Inhibitors of Testosterone Biosynthetic and Metabolic Activation Enzymes
Abstract
:1. Introduction
2. T Biosynthetic and Metabolic Activation Pathways
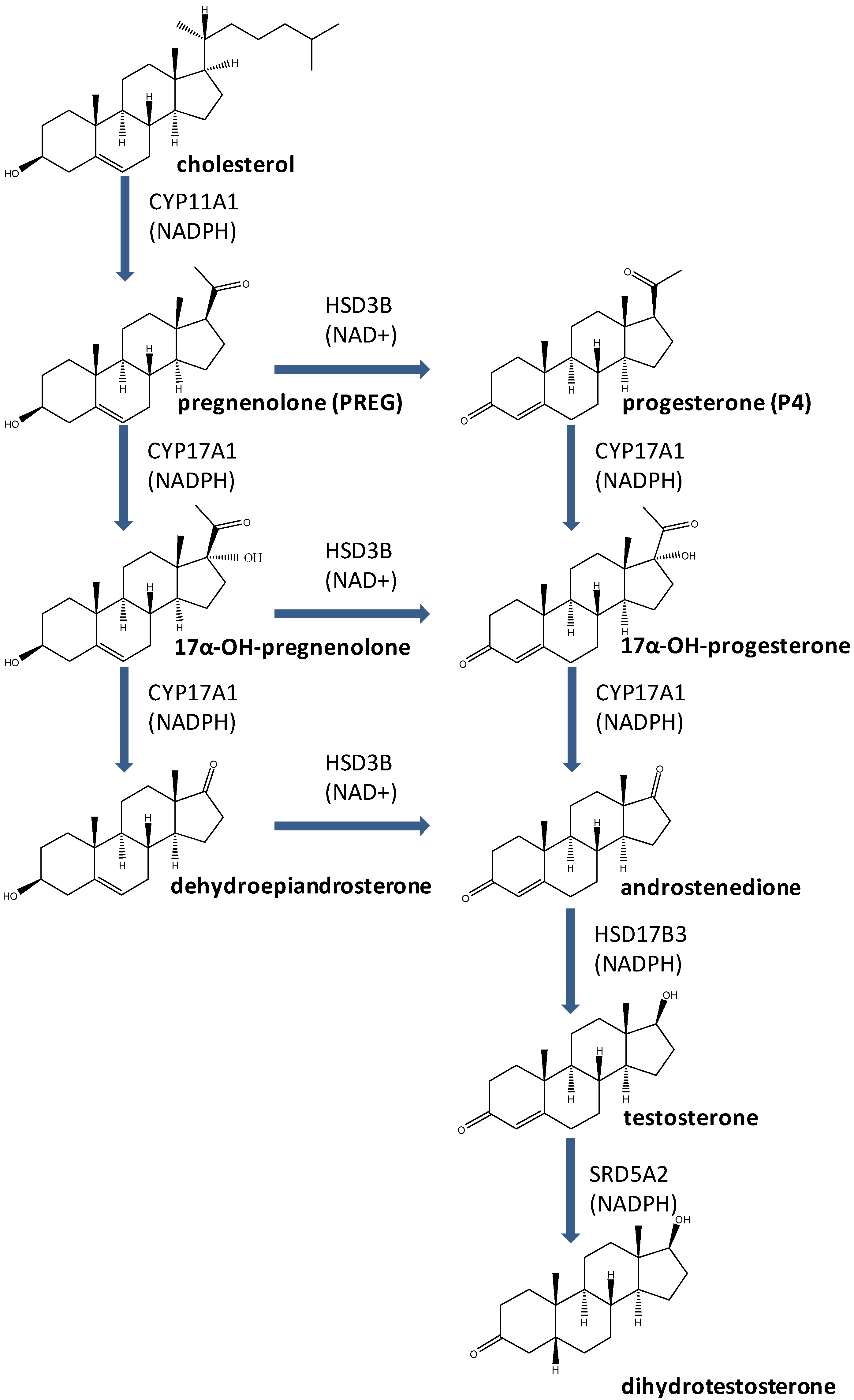
3. Enzymes for T Biosynthesis and Metabolic Activation
3.1. CYP11A1
3.2. HSD3B
3.3. CYP17A1
3.4. HSD17B3
3.5. SRD5A2
4. EDs with Direct Inhibition on Enzymes for T Biosynthesis and Metabolic Activation
| Enzyme | Chemicals | Use | Mode of inhibition |
|---|---|---|---|
| Enzymes for Testosterone Biosynthesis | |||
| CYP11A1 | Methoxychlor & HPTE | Insecticide | Non-competitive |
| Gossypol | Plant constituent | Mixed type | |
| Lindane | Insecticide | Unknown | |
| HSD3B | Perfluorooctane sulfonate | Surfactant | Competitive |
| Perfluorooctane acid | Surfactant | Competitive | |
| Phthalates | Plasticizers | Competitive | |
| Bisphenol A | Plasticizer | Competitive | |
| Methoxychlor & HPTE | Insecticide | Non-competitive | |
| Triphenyltin | Biocide | Unknown | |
| Tributyltin | Biocide | Unknown | |
| Genistein | Plant constituent | Competitive | |
| Gossypol | Plant constituent | Competitive | |
| CYP17A1 | Bisphenol A | Plasticizer | Competitive |
| Triphenyltin | Biocide | Unknown | |
| Tributyltin | Biocide | Unknown | |
| 1,2-Dibromo-3-chloropropane | Insecticide | Unknown | |
| Prochloraz | Biocide | Unknown | |
| Gossypol | Plant constituent | Unknown | |
| HSD17B3 | Perfluorooctane sulfonate | Surfactant | Non-competitive |
| Perfluorooctane acid | Surfactant | Non-competitive | |
| Phthalates | Plasticizers | Unknown | |
| Bisphenol A | Plasticizer | Competitive | |
| Benzophenones | UV blocker | Unknown | |
| Methoxychlor & HPTE | Insecticide | Non-competitive | |
| Triphenyltin | Biocide | Unknown | |
| Tributyltin | Biocide | Unknown | |
| Gossypol | Plant constituent | Competitive | |
| Enzyme for Testosterone Metabolic Activation | |||
| SRD5A2 | Triphenyltin | Biocide | Non-competitive |
| Tributyltin | Biocide | Non-competitive | |
| Genistein | Food constituent | Unknown | |
| Gossypol | Plant constituent | Unknown | |
4.1. Industrial Materials
4.1.1. Perfluoroalkyl Substance (PFASs)
4.1.2. Phthalates
4.1.3. Bisphenol A (BPA)
4.1.4. Benzophenone (BP)
4.2. Insecticides and Fungicides
4.2.1. Methoxychlor (MXC)
4.2.2. Organotins
4.2.3. 1,2-Dibromo-3-chloropropane (DBCP)
4.2.4. Lindane
4.2.5. Prochloraz
4.3. Plant Active Constituents
4.3.1. Isoflavone (Genistein)
4.3.2. Gossypol
5. Summary and Conclusions
Acknowledgements
References and Notes
- Huhtaniemi, I.; Pelliniemi, L.J. Fetal Leydig cells: Cellular origin, morphology, life span, and special functional features. Proc. Soc. Exp. Biol. Med. 1992, 201, 125–140. [Google Scholar]
- Awoniyi, C.A.; Santulli, R.; Sprando, R.L.; Ewing, L.L.; Zirkin, B.R. Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: Quantitative relationship to testosterone concentration within the testis. Endocrinology 1989, 124, 1217–1223. [Google Scholar] [CrossRef]
- Fujii, T. Roles of age and androgen in the regulation of sex accessory organs. Adv. Sex Horm. Res. 1977, 3, 103–137. [Google Scholar]
- Wilson, J.D. Prospects for research for disorders of the endocrine system. J. Am. Med. Assoc. 2001, 285, 624–627. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Carlsen, E.; Swan, S.H.; Petersen, J.H.; Skakkebaek, N.E. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum. Reprod. 2005, 20, 942–949. [Google Scholar]
- Moline, J.M.; Golden, A.L.; Bar-Chama, N.; Smith, E.; Rauch, M.E.; Chapin, R.E.; Perreault, S.D.; Schrader, S.M.; Suk, W.A.; Landrigan, P.J. Exposure to hazardous substances and male reproductive health: A research framework. Environ. Health Persp. 2000, 108, 803–813. [Google Scholar]
