Abstract
Endohedral metallofullerene glycoconjugates were synthesized under mild conditions by carbene addition using appropriate glycosylidene-derived diazirine with La2@Ih-C80. NMR spectroscopic studies revealed that the glycoconjugate consists of two diastereomers of [6,6]-open mono-adducts. The electronic properties were characterized using Vis/NIR absorption spectroscopy and electrochemical measurements. This study demonstrates that glycosylidene carbene is useful to incorporate carbohydrate moieties onto endohedral metallofullerene surfaces.
1. Introduction
Recent developments in the chemistry of endohedral metallofullerenes (EMFs) [1,2,3,4] have sparked increasing interest in their biochemical and medicinal applications. Particularly, great interest has been directed toward development of magnetic resonance imaging (MRI) contrast and therapeutic agents based on EMF scaffolds [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Robust fullerene cages protect encaged metal ions from any potential metabolic process, therefore, EMFs can act as nanocarriers with no release of toxic metal ions. In this context, chemical derivatization of EMFs to introduce functions such as solubility, permeability, and site-specific recognition ability is indispensable. To date, however, exohedral chemical functionalization of EMFs has remained limited to introduction of groups that do not introduce additional features because of the different reactivity from that of C60 [19].
We explored the reactivity of EMFs and found that reactions of EMFs with electrophilic carbenes proceed smoothly to afford the formation of corresponding EMF derivatives quantitatively [20,21,22,23]. These results encouraged us to synthesize functionalized EMF conjugates by such carbene addition. A carbohydrate moiety was selected as a functional group for this study because carbohydrate–protein interactions are encountered in many biological events. In addition, deprotection of the carbohydrate residues could potentially generate ambiphilic EMFs, leading to biochemical and pharmacological investigations [24,25,26,27,28,29,30,31,32,33]. This report describes the synthesis of endohedral metallofullerene glycoconjugates by carbene addition for the first time.
2. Results and Discussion
We adopted La2@Ih-C80 as a representative EMF scaffold because: (1) La2@Ih-C80 has icosahedral symmetry, which enables reduction of the number of possible isomers of the adducts; (2) its diamagnetic character enables characterization of the molecular structure using NMR spectroscopy; and (3) among lanthanum EMFs La2@Ih-C80 is obtainable in the second highest yield by direct-current arc-discharge process, whereas La@C2v-C82 is the main product.
Glycosylidene-derived diazirine 1 was synthesized according to reports in the literature by Vasella et al., as summarized in Scheme 1 [34,35].
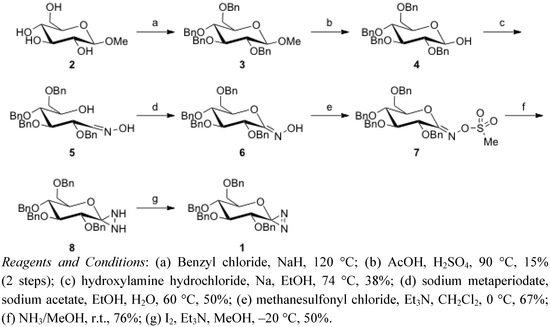
Scheme 1.
Synthesis of glycosylidene diazirine 1.
Scheme 1.
Synthesis of glycosylidene diazirine 1.
Reaction of commercially available methyl-α-D-glucopyranoside 2 with benzyl chloride in the presence of sodium hydride yielded O-benzyl derivative 3 [36]. The pyranoside anomeric hydroxyl group was deprotected with sulfuric acid to give 2,3,4,6-tetra-O-benzyl-D-glucopyranose (4) [37]. This product was condensed with hydroxylamine hydrochloride in the presence of sodium to provide open-chain oxime5 as a mixture of stereoisomers [38]. Oxidative cyclization of 5 with sodium metaperiodate provided the desired ring-closed material 6. Treating hydroximinolactone 6 with methanesulfonyl chloride under basic conditions yielded the corresponding methanesulfonate 7. Reaction of 7 with ammonia yielded diaziridine 8, which was subsequently oxidized by iodine to afford diazirine 1 [34,35].
