Snailase Preparation of Ginsenoside M1 from Protopanaxadiol-Type Ginsenoside and Their Protective Effects Against CCl4-Induced Chronic Hepatotoxicity in Mice
Abstract
:1. Introduction
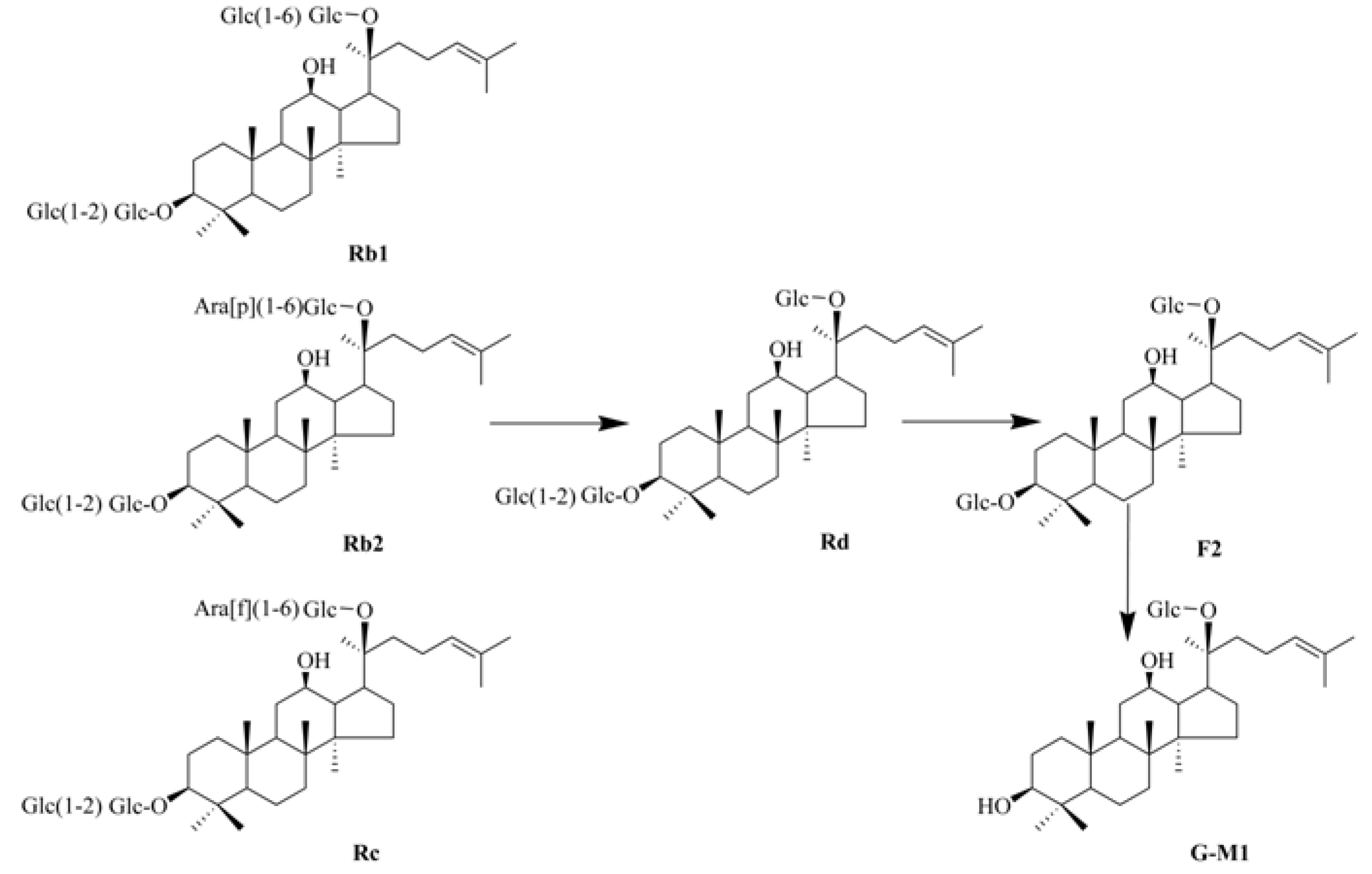
2. Results and Discussion
2.1. Biotransformation of PDG to G-M1
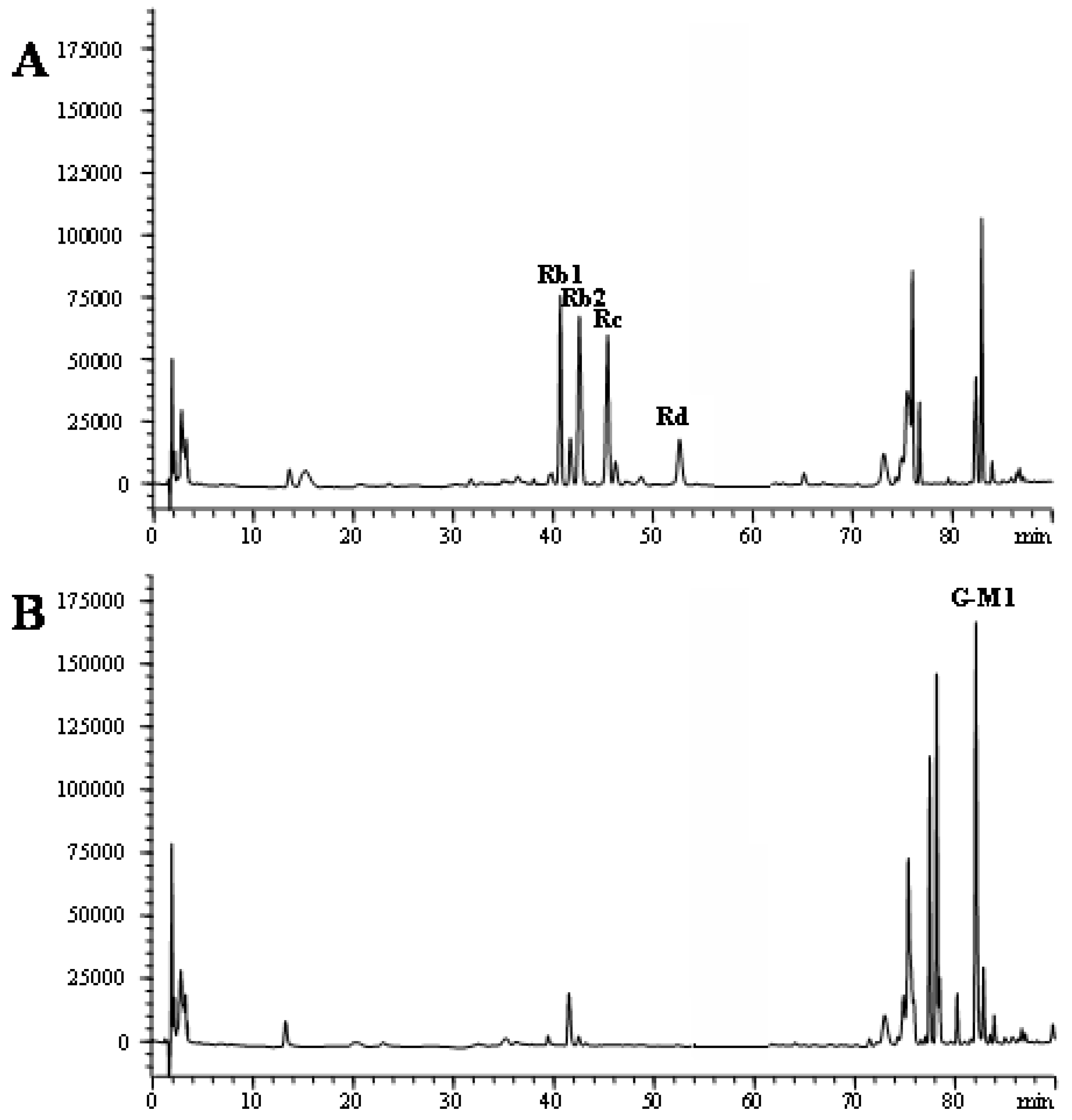
2.2. Identification of G-M1 by ESI-MS
2.3. Effects of PDG and G-M1 on Body and Organ Weights

| Group | Body weight (g) | Relative weight (g/g body weight, %) | ||
|---|---|---|---|---|
| Heart | Liver | Spleen | ||
| Normal control | 38.26 ± 2.15 | 0.51 ± 0.05 | 5.25 ± 0.31 | 0.42 ± 0.03 |
| CCl4 control | 37.25 ± 3.56 | 0.48 ± 0.06 | 6.98 ± 0.22 # | 0.53 ± 0.09 # |
| G-M1 + CCl4 | 39.15 ± 3.33 | 0.47 ± 0.08 | 5.78 ± 0.12 * | 0.44 ± 0.07 |
| PDG + CCl4 | 40.05 ± 4.15 | 0.50 ± 0.04 | 6.23 ± 0.52 * | 0.47 ± 0.11 |
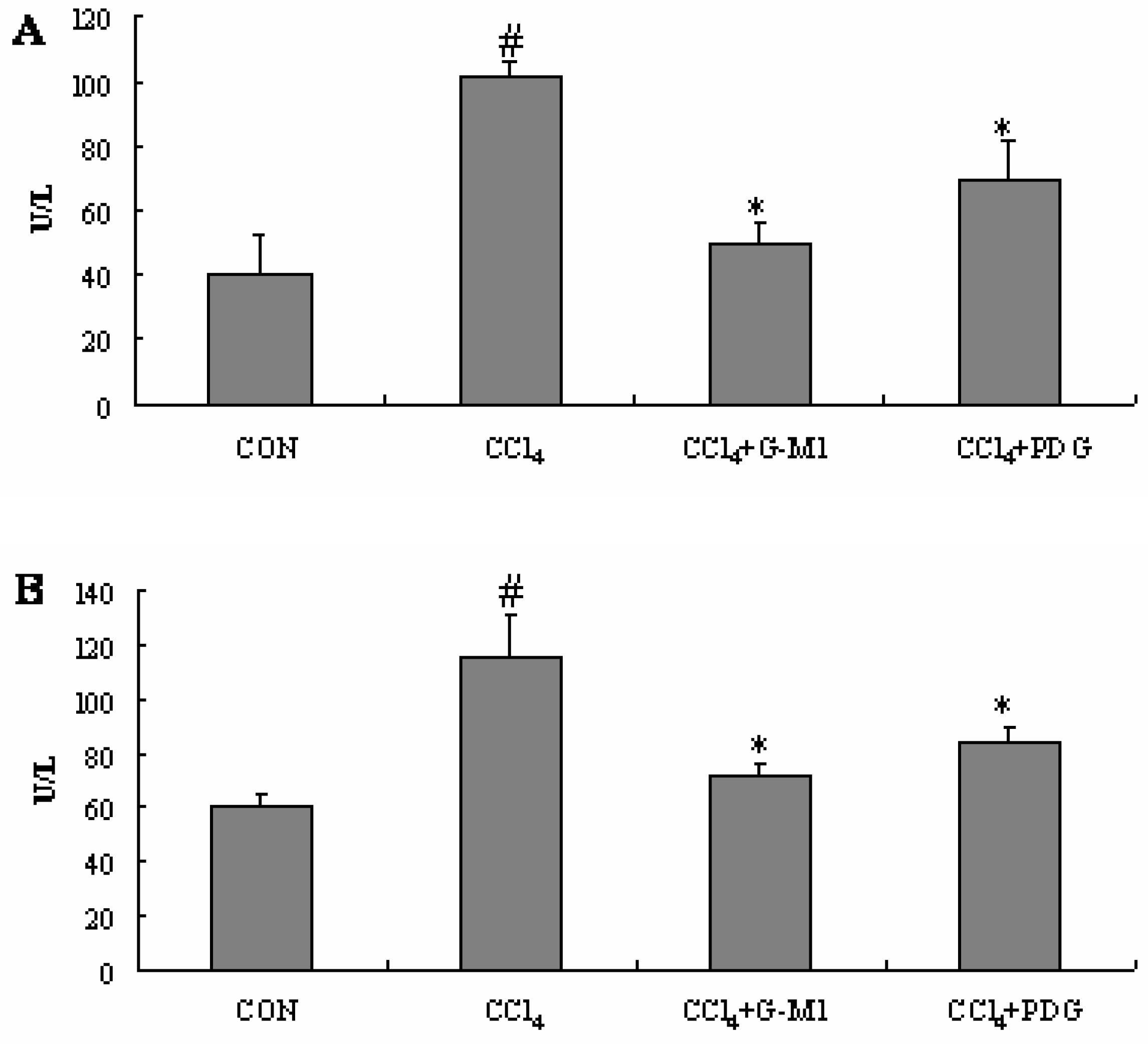
2.4. Effect of PDG and G-M1 on the Serum ALT and AST Levels
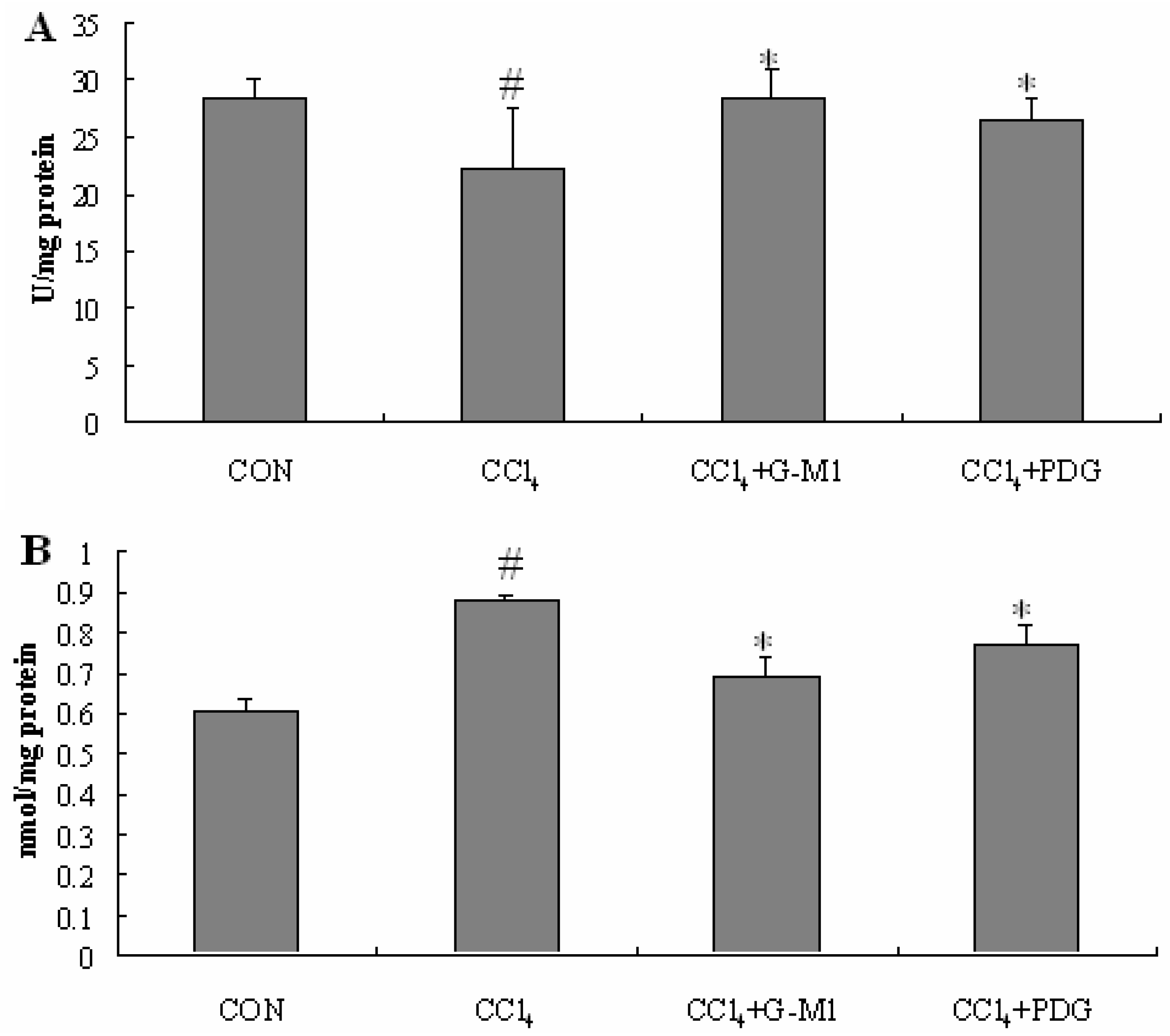
2.5. Effect of PDG and G-M1 on the Level of SOD and MDA in Liver Homogenate
2.6. Histopathological Evaluation
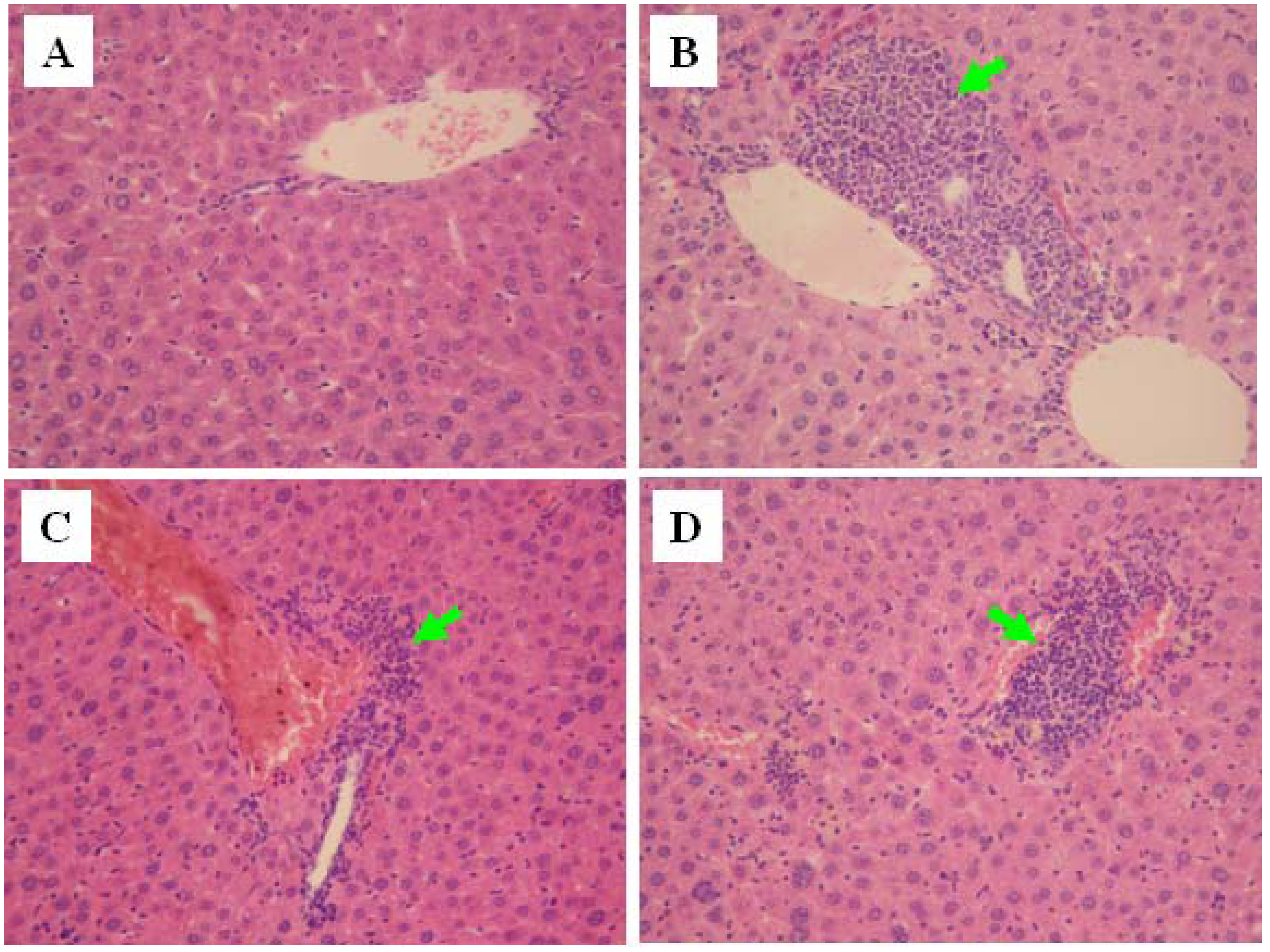
3. Experimental
3.1. Chemicals
3.2. Animals
3.3. Carbon Tetrachloride (CCl4)-Induced Chronic Liver Injury in Mice