- Ge, R.S.; Hardy, M.P. Regulation of Leydig cells during pubertal developmen. In The Leydig Cell in Health and Disease; Payne, A.H., Hardy, M.P., Eds.; Humana Press: New York, NY, USA, 2007; pp. 55–70. [Google Scholar]
- Payne, A.H.; O'Shaughnessy, P.J. Structure, function and regulation of steroidogenic enzymes in the Leydig cell. In The Leydig Cell; Payne, A.H., Hardy, M.P., Russell, L.D., Eds.; Cache River Press: Vienna, IL, USA, 1996; pp. 259–286. [Google Scholar]
- Eik-Nes, K.B. Production and secretion of 5-reduced testosterone (DHT) by male reproductive organs. J. Steroid Biochem. 1975, 6, 337–339. [Google Scholar] [CrossRef]
- Samtani, R.; Bajpai, M.; Ghosh, P.K.; Saraswathy, K.N. SRD5A2 gene mutations—A population-based review. Pediatr. Endocrinol. Rev. 2010, 8, 34–40. [Google Scholar]
- Maimoun, L.; Philibert, P.; Cammas, B.; Audran, F.; Bouchard, P.; Fenichel, P.; Cartigny, M.; Pienkowski, C.; Polak, M.; Skordis, N.; et al. Phenotypical, biological, and molecular heterogeneity of 5alpha-reductase deficiency: An extensive international experience of 55 patients. J. Clin. Endocr. Metab. 2011, 96, 296–307. [Google Scholar] [CrossRef]
- Tang, P.Z.; Tsai-Morris, C.H.; Dufau, M.L. Regulation of 3beta-hydroxysteroid dehydrogenase in gonadotropin-induced steroidogenic desensitization of Leydig cells. Endocrinology 1998, 139, 4496–4505. [Google Scholar] [CrossRef]
- Mendonca, B.B.; Bloise, W.; Arnhold, I.J.; Batista, M.C.; Toledo, S.P.; Drummond, M.C.; Nicolau, W.; Mattar, E. Male pseudohermaphroditism due to nonsalt-losing 3beta-hydroxysteroid dehydrogenase deficiency: Gender role change and absence of gynecomastia at puberty. J. Steroid Biochem. 1987, 28, 669–675. [Google Scholar] [CrossRef]
- Lutfallah, C.; Wang, W.; Mason, J.I.; Chang, Y.T.; Haider, A.; Rich, B.; Castro-Magana, M.; Copeland, K.C.; David, R.; Pang, S. Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J. Clin. Endocr. Metab. 2002, 87, 2611–2622. [Google Scholar] [CrossRef]
- Penning, T.M. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr. Rev. 1997, 18, 281–305. [Google Scholar] [CrossRef]
- Gilep, A.A.; Sushko, T.A.; Usanov, S.A. At the crossroads of steroid hormone biosynthesis: The role, substrate specificity and evolutionary development of CYP17. Biochim. Biophys. Acta 2011, 1814, 200–209. [Google Scholar]
- Geller, D.H.; Auchus, R.J.; Mendonca, B.B.; Miller, W.L. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat. Genet. 1997, 17, 201–205. [Google Scholar] [CrossRef]
- Jones, K.L.; Freidenberg, G.R.; Buchta, R.; Derenoncourt, A. Male pseudohermaphroditism resulting from 17 alpha-monooxygenase (P-450C17) deficiency in two unrelated Guamanians. Am. J. Dis. Child. 1992, 146, 592–595. [Google Scholar]
- Ge, R.S.; Hardy, M.P. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology 1998, 139, 3787–3795. [Google Scholar] [CrossRef]
- Andersson, S.; Geissler, W.M.; Wu, L.; Davis, D.L.; Grumbach, M.M.; New, M.I.; Schwarz, H.P.; Blethen, S.L.; Mendonca, B.B.; Bloise, W.; et al. Molecular genetics and pathophysiology of 17beta-hydroxysteroid dehydrogenase 3 deficiency. J. Clin. Endocr. Metab. 1996, 81, 130–136. [Google Scholar] [CrossRef]
- Rosler, A.; Silverstein, S.; Abeliovich, D.A. (R80Q) mutation in 17 beta-hydroxysteroid dehydrogenase type 3 gene among Arabs of Israel is associated with pseudohermaphroditism in males and normal asymptomatic females. J. Clin. Endocr. Metab. 1996, 81, 1827–1831. [Google Scholar] [CrossRef]
