Protective Effect of Salidroside from Rhodiolae Radix on Diabetes-Induced Oxidative Stress in Mice
Abstract
:1. Introduction
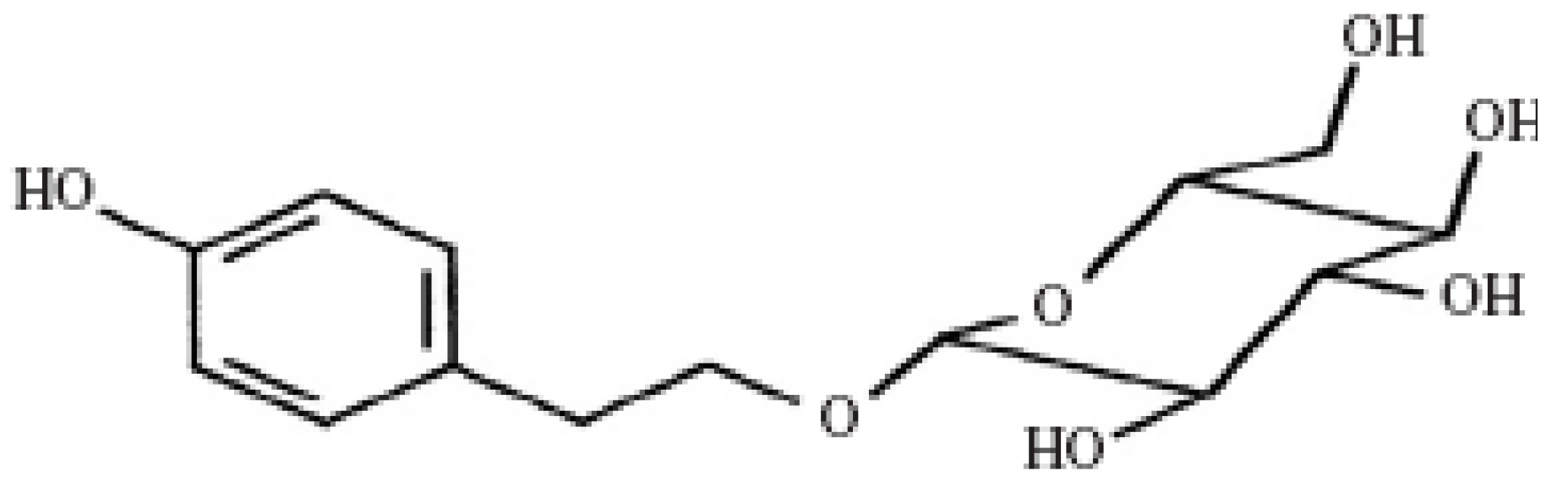
2. Results and Discussion
2.1. Effect of Salidroside Administration on Body Weight in Mice
| Groups | 0 days | 7 days | 14 days | 21 days | 28 days |
|---|---|---|---|---|---|
| NC | 24.41 ± 2.16 | 27.84 ± 2.47 b | 28. 97 ± 2.68 b | 30.64 ± 1.97 b | 31.16 ± 2.53 b |
| DC | 24.17 ± 1.48 | 22.39 ± 3.24 a | 22.81 ± 3.65 a | 23.76 ± 2.43 a | 23.31 ± 3.15 a |
| DLT, | 23.86 ± 2.34 | 23.25 ± 2.17 a | 24.29 ± 2.87 a | 28.57 ± 1.94 b | 29.14 ± 2.47 b |
| DMT | 23.79 ± 1.67 | 23.16 ± 1.49 a | 25.25 ± 2.12 a b | 28.41 ± 2.45 b | 29.39 ± 2.13 b |
| DHT | 24.33 ± 1.95 | 23.48 ± 1.83 a | 26.26 ± 2.08 b | 29.33 ± 1.87 b | 30.51 ± 2.52 b |
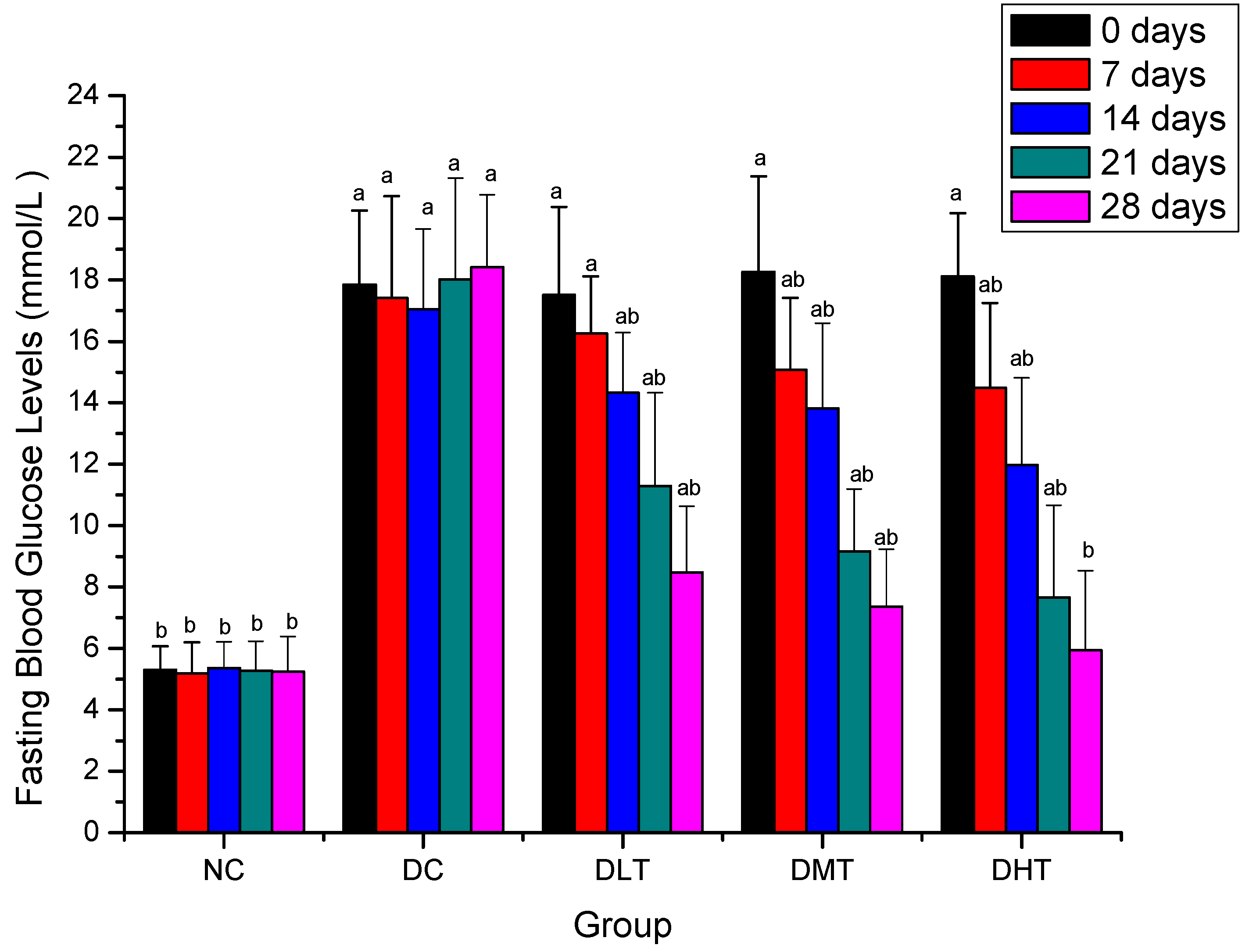
2.2. Effect of Salidroside Administration on Fasting Blood Glucose Levels in Mice
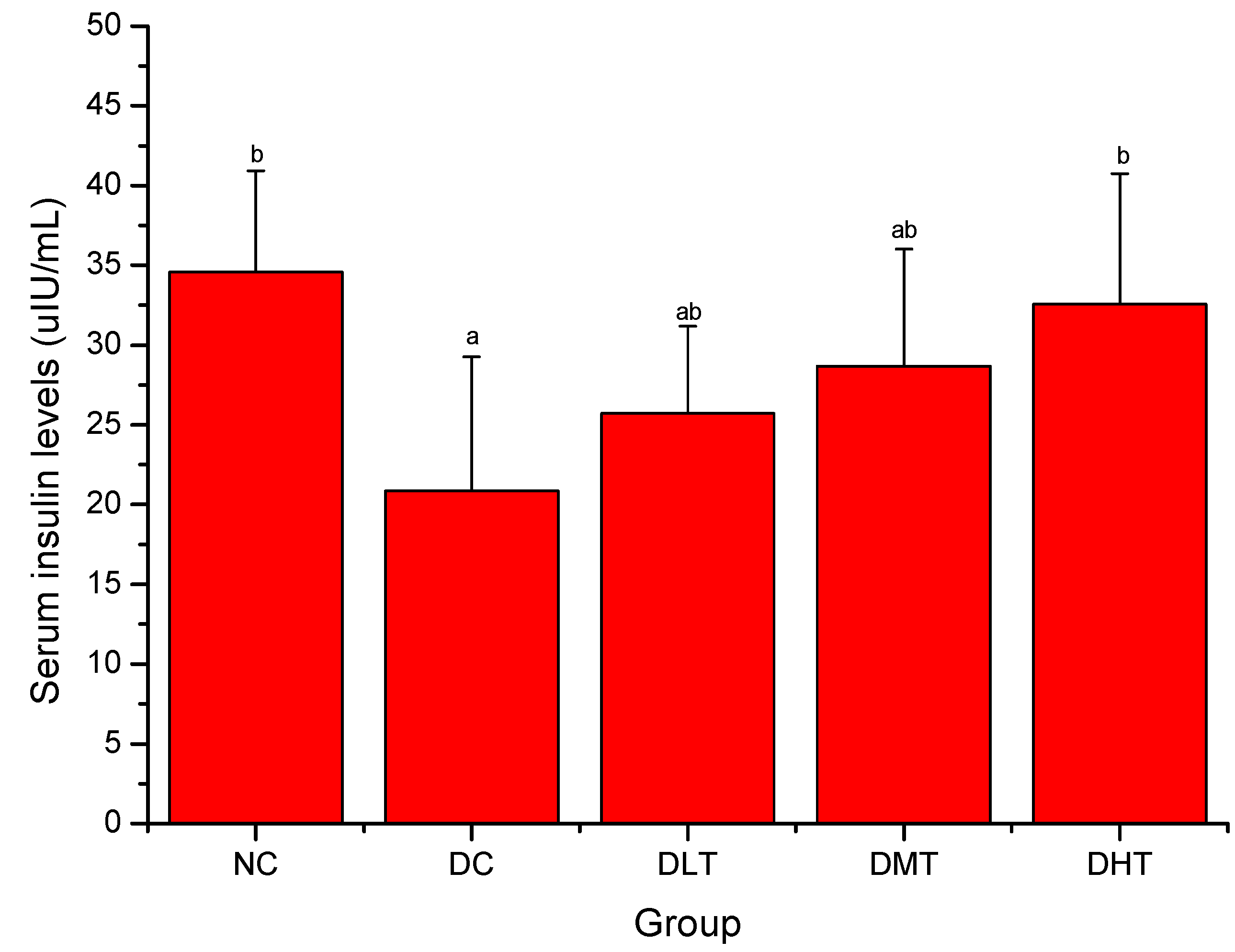
2.3. Effect of Salidroside Administration on Serum Insulin Levels in Mice
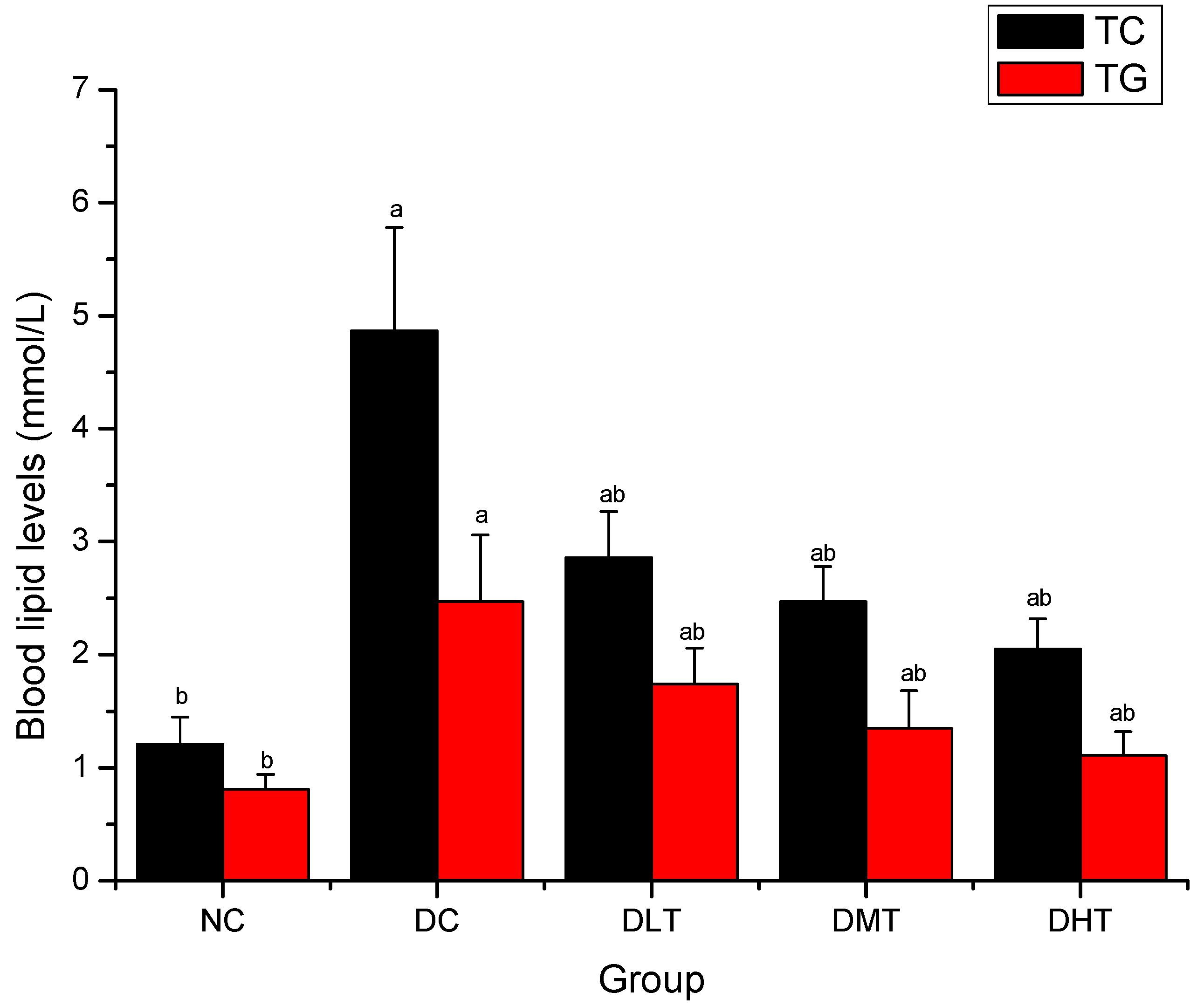
2.4. Effect of Salidroside Administration on Blood Lipid Levels in Mice
2.5. Effect of Salidroside Administration on Antioxidant Enzymes Activities and MDA Levels in Kidney and Liver in Mice
| Groups | Kidney | |||
|---|---|---|---|---|
| SOD (U/mg protein) | GPx (U/mg protein) | CAT (U/mg protein) | MDA (nmol/mg protein) | |
| NC | 10.87 ± 1.08 b | 7.43 ± 0.98 b | 14.29 ± 2.07 b | 1.57 ± 0.16 b |
| DC | 4.64 ± 0.95 a | 4.02 ± 0.67 a | 8.23 ± 0.96 a | 4.11 ± 0.12 a |
| DLT | 6.99 ± 1.21 a b | 7.11 ± 1.24 b | 13.94 ± 1.98 b | 2.84 ± 0.27 a b |
| DMT | 8.03 ± 0.84 a b | 7.93 ± 0.85 b | 15.31 ± 1.87 b | 2.36 ± 0.31 a b |
| DHT | 8.16 ± 1.35 a b | 8.26 ± 1.17 b | 18.49 ± 2.23 a b | 1.79 ± 0.25 b |
| Groups | Liver | |||
|---|---|---|---|---|
| SOD (U/mg protein) | GPx (U/mg protein) | CAT (U/mg protein) | MDA (nmol/mg protein) | |