Endohedral metallofullerene glycoconjugate was synthesized by the reaction of La2@Ih-C80 with 1, as shown in Scheme 2.
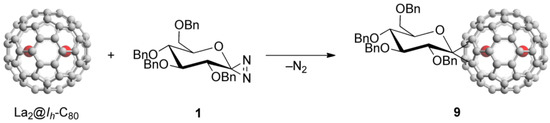
Scheme 2.
Reaction of La2@Ih-C80 with diazirine 1.
Scheme 2.
Reaction of La2@Ih-C80 with diazirine 1.
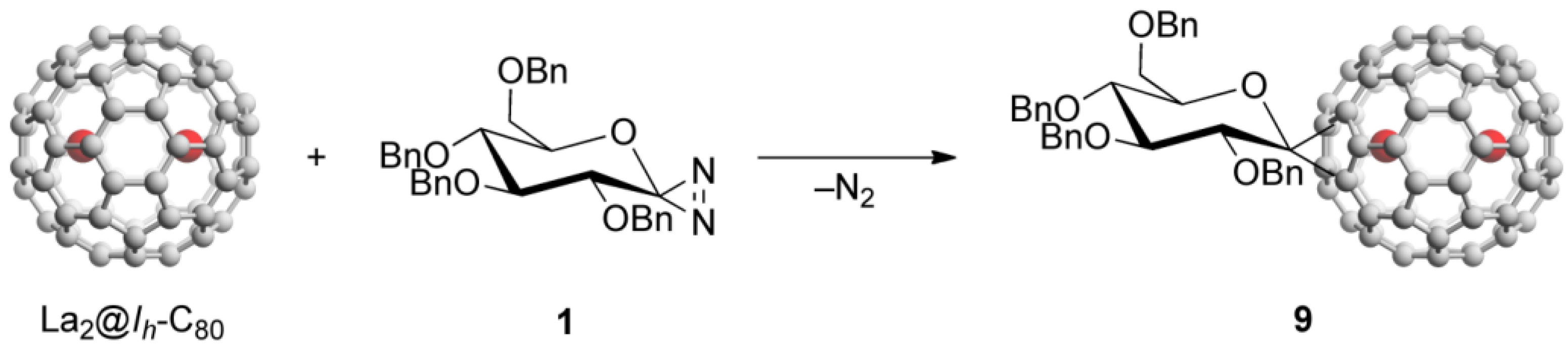
Compound 1 easily generates the corresponding glycosilydene carbene at room temperature, which is allowed to react smoothly with La2@Ih-C80 to afford the formation of La2@Ih-C80 glycoconjugate 9. The HPLC analysis of the reaction mixture suggested that 9 was formed predominantly. The mixture was subjected to HPLC separation to purify 9. As shown in Figure 1(a), the HPLC profiles of the purified 9 using different columns exhibited single peaks.
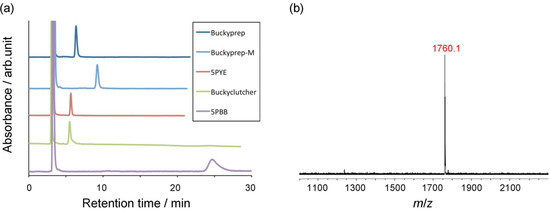
Figure 1.
(a) HPLC traces of purified 9. Conditions: 4.6 mm × 250 mm i.d. columns; eluent, toluene 1.0 mL/min; (b) Negative-mode MALDI-TOF mass spectrum of 9. 9-Nitroanthracene was used as matrix.
Figure 1.
(a) HPLC traces of purified 9. Conditions: 4.6 mm × 250 mm i.d. columns; eluent, toluene 1.0 mL/min; (b) Negative-mode MALDI-TOF mass spectrum of 9. 9-Nitroanthracene was used as matrix.