- Jenkins, E.P.; Hsieh, C.L.; Milatovich, A.; Normington, K.; Berman, D.M.; Francke, U.; Russell, D.W. Characterization and chromosomal mapping of a human steroid 5 alpha-reductase gene and pseudogene and mapping of the mouse homologue. Genomics 1991, 11, 1102–1112. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Davis, D.L.; Milatovich, A.; Mendonca, B.B.; Imperato-McGinley, J.; Griffin, J.E.; Francke, U.; Wilson, J.D.; Russell, D.W. Molecular genetics of steroid 5 alpha-reductase 2 deficiency. J. Clin. Invest. 1992, 90, 799–809. [Google Scholar] [CrossRef]
- Cantagrel, V.; Lefeber, D.J.; Ng, B.G.; Guan, Z.; Silhavy, J.L.; Bielas, S.L.; Lehle, L.; Hombauer, H.; Adamowicz, M.; Swiezewska, E.; et al. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell 2010, 142, 203–217. [Google Scholar] [CrossRef]
- Wilson, J.D.; Goldstein, J.L. Classification of hereditary disorders of sexual development. Birth Defects Orig. Artic. Ser. 1975, 11, 1–16. [Google Scholar]
- Andersson, S.; Russell, D.W. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc. Natl. Acad. Sci. USA 1990, 87, 3640–3644. [Google Scholar] [CrossRef]
- Andersson, S.; Berman, D.M.; Jenkins, E.P.; Russell, D.W. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature 1991, 354, 159–161. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Davis, D.L.; Gautier, T.; Imperato-McGinley, J.; Russell, D.W. Brief report: The molecular basis of steroid 5 alpha-reductase deficiency in a large Dominican kindred. N. Engl. J. Med. 1992, 327, 1216–1219. [Google Scholar] [CrossRef]
- Uemura, M.; Tamura, K.; Chung, S.; Honma, S.; Okuyama, A.; Nakamura, Y.; Nakagawa, H. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008, 99, 81–86. [Google Scholar]
- Hu, G.X.; Lian, Q.Q.; Ge, R.S.; Hardy, D.O.; Li, X.K. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrin. Metab. 2009, 20, 139–145. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, G.X.; Chu, Y.; Jin, X.; Gong, S.; Akingbemi, B.T.; Zhang, Z.; Zirkin, B.R.; Ge, R.S. Inhibition of human and rat 3beta-hydroxysteroid dehydrogenase and 17 beta-hydroxysteroid dehydrogenase 3 activities by perfluoroalkylated substances. Chem. Biol. Interact. 2010, 188, 38–43. [Google Scholar] [CrossRef]
- Abdellatif, A.G.; Preat, V.; Vamecq, J.; Nilsson, R.; Roberfroid, M. Peroxisome proliferation and modulation of rat liver carcinogenesis by 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopin. Carcinogenesis 1990, 11, 1899–1902. [Google Scholar] [CrossRef]
- Jensen, A.A.; Leffers, H. Emerging endocrine disrupters: perfluoroalkylated substances. Int. J. Androl. 2008, 31, 161–169. [Google Scholar] [CrossRef]
- Johnson, J.D.; Gibson, S.J.; Ober, R.E. Cholestyramine-enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14C] perfluorooctanoate or potassium [14C] perfluorooctanesulfonate. Fundam. Appl. Toxicol. 1984, 4, 972–976. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef]
- Olsen, G.W.; Mair, D.C.; Church, T.R.; Ellefson, M.E.; Reagen, W.K.; Boyd, T.M.; Herron, R.M.; Medhdizadehkashi, Z.; Nobiletti, J.B.; Rios, J.A.; et al. Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American Red Cross adult blood donors, 2000–2006. Environ. Sci. Technol. 2008, 42, 4989–4995. [Google Scholar]
- Chengelis, C.P.; Kirkpatrick, J.B.; Myers, N.R.; Shinohara, M.; Stetson, P.L.; Sved, D.W. Comparison of the toxicokinetic behavior of perfluorohexanoic acid (PFHxA) and nonafluorobutane-1-sulfonic acid (PFBS) in cynomolgus monkeys and rats. Reprod. Toxicol. 2009, 27, 400–406. [Google Scholar] [CrossRef]
- Olsen, G.W.; Gilliland, F.D.; Burlew, M.M.; Burris, J.M.; Mandel, J.S.; Mandel, J.H. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J. Occup. Environ. Med. 1998, 40, 614–622. [Google Scholar]