| NC | 12.08 ± 2.19 b | 4.14 ± 0.54 b | 19.16 ± 2.81 b | 6.61 ± 0.47 b |
| DC | 6.16 ± 0.98 a | 1.12 ± 0.26 a | 11.43 ± 2.35 a | 9.27 ± 0.84 a |
| DLT, | 8.95 ± 1.17 a b | 3.47 ± 0.31 b | 15.60 ± 1.91 a b | 8.89 ± 0.58 a |
| DMT | 10.99 ± 1.64 b | 4.09 ± 0.48 b | 18.47 ± 2.13 b | 7.26 ± 0.67 b |
| DHT | 11.32 ± 2.25 b | 4.63 ± 0.41 b | 20.24 ± 1.68 b | 7.01 ± 0.49 b |
3. Experimental
3.1. Chemicals and Reagents
3.2. Animals
3.3. Preparation of Diabetic Mice
3.4. Experimental Design
3.5. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic activity of Prunella vulgaris L. in streptozotocin-induced diabetic mice. Asia Pac. J. Clin. Nutr. 2007, 1, 427–431. [Google Scholar]
- He, Z.; King, G.L. Microvascular complications of diabete. Endocrin. Metab. Clin. 2004, 33, 215–238. [Google Scholar] [CrossRef]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De, K.N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Farag, Y.M.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transplant. 2011, 26, 28–35. [Google Scholar] [CrossRef]
- Dias, A.S.; Porawski, M.; Alonso, M.; Marroni, N.; Collado, P.S.; González-Gallego, J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J. Nutr. 2005, 135, 2299–2304. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1–10. [Google Scholar] [CrossRef]
- Pennathur, S.; Heinecke, J.W. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid. Redox Signal. 2007, 9, 955–969. [Google Scholar] [CrossRef]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef]
- Bikopoulos, G.; Pimenta, A.S.; Lee, S.C.; Lakey, J.R.; Der, S.D.; Chan, C.B.; Ceddia, R.B.; Wheeler, M.B.; Rozakis-Adcock, M. Ex vivo transcriptional profiling of human pancreatic islets following chronic exposure tomonounsaturated fatty acids. J. Endocrinol. 2008, 196, 455–464. [Google Scholar] [CrossRef]