The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum of 9 clearly displayed the expected molecular ion peak at m/z 1760.1 (calcd. for C114H34O5La2: 1760.05), as shown in Figure 2(b). In addition, circular dichroism (CD) bands were observed at 390–550 nm, confirming that the chiral glucopyranose moiety was introduced successfully onto the EMF surface (see Figure S1 in the Supporting Information). The solubility of 9 in common organic solvents is higher than that of La2@Ih-C80(Ad) (Ad = adamantylidene), presumably because of the introduction of polarity with the sugar-like structure.
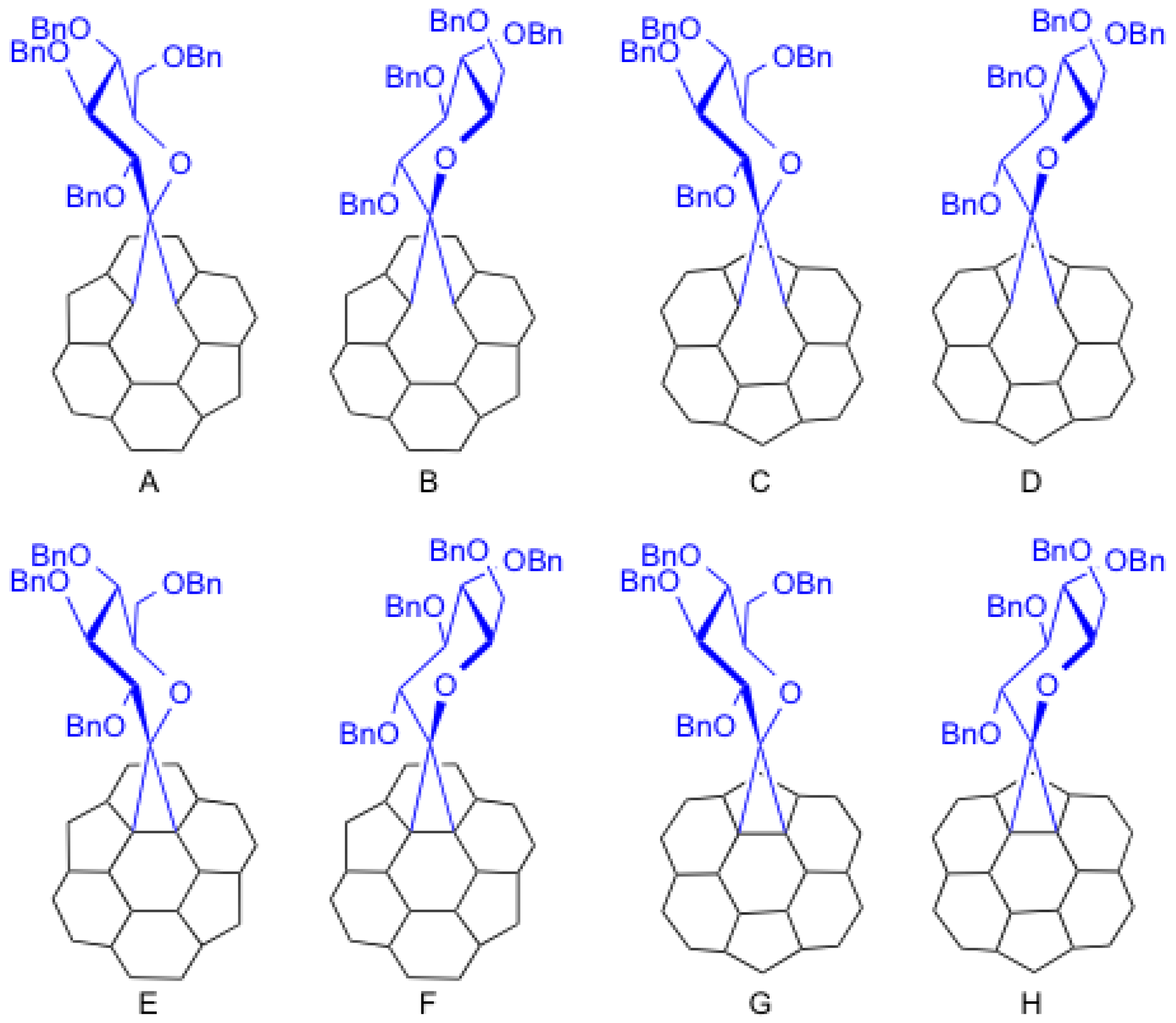
Theoretically, eight possible isomers (A–H) exist for conjugate 9, as shown in Figure 2. All isomers have C1 symmetry. In isomers A, B, E, and F, the addition took place at a C–C bond that bisects two hexagonal rings (so-called [6,6]-addition). In C, D, G, and H, the addition took place at a C–C bond that bisects hexagonal and pentagonal rings (so-called [5,6]-addition). In addition, the C–C bond was cleaved by the addition in isomers A–D (so-called open form). The addition yielded a cyclopropane ring on the cage in isomers E–F (so-called closed form).
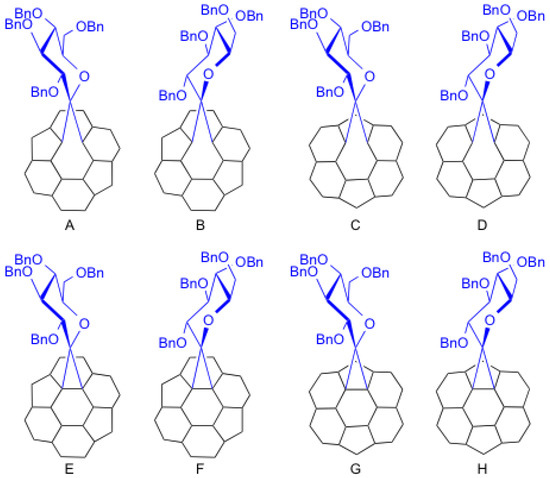
Figure 2.
Partial structures of the eight possible isomers A–H.
Figure 2.
Partial structures of the eight possible isomers A–H.
NMR spectroscopic studies revealed that 9 contains two inseparable diastereomers in a ratio of ca. 1:1 because two sets of signals were observed in the 1H- and 13C-NMR spectra although a single signal was observed in the 139La-NMR spectrum (see Figures S2,3 in the Supporting Information). In fact, 117 quaternary carbon signals appeared in the 13C-NMR spectrum, which