- Gilliland, F.D.; Mandel, J.S. Mortality among employees of a perfluorooctanoic acid production plant. J. Occup. Med. 1993, 35, 950–954. [Google Scholar] [CrossRef]
- Biegel, L.B.; Liu, R.C.; Hurtt, M.E.; Cook, J.C. Effects of ammonium perfluorooctanoate on Leydig cell function: In vitro, in vivo, and ex vivo studies. Toxicol. Appl. Pharmacol. 1995, 134, 18–25. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, H.; Liu, Y.; Xu, M.; Dai, J. Alterations in gene expression and testosterone synthesis in the testes of male rats exposed to perfluorododecanoic acid. Toxicol. Sci. 2007, 98, 206–215. [Google Scholar] [CrossRef]
- Zhao, B.; Chu, Y.; Hardy, D.O.; Li, X.K.; Ge, R.S. Inhibition of 3beta- and 17beta-hydroxysteroid dehydrogenase activities in rat Leydig cells by perfluorooctane acid. J. Steroid. Biochem. Mol. Biol. 2010, 118, 13–17. [Google Scholar] [CrossRef]
- Kohn, M.C.; Parham, F.; Masten, S.A.; Portier, C.J.; Shelby, M.D.; Brock, J.W.; Needham, L.L. Human exposure estimates for phthalates. Environ. Health Perspect. 2000, 108, A440–A442. [Google Scholar]
- Binder, P.S.; Baumgartner, S.D.; Zavala, E.Y.; Deg, J.K.; Grossman, K.R. Refractive keratoplasty: Myopic keratomileusis in baboons. Curr. Eye Res. 1984, 3, 1187–1197. [Google Scholar] [CrossRef]
- Boxmeer, J.C.; Smit, M.; Weber, R.F.; Lindemans, J.; Romijn, J.C.; Eijkemans, M.J.; Macklon, N.S.; Steegers-Theunissen, R.P. Seminal plasma cobalamin significantly correlates with sperm concentration in men undergoing IVF or ICSI procedures. J. Androl. 2007, 28, 521–527. [Google Scholar] [CrossRef]
- Ge, R.S.; Chen, G.R.; Tanrikut, C.; Hardy, M.P. Phthalate ester toxicity in Leydig cells: Developmental timing and dosage considerations. Reprod. Toxicol. 2007, 23, 366–373. [Google Scholar] [CrossRef]
- Albro, P.W.; Corbett, J.T.; Schroeder, J.L.; Jordan, S.; Matthews, H.B. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ. Health Perspect. 1982, 45, 19–25. [Google Scholar] [CrossRef]
- Albro, P.W.; Chapin, R.E.; Corbett, J.T.; Schroeder, J.; Phelps, J.L. Mono-2-ethylhexyl phthalate, a metabolite of di-(2-ethylhexyl) phthalate, causally linked to testicular atrophy in rats. Toxicol. Appl. Pharmacol. 1989, 100, 193–200. [Google Scholar] [CrossRef]
- Howdeshell, K.L.; Furr, J.; Lambright, C.R.; Rider, C.V.; Wilson, V.S.; Gray, L.E., Jr. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: Altered fetal steroid hormones and genes. Toxicol. Sci. 2007, 99, 190–202. [Google Scholar] [CrossRef]
- Creasy, D.M.; Beech, L.M.; Gray, T.J.; Butler, W.H. The ultrastructural effects of di-n-pentyl phthalate on the testis of the mature rat. Exp. Mol. Pathol. 1987, 46, 357–371. [Google Scholar] [CrossRef]
- Gray, T.J.; Gangolli, S.D. Aspects of the testicular toxicity of phthalate esters. Environ. Health Perspect. 1986, 65, 229–235. [Google Scholar]
- Sjoberg, P.; Bondesson, U.; Gray, T.J.; Ploen, L. Effects of di-(2-ethylhexyl) phthalate and five of its metabolites on rat testis in vivo and in vitro. Acta Pharmacol. Toxicol. (Copenh). 1986, 58, 225–233. [Google Scholar]
- Gray, T.J.; Beamand, J.A. Effect of some phthalate esters and other testicular toxins on primary cultures of testicular cells. Food Chem. Toxicol. 1984, 22, 123–131. [Google Scholar] [CrossRef]
- Gray, T.J.; Butterworth, K.R. Testicular atrophy produced by phthalate esters. Arch. Toxicol. Suppl. 1980, 4, 452–455. [Google Scholar] [CrossRef]
- Sharpe, R.M. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol. Lett. 2001, 120, 221–232. [Google Scholar] [CrossRef]