- Omar, H.S.; El-Beshbishy, H.A.; Moussa, Z.; Taha, K.F.; Singab, A.N. Antioxidant activity of Artocarpus heterophyllus Lam. (Jack Fruit) leaf extracts: Remarkable attenuations of hyperglycemia and hyperlipidemia in streptozotocin-diabetic rats. Sci. World J. 2011, 11, 788–800. [Google Scholar] [CrossRef]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Attenuation of oxidative stress and alteration of hepatic tissue ultrastructure by D-pinitol in streptozotocin-induced diabetic rats. Free Radic. Res. 2010, 44, 668–678. [Google Scholar] [CrossRef]
- Cemek, M.; Kağa, S.; Simşek, N.; Büyükokuroğlu, M.E.; Konuk, M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 2008, 62, 284–293. [Google Scholar]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. J. Am. Med. Assoc. 2002, 287, 360–372. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar]
- Scheen, A.J.; Lefèbvre, P.J. Oral antidiabetic agents: A guide to selection. Drugs 1998, 55, 225–236. [Google Scholar] [CrossRef]
- Mizuno, C.S.; Chittiboyina, A.G.; Kurtz, T.W.; Pershadsingh, H.A.; Avery, M.A. Type 2 diabetes and oral antihyperglycemic drugs. Curr. Med. Chem. 2008, 15, 61–74. [Google Scholar] [CrossRef]
- Lau, C.H.; Chan, C.M.; Chan, Y.W.; Lau, K.M.; Lau, T.W.; Lam, F.C.; Che, C.T.; Leung, P.C.; Fung, K.P.; Ho, Y.Y.; et al. In vitro antidiabetic activities of five medicinal herbs used in Chinese medicinal formulae. Phytother. Res. 2008, 22, 1384–1388. [Google Scholar] [CrossRef]
- Wen, X.Y.; Hu, Q.J.; Wang, F. Amelioration effect of Zhenqing Capsule on peripheral neuropathy in type 1 diabetic rats. Zhong Xi Yi Jie He Xue Bao 2008, 6, 289–293. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Yamabe, N. Amelioration of diabetic nephropathy by dried Rehmanniae Radix (Di Huang) extract. Am. J. Chin. Med. 2004, 32, 829–839. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Shi, X.; Li, X.; Hong, J.; Chen, J.; Gu, W.; Lu, X.; Xu, G.; Ning, G. Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 2010, 81, 766–772. [Google Scholar] [CrossRef]