are associated with the sp2 cage carbon atoms and benzene rings. In addition, two 13C signals at 91.09 and 89.51 ppm are attributed to spiro carbon atoms (designated as C3 and C3′) on the glycosilydene moiety, indicating the presence of two isomers. The 13C signals of the cage carbon atoms bonded to the glycosilydene moiety (designated as C1 and C1’) appeared at 104.16 and 104.11 ppm. In fact, the two signals are correlated with the axial proton atoms (designated as H4 and H4′) on the glycosilydene ring in the HMBC NMR spectrum as shown in Figure 3. Observations also indicate that the diastereomers possess not closed forms but open forms because C1 and C1’ carbon atoms can be regarded as sp2-carbon atoms. In contrast, correlation between H4 and the other carbon atoms designated as C2 (or C2′) at 117.16 and 115.75 ppm in Figure 3, was not observed.
The absence of the cross peaks is reasonable because of the fact that the dihedral angle between H4 and C2 is close to 90°, leading to the coupling constant of zero based on Karplus equation [39,40,41]. It is noteworthy that the chemical shifts of the bonded cage carbons (C1 and C2, or C1′ and C2′) of 9 closely resemble those of the bonded cage carbons of La2@Ih-C80(Ad) having [6,6]-open form [21]. Therefore, we concluded that the two diastereomers of 9 are associated with isomers A and B. Positive evidence of the possession of the [6,6]-open form is also provided by the similarity in the absorption spectra of 9 and La2@Ih-C80(Ad). As shown in Figure 4, the absorption spectrum of 9 resembles those of La2@Ih-C80 and La2@Ih-C80(Ad), demonstrating that the intrinsic electronic structure of La2@Ih-C80 is only slightly altered by the carbene addition.