- Akingbemi, B.T.; Hardy, M.P. Oestrogenic and antiandrogenic chemicals in the environment: Effects on male reproductive health. Ann. Med. 2001, 33, 391–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Cao, Y.; Chen, B.; Zheng, L.; Ge, R.S. Phthalate levels and low birth weight: A nested case-control study of Chinese newborns. J. Pediatr. 2009, 155, 500–504. [Google Scholar] [CrossRef]
- Foster, P.M.; Thomas, L.V.; Cook, M.W.; Walters, D.G. Effect of DI-n-pentyl phthalate treatment on testicular steroidogenic enzymes and cytochrome P-450 in the rat. Toxicol. Lett. 1983, 15, 265–271. [Google Scholar] [CrossRef]
- Hallmark, N.; Walker, M.; McKinnell, C.; Mahood, I.K.; Scott, H.; Bayne, R.; Coutts, S.; Anderson, R.A.; Greig, I.; Morris, K.; et al. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ. Health Perspect. 2007, 115, 390–396. [Google Scholar]
- Stroheker, T.; Cabaton, N.; Nourdin, G.; Regnier, J.F.; Lhuguenot, J.C.; Chagnon, M.C. Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology 2005, 208, 115–121. [Google Scholar] [CrossRef]
- Loganathan, S.N.; Kannan, K. Occurrence of bisphenol a in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol. 2011, 61, 68–73. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar]
- Ouchi, K.; Watanabe, S. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J. Chromatogr. B 2002, 780, 365–370. [Google Scholar] [CrossRef]
- Takeuchi, T.; Tsutsumi, O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 2002, 291, 76–78. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef]
- Akingbemi, B.T.; Sottas, C.M.; Koulova, A.I.; Klinefelter, G.R.; Hardy, M.P. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 2004, 145, 592–603. [Google Scholar]
- Murono, E.P.; Derk, R.C.; de Leon, J.H. Differential effects of octylphenol, 17beta-estradiol, endosulfan, or bisphenol A on the steroidogenic competence of cultured adult rat Leydig cells. Reprod. Toxicol. 2001, 15, 551–560. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Liao, L.; Han, S. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008, 283, 12–18. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, B.; Hu, G.; Chu, Y.; Ge, R.S. Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. Toxicol. Lett. 2011, 207, 137–142. [Google Scholar] [CrossRef]
- Niwa, T.; Fujimoto, M.; Kishimoto, K.; Yabusaki, Y.; Ishibashi, F.; Katagiri, M. Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol. Pharm. Bull. 2001, 24, 1064–1067. [Google Scholar] [CrossRef]
- Rodriguez-Bernaldo de Quiros, A.; Paseiro-Cerrato, R.; Pastorelli, S.; Koivikko, R.; Simoneau, C.; Paseiro-Losada, P. Migration of photoinitiators by gas phase into dry foods. J. Agric. Food Chem. 2009, 57, 10211–10215. [Google Scholar] [CrossRef]
- Nashev, L.G.; Schuster, D.; Laggner, C.; Sodha, S.; Langer, T.; Wolber, G.; Odermatt, A. The UV-filter benzophenone-1 inhibits 17beta-hydroxysteroid dehydrogenase type 3: Virtual screening as a strategy to identify potential endocrine disrupting chemicals. Biochem. Pharmacol. 2010, 79, 1189–1199. [Google Scholar] [CrossRef]
- Gray, L.E., Jr.; Ostby, J.; Ferrell, J.; Sigmon, R.; Cooper, R.; Linder, R.; Rehnberg, G.; Goldman, J.; Laskey, J. Correlation of sperm and endocrine measures with reproductive success in rodents. Prog. Clin. Biol. Res. 1989, 302, 193–206; discussion 206-199. [Google Scholar]
- Akingbemi, B.T.; Ge, R.S.; Klinefelter, G.R.; Gunsalus, G.L.; Hardy, M.P. A metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, reduces testosterone biosynthesis in rat leydig cells through suppression of steady-state messenger ribonucleic acid levels of the cholesterol side-chain cleavage enzyme. Biol. Reprod. 2000, 62, 571–578. [Google Scholar] [CrossRef]