- Wu, Y.L.; Piao, D.M.; Han, X.H.; Nan, J.X. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol. Pharm. Bull. 2008, 31, 1523–1529. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Wang, X.; Liu, B.; Xu, H. Salidroside production by hairy roots of Rhodiola sachalinensis obtained after transformation with Agrobacterium rhizogenes. Biol. Pharm. Bull. 2007, 30, 439–442. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, F. Preparative isolation and purification of salidroside from the Chinese medicinal plant Rhodiolasachalinensis by high-speed counter-current chromatography. J. Chromatogr. A 2001, 932, 91–95. [Google Scholar] [CrossRef]
- Gao, D.; Li, Q.; Liu, Z.; Feng, J.; Li, J.; Han, Z.; Duan, Y. Antidiabetic potential of Rhodiola sachalinensis root extract in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 375–381. [Google Scholar]
- Cui, X.; Piao, J.H.; Liu, Y.; Quan, X.F. Effects of Rhodiola sachalinensis Aqueous Extract on the Activity of GSH-Px and the Content of MDA in Diabetic Rats. Lishizhen Med. Mater. Med. Res. 2007, 18, 611–613. [Google Scholar]
- Kucinskaite, A.; Briedis, V.; Savickas, A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina (Kaunas) 2004, 40, 614–619. [Google Scholar]
- Skopińska-Rózewska, E.; Malinowski, M.; Wasiutyński, A.; Sommer, E.; Furmanowa, M.; Mazurkiewicz, M.; Siwicki, A.K. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol. J. Vet. Sci. 2008, 11, 97–110. [Google Scholar]
- Gupta, V.; Saggu, S.; Tulsawani, R.K.; Sawhney, R.C.; Kumar, R. A dose dependent adaptogenic and safety evaluation of Rhodiola imbricata Edgew, a high altitude rhizome. Food Chem. Toxicol. 2008, 46, 1645–1652. [Google Scholar] [CrossRef]