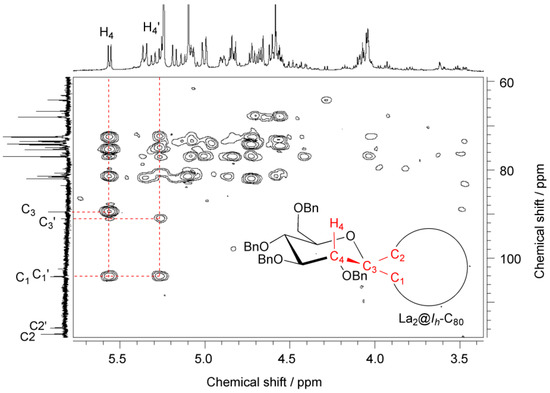
Figure 3.
500 MHz HMBC NMR spectrum of 9 in CD2Cl2/CS2 (v/v 1:3) at 303 K. Inset shows the schematic structure of 9.
Figure 3.
500 MHz HMBC NMR spectrum of 9 in CD2Cl2/CS2 (v/v 1:3) at 303 K. Inset shows the schematic structure of 9.
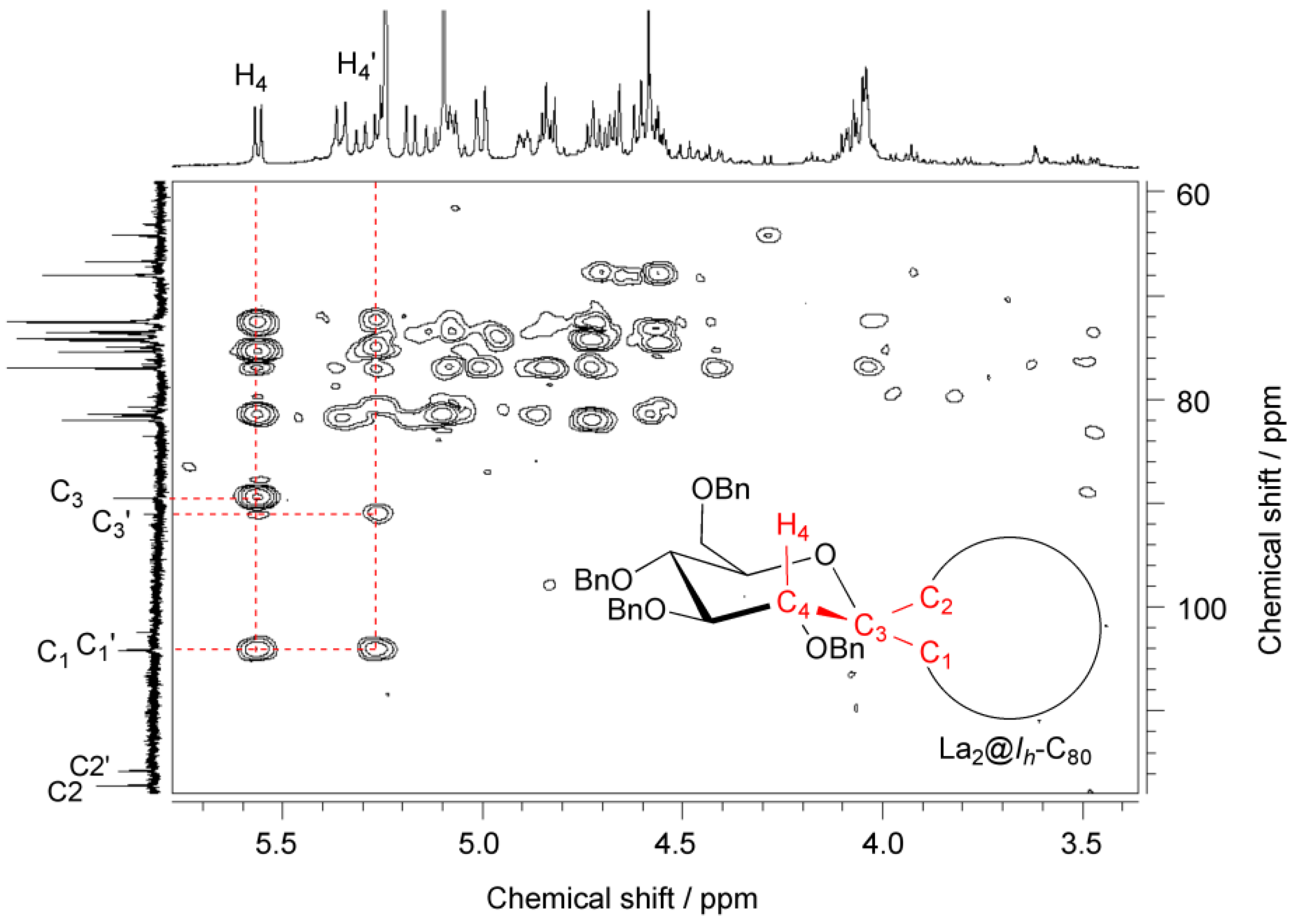
Figure 4.
Vis/NIR absorption spectra of 9 (red), La2@Ih-C80(Ad) (green), and La2@Ih-C80 (blue) in CS2.
Figure 4.
Vis/NIR absorption spectra of 9 (red), La2@Ih-C80(Ad) (green), and La2@Ih-C80 (blue) in CS2.
To characterize the electrochemical properties, cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were performed as shown in Figure S4 in the Supporting Information. It is reasonable to consider that the two diastereomers of 9 have identical redox potentials because the stereochemistry does not affect the electronic structure of La2@Ih-C80[42]. Therefore, we assume that the waves of two diastereomers are entirely overlapped. As presented in Table 1, the first reduction potential of 9 is only shifted cathodically to 40 mV as compared to pristine La2@Ih-C80. This trend is similar to the electrochemical behavior of La2@Ih-C80(Ad) [21]. Results indicate that introduction of a glucopyranose moiety decreases the electron-accepting property because of the inductive effect. However, other reduction and oxidation waves were not identified because 9 was decomposed gradually during electrochemical measurements. Separation of the two diastereomers and deprotection of the glucopyranose moieties are currently under investigation.

Table 1.
Redox potentials of La2@Ih-C80 and its derivatives a.
| compound | oxE1 | redE1 |
|---|---|---|
| 9 | –0.35 | |
| La2@Ih-C80(Ad) b | 0.49 | –0.36 |
| La2@Ih-C80c | 0.56 | –0.31 |
a Values are in volts relative to Fc/Fc+ couple and obtained by DPV. b Data from ref. [21]. c Data from ref. [42].