- Murono, E.P.; Derk, R.C. The reported active metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits testosterone formation by cultured Leydig cells from neonatal rats. Reprod. Toxicol. 2005, 20, 503–513. [Google Scholar] [CrossRef]
- Matthews, J.; Celius, T.; Halgren, R.; Zacharewski, T. Differential estrogen receptor binding of estrogenic substances: A species comparison. J. Steroid Biochem. Mol. Biol. 2000, 74, 223–234. [Google Scholar] [CrossRef]
- Murono, E.P.; Derk, R.C. The effects of the reported active metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, on testosterone formation by cultured Leydig cells from young adult rats. Reprod. Toxicol. 2004, 19, 135–146. [Google Scholar] [CrossRef]
- Tsujita, M.; Ichikawa, Y. Substrate-binding region of cytochrome P-450SCC (P-450 XIA1). Identification and primary structure of the cholesterol binding region in cytochrome P-450SCC. Biochim. Biophys. Acta 1993, 1161, 124–130. [Google Scholar] [CrossRef]
- Bhosle, N.B.; Garg, A.; Jadhav, S.; Harjee, R.; Sawant, S.S.; Venkat, K.; Anil, A.C. Butyltins in water, biofilm, animals and sediments of the west coast of India. Chemosphere 2004, 57, 897–907. [Google Scholar] [CrossRef]
- Ohno, S.; Nakajima, Y.; Nakajin, S. Triphenyltin and Tributyltin inhibit pig testicular 17beta-hydroxysteroid dehydrogenase activity and suppress testicular testosterone biosynthesis. Steroids 2005, 70, 645–651. [Google Scholar] [CrossRef]
- McVey, M.J.; Cooke, G.M. Inhibition of rat testis microsomal 3beta-hydroxysteroid dehydrogenase activity by tributyltin. J. Steroid Biochem. Mol. Biol. 2003, 86, 99–105. [Google Scholar] [CrossRef]
- Lo, S.; Allera, A.; Albers, P.; Heimbrecht, J.; Jantzen, E.; Klingmuller, D.; Steckelbroeck, S. Dithioerythritol (DTE) prevents inhibitory effects of triphenyltin (TPT) on the key enzymes of the human sex steroid hormone metabolism. J. Steroid Biochem. Mol. Biol. 2003, 84, 569–576. [Google Scholar] [CrossRef]
- Doering, D.D.; Steckelbroeck, S.; Doering, T.; Klingmuller, D. Effects of butyltins on human 5alpha-reductase type 1 and type 2 activity. Steroids 2002, 67, 859–867. [Google Scholar] [CrossRef]
- Kano, Y.; Natori, S. Change in free amino acids and phospholipids in the head of adult Sarcophaga peregrina with age. Differentiation 1983, 24, 9–12. [Google Scholar] [CrossRef]
- Rao, K.S.; Burek, J.D.; Murray, F.J.; John, J.A.; Schwetz, B.A.; Bell, T.J.; Potts, W.J.; Parker, C.M. Toxicologic and reproductive effects of inhaled 1,2-dibromo-3-chloropropane in rats. Fundam. Appl. Toxicol. 1983, 3, 104–110. [Google Scholar] [CrossRef]
- Shemi, D.; Marx, Z.; Kaplanski, J.; Potashnik, G.; Sod-Moriah, U.A. Testicular damage development in rats injected with dibromochloropropane (DBCP). Andrologia 1988, 20, 331–337. [Google Scholar]
- Potashnik, G.; Yanai-Inbar, I.; Sacks, M.I.; Israeli, R. Effect of dibromochloropropane on human testicular function. Isr. J. Med. Sci. 1979, 15, 438–442. [Google Scholar]
- Egnatz, D.G.; Ott, M.G.; Townsend, J.C.; Olson, R.D.; Johns, D.B. DBCP and testicular effects in chemical workers: An epidemiological survey in Midland, Michigan. J. Occup. Med. 1980, 22, 727–732. [Google Scholar]
- Potashnik, G.; Yanai-Inbar, I. Dibromochloropropane (DBCP): An 8-year reevaluation of testicular function and reproductive performance. Fertil. Steril. 1987, 47, 317–323. [Google Scholar]
- Chayoth, R.; Kaplanski, J.; Sror, U.; Shemi, D.; Shaked, I.; Potashnik, G.; Sod-Moriah, U.A. The effect of dibromochloropropane (DBCP) on in vitro cyclic AMP levels and testosterone production in rat testes. Andrologia 1988, 20, 232–237. [Google Scholar]