3.4. Measurement of Serum ALT and AST
3.5. Measurement of SOD and MDA in Liver Homogenate
3.6. Histopathological Evaluation
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Xu, G.S.; Liu, H.N.; Li, J.; Wu, X.L.; Dai, X.M.; Liu, Y.H. Hepatic injury induced by carbon dioxide pneumoperitoneum in experimental rats. World J. Gastroenterol. 2009, 15, 3060–3064. [Google Scholar] [CrossRef]
- Bleibel, W.; Kim, S.; D’Silva, K.; Lemmer, E.R. Drug-induced liver injury: Review article. Dig. Dis. Sci. 2007, 52, 2463–2471. [Google Scholar]
- Benjamin, S.B.; Ishak, K.G.; Zimmerman, H.J.; Grushka, A. Phenylbutazone liver injury: A clinical-pathologic survey of 23 cases and review of the literature. Hepatology 1981, 1, 255–263. [Google Scholar] [CrossRef]
- Au, J.S.; Navarro, V.J.; Rossi, S. Review article: Drug-induced liver injury-Its pathophysiology and evolving diagnostic tools. Aliment. Pharmacol. Ther. 2011, 34, 11–20. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation. oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Ninomiya, K.; Shimoda, H.; Nishida, N.; Matsuda, H. Hepatoprotective and antioxidative properties of Salacia reticulata: Preventive effects of phenolic constituents on CCl4-induced liver injury in mice. Biol. Pharm. Bull. 2002, 25, 72–76. [Google Scholar] [CrossRef]