- Mao, G.X.; Wang, Y.; Qiu, Q.; Deng, H.B.; Yuan, L.G.; Li, R.G.; Song, D.Q.; Li, Y.Y.; Li, D.D.; Wang, Z. Salidroside protects human fibroblast cells from premature senescence induced by H(2)O(2) partly through modulating oxidative status. Mech. Ageing Dev. 2010, 131, 723–731. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lian, L.H.; Jiang, Y.Z.; Nan, J.X. Hepatoprotective effects of salidroside on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J. Pharm. Pharmacol. 2009, 61, 1375–1382. [Google Scholar]
- Tong, B.D.; Ma, L.; Cai, D.L.; Shen, X.; Lu, Q.H.; Xiong, J.P. Effect of Salidroside on Energy Metabolism of Mice. Chin. J. Clin. Nutr. 2008, 16, 129–133. [Google Scholar]
- Sharma, V.K.; Kumar, S.; Patel, H.J.; Hugar, S. Hypoglycemic activity of ficus glomerata in alloxan induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 18–22. [Google Scholar]
- Qi, X.Y.; Chen, W.J.; Zhang, L.Q.; Xie, B.J. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr. Res. 2008, 28, 278–284. [Google Scholar] [CrossRef]
- Rajagopal, K.; Sasikala, K. Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rat. Singapore Med. J. 2008, 49, 137–141. [Google Scholar]
- Kalaiarasi, P.; Pugalendi, K.V. Antihyperglycemic effect of 18 beta-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 2009, 606, 269–273. [Google Scholar] [CrossRef]
- Varma, S.B.; Jaybhaye, D.L. Antihyperglycemic activity of Tectona grandis Linn. bark extract on alloxan induced diabetes in rats. Int. J. Ayurveda Res. 2010, 1, 163–166. [Google Scholar] [CrossRef]
- Attele, A.S.; Zhou, Y.P.; Xie, J.T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef]
- Xie, J.-T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.-Z.; Wu, J.A.; Aung, H.H.; Rue, P.A.; Bell, G.I.; et al. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim. Biophys. Acta 2005, 1740, 319–325. [Google Scholar]
- Pari, L.; Latha, M. Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids instreptozotocin diabetic rats. Singapore Med. J. 2002, 43, 617–621. [Google Scholar]
