, J.; Bouchard, J.; Pichette, A. Useful approach to access germanicanes from betulin. Tetrahedron Lett. 2007, 48, 8416–8419. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Medvedeva, N.I.; Tolstikov, G.A.; Galin, F.Z.; Yunusov, M.S.; Mai, H.N.T.; Tien, L.V.; Savinova, I.V.; Boreko, E.I.; Titov, L.P.; Glukhov, I.V. Synthesis of olean-18-(19)-ene derivatives from betulin. Russ. J. Bioorg. Chem. 2009, 35, 233–239. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Tolstikov, G.A.; Suponitskii, K.Y. A one-step approach to the synthesis of germanicane triterpenoids from allobetulin. Russ. J. Bioorg. Chem. 2010, 36, 133–135. [Google Scholar] [CrossRef]
- Platanov, V.G.; Zorina, A.D.; Gordon, M.A.; Chizhov, N.P.; Balykina, L.V.; Mikhailov, Y.D.; Ivanen, D.R.; Kvi, T.K.; Shavva, A.G. Triterpenoid antiviral activity against influenza A and B viruses. Pharm. Chem. J. 1995, 29, 42–46. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Nigmatullina, L.R.; Baltina, L.A.; Karachurina, L.T.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V. Synthesis of betulinic acid from betulin extract and study of the antiviral and antiulcer activity of some related terpenoids. Pharm. Chem. J. 2002, 36, 484–487. [Google Scholar] [CrossRef]
- Anikina, L.V.; Tolmacheva, I.A.; Vikharev, Y.B.; Grisko, V.V. Effects of lupane and oleanane β-enaminoketones on the number and morphology of white blood cells. Pharm. Chem. J. 2009, 43, 378–380. [Google Scholar] [CrossRef]
- Salin, O.; Alakurtti, S.; Pohjala, L.; Siiskonen, A.; Maass, V.; Maass, M.; Yli-Kauhaluoma, J.; Vuorela, P. Inhibitory effect of the natural product betulin and its derivatives against the intracellular bacterium Chlamydia pneumoniae. Biochem. Pharmacol. 2010, 80, 1141–1151. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Medvedeva, N.I.; Karachurina, L.T.; Baltina, L.A.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A. Synthesis and Pharmacological Activity of Betulin, Betulinic Acid, and Allobetulin Esters. Pharm. Chem. J. 2005, 39, 401–404. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dehaen, W.; Mashentseva, A.A.; Seitembetov, T.S. Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules 2011, 16, 2443-2466. https://doi.org/10.3390/molecules16032443
Dehaen W, Mashentseva AA, Seitembetov TS. Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules. 2011; 16(3):2443-2466. https://doi.org/10.3390/molecules16032443
Chicago/Turabian StyleDehaen, Wim, Anastassiya A. Mashentseva, and Talgat S. Seitembetov. 2011. "Allobetulin and Its Derivatives: Synthesis and Biological Activity" Molecules 16, no. 3: 2443-2466. https://doi.org/10.3390/molecules16032443
APA StyleDehaen, W., Mashentseva, A. A., & Seitembetov, T. S. (2011). Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules, 16(3), 2443-2466. https://doi.org/10.3390/molecules16032443