- Domitrovic, R.; Jakovac, H.; Blagojevic, G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology 2011, 280, 33–43. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.Z.; Ye, J.Z.; Li, H.T. Hepatoprotective effects of polyprenols from Ginkgo biloba L. leaves on CCl4-induced hepatotoxicity in rats. Fitoterapia 2011, 82, 834–840. [Google Scholar] [CrossRef]
- Yurt, B.; Celik, I. Hepatoprotective effect and antioxidant role of sun, sulphited-dried apricot (Prunus armeniaca L.) and its kernel against ethanol-induced oxidative stress in rats. Food Chem. Toxicol. 2011, 49, 508–513. [Google Scholar] [CrossRef]
- Lin, H.J.; Chen, J.Y.; Lin, C.F.; Kao, S.T.; Cheng, J.C.; Chen, H.L.; Chen, C.M. Hepatoprotective effects of Yi Guan Jian, an herbal medicine, in rats with dimethylnitrosamine-induced liver fibrosis. J. Ethnopharmacol. 2011, 134, 953–960. [Google Scholar] [CrossRef]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Hasegawa, H.; Murata, J.; Saiki, I. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol. Res. 1997, 9, 411–417. [Google Scholar]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar]
- Lee, I.K.; Kang, K.A.; Lim, C.M.; Kim, K.C.; Kim, H.S.; Kim, D.H.; Kim, B.J.; Chang, W.Y.; Choi, J.H.; Hyun, J.W. Compound K, a metabolite of ginseng saponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. Int. J. Mol. Sci. 2010, 11, 4916–4931. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, M.W.; Yuan, H.D.; Lee, H.J.; Kim, S.H.; Chung, S.H. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J. Agric. Food Chem. 2009, 57, 10573–10578. [Google Scholar] [CrossRef]
- Joh, E.H.; Lee, I.A.; Jung, I.H.; Kim, D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation-The key step of inflammation. Biochem. Pharmacol. 2011, 82, 278–286. [Google Scholar]
- Yoon, S.H.; Han, E.J.; Sung, J.H.; Chung, S.H. Anti-diabetic effects of compound K versus metformin versus compound K-metformin combination therapy in diabetic db/db mice. Biol. Pharm. Bull. 2007, 30, 2196–2200. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yuan, H.D.; Chung, I.K.; Chung, S.H. Compound K, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in human hepatoma cells. J. Agric. Food Chem. 2009, 57, 1532–1537. [Google Scholar]