- Ahmed, I.; Adeghate, E.; Sharma, A.K.; Pallot, D.J.; Singh, J. Effects of Momordica charantia fruit juice on islet morphology in the pancreas of thestreptozotocin-diabetic rat. Diabetes Res. Clin. Pract. 1998, 40, 145–151. [Google Scholar] [CrossRef]
- Jain, N.; Vijayaraghavan, R.; Pant, S.C.; Lomash, V.; Ali, M. Aloe vera gel alleviates cardiotoxicity in streptozocin-induced diabetes in rats. J. Pharm. Pharmacol. 2010, 62, 115–123. [Google Scholar]
- Davidson, M.H.; Yannicelli, H.D. New concepts in dyslipidemia in the metabolic syndrome and diabetes. Metab. Syndr. Relat. Disord. 2006, 4, 299–314. [Google Scholar] [CrossRef]
- Mitra, S.K.; Gopumadhavan, S.; Muralidhar, T.S.; Anturlikar, S.D.; Sujatha, M.B. Effect of D-400, a herbomineral preparation on lipid profile, glycated haemoglobin and glucose tolerance in streptozotocin induced diabetes in rats. Indian J. Exp. Biol. 1995, 33, 798–800. [Google Scholar]
- Triggiani, V.; Resta, F.; Guastamacchia, E.; Sabbà, C.; Licchelli, B.; Ghiyasaldin, S.; Tafaro, E. Role of antioxidants, essential fatty acids, carnitine, vitamins, phytochemicals and traceelements in the treatment of diabetes mellitus and its chronic complications. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 77–93. [Google Scholar]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar]
- Parhizkar, S.; Latiff, L.A.; Rahman, S.A.; Dollah, M.A. Preventive effect of Nigella sativa on metabolic syndrome in menopause induced rats. J. Med. Plants Res. 2011, 5, 1478–1484. [Google Scholar]
- Kanter, M.; Akpolat, M.; Aktas, C. Protective effects of the volatile oil of Nigella sativa seeds on beta-cell damage instreptozotocin-induced diabetic rats: A light and electron microscopic study. J. Mol. Histol. 2009, 40, 379–385. [Google Scholar] [CrossRef]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.H.; Chen, X.; Sang, S.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: Effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Xue, S.X.; Chen, X.M.; Lu, J.X.; Jin, L.Q. Protective effect of sulfated Achyranthes bidentata polysaccharides on streptozotocin-induced oxidative stress in rats. Carbohydr. Polym. 2009, 75, 415–419. [Google Scholar] [CrossRef]