- Heindel, J.J.; Berkowitz, A.S.; Kyle, G.; Luthra, R.; Bruckner, J.V. Assessment in rats of the gonadotoxic and hepatorenal toxic potential of dibromochloropropane (DBCP) in drinking water. Fundam. Appl. Toxicol. 1989, 13, 804–815. [Google Scholar] [CrossRef]
- Leone, M.; Costa, M.; Capitanio, G.L.; Palmero, S.; Prati, M.; Leone, M.M. Dibromochloropropane (DBCP) effects on the reproductive function of the adult male rat. Acta Eur. Fertil. 1988, 19, 99–103. [Google Scholar]
- Sod-Moriah, U.A.; Shemi, D.; Potashnik, G.; Kaplanski, J. Age-dependent differences in the effects of 1,2-dibromo-3-chloropropane (DBCP) on fertility, sperm count, testicular histology and hormonal profile in rats. Andrologia 1990, 22, 455–462. [Google Scholar]
- Kelce, W.R.; Raisbeck, M.F.; Ganjam, V.K. Gonadotoxic effects of 2-hexanone and 1,2-dibromo-3-chloropropane on the enzymatic activity of rat testicular 17 alpha-hydroxylase/C17,20-lyase. Toxicol. Lett. 1990, 52, 331–338. [Google Scholar] [CrossRef]
- Shivanandappa, T.; Krishnakumari, M.K. Hexachlorocyclohexane-induced testicular dysfunction in rats. Acta Pharmacol. Toxicol. (Copenh). 1983, 52, 12–17. [Google Scholar] [CrossRef]
- Dalsenter, P.R.; Faqi, A.S.; Webb, J.; Merker, H.J.; Chahoud, I. Reproductive toxicity and tissue concentrations of lindane in adult male rats. Hum. Exp. Toxicol. 1996, 15, 406–410. [Google Scholar] [CrossRef]
- Traina, M.E.; Rescia, M.; Urbani, E.; Mantovani, A.; Macri, C.; Ricciardi, C.; Stazi, A.V.; Fazzi, P.; Cordelli, E.; Eleuteri, P.; et al. Long-lasting effects of lindane on mouse spermatogenesis induced by in utero exposure. Reprod. Toxicol. 2003, 17, 25–35. [Google Scholar] [CrossRef]
- Ronco, A.M.; Valdes, K.; Marcus, D.; Llanos, M. The mechanism for lindane-induced inhibition of steroidogenesis in cultured rat Leydig cells. Toxicology 2001, 159, 99–106. [Google Scholar] [CrossRef]
- Walsh, L.P.; Stocco, D.M. Effects of lindane on steroidogenesis and steroidogenic acute regulatory protein expression. Biol. Reprod. 2000, 63, 1024–1033. [Google Scholar] [CrossRef]
- Suwalsky, M.; Villena, F.; Marcus, D.; Ronco, A.M. Plasma absorption and ultrastructural changes of rat testicular cells induced by lindane. Hum. Exp. Toxicol. 2000, 19, 529–533. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Gautam, A.K. Steroidogenic impairment after lindane treatment in male rats. J. UOEH 1994, 16, 145–152. [Google Scholar]
- Wango, E.O.; Onyango, D.W.; Odongo, H.; Okindo, E.; Mugweru, J. In vitro production of testosterone and plasma levels of luteinising hormone, testosterone and cortisol in male rats treated with heptachlor. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997, 118, 381–386. [Google Scholar] [CrossRef]
- Sircar, S.; Lahiri, P. Effect of lindane on mitochondrial side-chain cleavage of cholesterol in mice. Toxicology 1990, 61, 41–46. [Google Scholar] [CrossRef]
- Henry, M.J.; Sisler, H.D. Effects of sterol biosynthesis-inhibiting (SBI) fungicides on cytochrome P-450 oxygenations in fungi. Pestic. Biochem. Physiol. 1984, 22, 262–275. [Google Scholar] [CrossRef]
- Blystone, C.R.; Lambright, C.S.; Howdeshell, K.L.; Furr, J.; Sternberg, R.M.; Butterworth, B.C.; Durhan, E.J.; Makynen, E.A.; Ankley, G.T.; Wilson, V.S.; et al. Sensitivity of fetal rat testicular steroidogenesis to maternal prochloraz exposure and the underlying mechanism of inhibition. Toxicol. Sci. 2007, 97, 512–519. [Google Scholar] [CrossRef]