- Han, B.H.; Park, M.H.; Han, Y.N.; Woo, L.K.; Sankawa, U.; Yahara, S.; Tanaka, O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982, 44, 146–149. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Choo, M.K.; Park, S.Y.; Kim, D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol. Pharm. Bull. 2002, 25, 58–63. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.M.; Sung, B.H.; An, D.S.; Lee, H.G.; Kim, A.G.; Kim, S.C.; Lee, S.T.; Lm, W.T. Bioconversion of ginsenosides Rb1, Rb2, Rc and Rd by novel β-glucosidase hydrolyzing outer 3-O glycoside from Sphingomonas sp. 2F2. Cloning, expression, and enzyme characterization. J. Biotechnol. 2011, 156, 125–133. [Google Scholar]
- You, J.Y.; Peng, C.; Liu, X.; Ji, X.J.; Lu, J.; Tong, Q.; Wei, P.; Cong, L.; Li, Z.; Huang, H. Enzymatic hydrolysis and extraction of arachidonic acid rich lipids from Mortierella alpina. Bioresour. Technol. 2011, 102, 6088–6094. [Google Scholar]
- Liu, X.; Cui, Y.; Yang, L.; Yang, S.L. Purification of a ginsenoside-Rb1 hydrolase from Helix snailase. Sheng Wu Gong Cheng Xue Bao 2005, 21, 929–933. [Google Scholar]
- Park, E.J.; Zhao, Y.Z.; Kim, J.; Sohn, D.H. A ginsenoside metabolite, 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol, triggers apoptosis in activated rat hepatic stellate cells via caspase-3 activation. Planta Med. 2006, 72, 1250–1253. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.Z.; Liu, W.C.; Kimura, Y.; Zheng, Y.N. Anti-obesity effects of protopanaxdiol types of ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high-fat diet. Fitoterapia 2010, 81, 1079–1087. [Google Scholar] [CrossRef]
- Sigala, F.; Kostopanagiotou, G.; Andreadou, I.; Kavatzas, N.; Felekouras, E.; Sigalas, P.; Bastounis, E.; Papalambros, E. Histological and lipid peroxidation changes after administration of 2-acetylaminofluorene in a rat liver injury model following selective periportal and pericentral damage. Toxicology 2004, 196, 155–163. [Google Scholar] [CrossRef]
- Pesh-Imam, M.; Recknagel, R.O. Lipid peroxidation and the concept of antioxygenic potential: Vitamin E changes in acute experimental CCl4-BrCCl3- and ethanol-induced liver injury. Toxicol. Appl. Pharmacol. 1977, 42, 463–475. [Google Scholar] [CrossRef]
- Quan, L.H.; Cheng, L.Q.; Kim, H.B.; Kim, J.H.; Son, N.R.; Kim, S.Y.; Jin, H.O.; Yang, D.C. Bioconversion of Ginsenoside Rd into Compound K by Lactobacillus pentosus DC101 Isolated from Kimchi. J. Ginseng Res. 2010, 34, 288–295. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Zhang, M.; Zheng, Y.-N.; Li, J.; Wang, Y.-P.; Wang, Y.-J.; Gu, J.; Jin, Y.; Wang, H.; Chen, L. Snailase Preparation of Ginsenoside M1 from Protopanaxadiol-Type Ginsenoside and Their Protective Effects Against CCl4-Induced Chronic Hepatotoxicity in Mice. Molecules 2011, 16, 10093-10103. https://doi.org/10.3390/molecules161210093