- Sathishsekar, D.; Subramanian, S. Antioxidant properties of Momordica Charantia (bitter gourd) seeds on Streptozotocin induced diabetic rats. Asia Pac. J. Clin. Nutr. 2005, 14, 153–158. [Google Scholar]
- Elmalí, E.; Altan, N.; Bukan, N. Effect of the sulphonylurea glibenclamide on liver and kidney antioxidant enzymes in streptozocin-induced diabetic rats. Drugs R D 2004, 5, 203–208. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Gao, D.; Peng, Y. The optimal extraction parameters and anti-diabetic activity of flavonoids from Ipomoeabatatas leaf. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 195–202. [Google Scholar]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, F.; Tang, H.; Xiao, F.; Gong, J.; Peng, Y.; Meng, X. Protective Effect of Salidroside from Rhodiolae Radix on Diabetes-Induced Oxidative Stress in Mice. Molecules 2011, 16, 9912-9924. https://doi.org/10.3390/molecules16129912
Li F, Tang H, Xiao F, Gong J, Peng Y, Meng X. Protective Effect of Salidroside from Rhodiolae Radix on Diabetes-Induced Oxidative Stress in Mice. Molecules. 2011; 16(12):9912-9924. https://doi.org/10.3390/molecules16129912
Chicago/Turabian StyleLi, Fenglin, Hong Tang, Furen Xiao, Jingli Gong, Yong Peng, and Xiangle Meng. 2011. "Protective Effect of Salidroside from Rhodiolae Radix on Diabetes-Induced Oxidative Stress in Mice" Molecules 16, no. 12: 9912-9924. https://doi.org/10.3390/molecules16129912
APA StyleLi, F., Tang, H., Xiao, F., Gong, J., Peng, Y., & Meng, X. (2011). Protective Effect of Salidroside from Rhodiolae Radix on Diabetes-Induced Oxidative Stress in Mice. Molecules, 16(12), 9912-9924. https://doi.org/10.3390/molecules16129912