- Blystone, C.R.; Furr, J.; Lambright, C.S.; Howdeshell, K.L.; Ryan, B.C.; Wilson, V.S.; Leblanc, G.A.; Gray, L.E., Jr. Prochloraz inhibits testosterone production at dosages below those that affect androgen-dependent organ weights or the onset of puberty in the male Sprague Dawley rat. Toxicol. Sci. 2007, 97, 65–74. [Google Scholar] [CrossRef]
- Ohlsson, A.; Ulleras, E.; Oskarsson, A. A biphasic effect of the fungicide prochloraz on aldosterone, but not cortisol, secretion in human adrenal H295R cells—Underlying mechanisms. Toxicol. Lett. 2009, 191, 174–180. [Google Scholar] [CrossRef]
- Akingbemi, B.T.; Braden, T.D.; Kemppainen, B.W.; Hancock, K.D.; Sherrill, J.D.; Cook, S.J.; He, X.; Supko, J.G. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology 2007, 148, 4475–4488. [Google Scholar] [CrossRef]
- Reinli, K.; Block, G. Phytoestrogen content of foods--a compendium of literature values. Nutr. Cancer 1996, 26, 123–148. [Google Scholar] [CrossRef]
- Svechnikov, K.; Supornsilchai, V.; Strand, M.L.; Wahlgren, A.; Seidlova-Wuttke, D.; Wuttke, W.; Soder, O. Influence of long-term dietary administration of procymidone, a fungicide with anti-androgenic effects, or the phytoestrogen genistein to rats on the pituitary-gonadal axis and Leydig cell steroidogenesis. J. Endocrinol. 2005, 187, 117–124. [Google Scholar] [CrossRef]
- Hu, G.X.; Zhao, B.H.; Chu, Y.H.; Zhou, H.Y.; Akingbemi, B.T.; Zheng, Z.Q.; Ge, R.S. Effects of genistein and equol on human and rat testicular 3beta-hydroxysteroid dehydrogenase and 17beta-hydroxysteroid dehydrogenase 3 activities. Asian J. Androl. 2010, 12, 519–526. [Google Scholar] [CrossRef]
- Hiipakka, R.A.; Zhang, H.Z.; Dai, W.; Dai, Q.; Liao, S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Yu, Z.H.; Chan, H.C. Gossypol as a male antifertility agent—Why studies should have been continued. Int. J. Androl. 1998, 21, 2–7. [Google Scholar]
- Waites, G.M.; Wang, C.; Griffin, P.D. Gossypol: Reasons for its failure to be accepted as a safe, reversible male antifertility drug. Int. J. Androl. 1998, 21, 8–12. [Google Scholar]
- Gu, Y.; Lin, Y.C.; Rikihisa, Y. Inhibitory effect of gossypol on steroidogenic pathways in cultured bovine luteal cells. Biochem. Biophys. Res. Commun. 1990, 169, 455–461. [Google Scholar] [CrossRef]
- Cuellar, A.; Diaz-Sanchez, V.; Ramirez, J. Cholesterol side-chain cleavage and 11 beta-hydroxylation are inhibited by gossypol in adrenal cortex mitochondria. J. Steroid Biochem. Mol. Biol. 1990, 37, 581–585. [Google Scholar] [CrossRef]
- Hu, G.X.; Zhou, H.Y.; Li, X.W.; Chen, B.B.; Xiao, Y.C.; Lian, Q.Q.; Liang, G.; Kim, H.H.; Zheng, Z.Q.; Hardy, D.O.; et al. The (+)- and (−)-gossypols potently inhibit both 3beta-hydroxysteroid dehydrogenase and 17beta-hydroxysteroid dehydrogenase 3 in human and rat testes. J. Steroid Biochem. Mol. Biol. 2009, 115, 14–19. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds including nestorone are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ye, L.; Su, Z.-J.; Ge, R.-S. Inhibitors of Testosterone Biosynthetic and Metabolic Activation Enzymes. Molecules 2011, 16, 9983-10001. https://doi.org/10.3390/molecules16129983
Ye L, Su Z-J, Ge R-S. Inhibitors of Testosterone Biosynthetic and Metabolic Activation Enzymes. Molecules. 2011; 16(12):9983-10001. https://doi.org/10.3390/molecules16129983
Chicago/Turabian StyleYe, Leping, Zhi-Jian Su, and Ren-Shan Ge. 2011. "Inhibitors of Testosterone Biosynthetic and Metabolic Activation Enzymes" Molecules 16, no. 12: 9983-10001. https://doi.org/10.3390/molecules16129983
APA StyleYe, L., Su, Z.-J., & Ge, R.-S. (2011). Inhibitors of Testosterone Biosynthetic and Metabolic Activation Enzymes. Molecules, 16(12), 9983-10001. https://doi.org/10.3390/molecules16129983