Li W, Zhang M, Zheng Y-N, Li J, Wang Y-P, Wang Y-J, Gu J, Jin Y, Wang H, Chen L. Snailase Preparation of Ginsenoside M1 from Protopanaxadiol-Type Ginsenoside and Their Protective Effects Against CCl4-Induced Chronic Hepatotoxicity in Mice. Molecules. 2011; 16(12):10093-10103. https://doi.org/10.3390/molecules161210093
Chicago/Turabian StyleLi, Wei, Ming Zhang, Yi-Nan Zheng, Jing Li, Ying-Ping Wang, Yun-Jing Wang, Jian Gu, Ying Jin, Hui Wang, and Li Chen. 2011. "Snailase Preparation of Ginsenoside M1 from Protopanaxadiol-Type Ginsenoside and Their Protective Effects Against CCl4-Induced Chronic Hepatotoxicity in Mice" Molecules 16, no. 12: 10093-10103. https://doi.org/10.3390/molecules161210093
APA StyleLi, W., Zhang, M., Zheng, Y.-N., Li, J., Wang, Y.-P., Wang, Y.-J., Gu, J., Jin, Y., Wang, H., & Chen, L. (2011). Snailase Preparation of Ginsenoside M1 from Protopanaxadiol-Type Ginsenoside and Their Protective Effects Against CCl4-Induced Chronic Hepatotoxicity in Mice. Molecules, 16(12), 10093-10103. https://doi.org/10.3390/molecules161210093