3. Experimental
3.1. General
Toluene was distilled over benzophenone sodium ketyl under an argon atmosphere before use for the reactions. 1,2-Dichlorobenzene (ODCB) was distilled over P2O5 under vacuum before use. CS2 was distilled over P2O5 under an argon atmosphere before use. High-performance liquid chromatography (HPLC) isolation was performed using a recycling preparative HPLC system (LC-908; Japan Analytical Industry Co., Ltd.) and monitored by ultraviolet (UV) absorption at 330 nm. Toluene was used as the eluent. Mass spectrometry (Biflex III; Bruker Analytik GmbH) was performed with 9-nitroanthracene as matrix. The Vis/NIR absorption spectra were measured in a CS2 solution using a spectrophotometer (UV-3150; Shimadzu Corp.). Circular dichroism (CD) spectra were recorded on a spectropolarimeter (J-720W; Jasco Corp.). CD: scanning mode, continuous; scanning speed, 200 nm min−1; response, 2.0 s; bandwidth, 1.0 nm. Cyclic voltammograms (CVs) and differential pulse voltammograms (DPVs) were recorded on a BAS CV50W electrochemical analyzer. Platinum wires were used, respectively, as the working electrode and the counter electrode. The reference electrode was a saturated calomel reference electrode (SCE) filled with 0.1 M (nBu)4NPF6 in ODCB. All potentials were referenced to the ferrocene/ferrocenium couple (Fc/Fc+) as the standard. CV: scan rate, 20 mV s−1. DPV: pulse amplitude, 50 mV; pulse width, 50 ms; pulse period, 200 ms; scan rate, 20 mV s−1. NMR spectra were obtained using an AVANCE-300 or AVANCE-500 spectrometer (Bruker Analytik GmbH) with a CryoProbe system (Bruker Analytik GmbH).
3.2. Preparation of La2@Ih-C80 Glycoconjugate (9)
To a solution of 1.0 mg (8.1 × 10−4 mmol) of La2@Ih-C80 in 20 mL of toluene was added 4.4 mg (8.0 × 10−3 mmol) of 8 at 0 °C followed by consecutive freeze–pump–thaw cycles. The mixture was stirred at room temperature for 1 h. The yield of 9 was estimated as 62% based on consumption of La2@Ih-C80. The solvent was removed under vacuum, and the residue was purified by HPLC using a Buckyprep column to give glycoconjugate 9 as a dark brown solid: 1H-NMR (500 MHz, CD2Cl2/CS2 1:3, 303 K) δ 7.4–7.0 (m), 5.57 (d, 7.7 Hz), 5.36 (d, 10.5 Hz), 5.28 (d, 10.5 Hz), 5.26 (d, 7.7 Hz), 5.17 (d, 10.5 Hz), 5.13 (d, 10.5 Hz), 5.05 (d, 7.7 Hz), 5.00 (d, 10.5 Hz), 4.9–4.8 (m), 4.7–4.5 (m), 4.1–4.0 (m) ppm; 13C-NMR (125 MHz, CD2Cl2/CS2 1:3, 303 K) δ 152.84 (q), 152.83 (q), 152.71 (q), 152.66 (q), 150.34 (q), 150.32 (q), 150.29 (q), 150.23 (q), 150.11 (q), 150.01 (q), 149.98 (q), 149.95 (q), 149.45 (q), 149.39 (q), 149.30 (q), 149.26 (q), 149.23 (q), 148.69 (q), 148.67 (q), 148.61 (q), 148.36 (q), 148.23 (q), 148.15 (q), 148.03 (q), 146.97 (q), 146.84 (q), 146.42 (q), 146.32 (q), 146.28 (q), 145.34 (q), 145.32 (q), 145.29 (q), 144.52 (q), 144.33 (q), 144.23 (q), 144.19 (q), 144.14 (q), 144.11 (q), 143.11 (q), 143.03 (q), 142.98 (q), 142.97 (q), 142.91 (q), 142.83 (q), 142.73 (q), 142.61 (q), 142.58 (q), 142.56 (q), 141.99 (q), 141.94 (q), 141.90 (q), 141.87 (q), 141.85 (q), 140.12 (q), 139.98 (q), 139.92 (q), 139.86 (q), 139.59 (q), 139.53 (q), 139.42 (q), 139.37 (q), 139.24 (q), 139.21 (q), 136.69 (q), 136.50 (q), 136.47 (q), 136.44 (q), 136.39 (q), 136.35 (q), 136.34 (q), 136.23 (q), 136.21 (q), 136.20 (q), 136.18 (q), 136.07 (q), 135.65 (q), 135.40 (q), 134.73 (q), 134.31 (q), 134.25 (q), 134.13 (q), 134.05 (q), 133.71 (q), 133.70 (q), 133.39 (q), 133.25 (q), 133.21 (q), 133.20 (q), 133.19 (q), 133.15 (q), 133.13 (q), 132.00 (q), 131.00 (q), 130.89 (q), 130.87 (q), 130.84 (q), 130.68 (q), 130.61 (q), 130.44 (q), 130.12 (q), 130.05 (q), 130.04 (q), 129.98 (q), 129.85 (q), 127.7–126.6 (Ph), 125.66 (q), 125.57 (q), 125.31 (q), 125.26 (q), 124.81 (q), 123.38 (q), 122.01 (q), 119.87 (q), 119.83 (q), 117.16 (q; C2), 115.75 (q; C2′), 104.16 (q; C1), 104.11 (q; C1′), 91.09 (q; C3′), 89.51 (q; C3), 82.01 (CH), 81.62 (CH), 81.43 (CH), 80.73 (CH), 77.02 (CH), 75.40 (CH), 75.01 (CH), 74.43 (CH2), 74.23 (CH2), 74.17 (CH2), 73.61 (CH2), 73.45 (CH2), 72.63 (CH2), 72.57 (CH2), 72.52 (CH2), 68.09 (CH2), 68.03 (CH2) ppm (q = quaternary carbon signal); 139La-NMR (84.8 MHz, CD2Cl2/CS2 1:3, 290 K) δ –360.2 ppm; MALDI-TOF MS (negative mode) calcd. for C114H34O5La2 [M] 1760.05, found [M–] 1760.1.
4. Conclusions
The results of this study demonstrate clearly that addition of electrophilic carbene is a powerful means to functionalize EMFs. The glycosilydene carbene generated in-situ from the corresponding diazirine precursor is highly reactive toward La2@Ih-C80 at room temperature to afford two inseparable diastereomers of the mono-adducts, which are the first example of EMF glycoconjugates. We believe that this work paves the way for development of functionalized EMFs for biological and pharmacological applications.
Supplementary Materials
Supplementary materials can be accessed on: http://www.mdpi.com/1420-3049/16/11/9495/s1.
Acknowledgements
We thank Tatsuya Nabeshima (University of Tsukuba) for CD measurements. This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas (No. 20108001, “pi-Space”), a Grant-in-Aid for Scientific Research (A) (No. 20245006), a Grant-in-Aid for Young Scientists (B) (No. 22750030), The Next Generation Super Computing Project (Nanoscience Project), Nanotechnology Support Project, Grants-in-Aid for Scientific Research on Priority Area (Nos. 20036008, 20038007), and Specially Promoted Research (No. 22000009) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, The Strategic Japanese-Spanish Cooperative Program funded by JST and MICINN.
References and Notes
- Akasaka, T.; Nagase, S. Endofullerenes: A New Family of Carbon Clusters; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef]
- Akasaka, T.; Wudl, F.; Nagase, S. Chemistry of Nanocarbons; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Yamada, M.; Tsuchiya, T.; Akasaka, T.; Nagase, S. In-depth understanding of π-electron systems: New vistas in fullerene endohedrals. Pure Appl. Chem. 2010, 82, 757–767. [Google Scholar] [CrossRef]
- Mikawa, M.; Kato, H.; Okumura, M.; Narazaki, M.; Kanazawa, Y.; Miwa, N.; Shinohara, H. Paramagnetic water soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjugate Chem. 2001, 12, 510–514. [Google Scholar] [CrossRef]
- Bolskar, R.D.; Benedetto, A.F.; Husebo, L.O.; Price, R.E.; Jackson, E.F.; Wallace, S.; Wilson, L.J.; Alford, J.M. First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J. Am. Chem. Soc. 2003, 125, 5471–5478. [Google Scholar]
- Kato, H.; Kanazawa, Y.; Okumura, M.; Taninaka, A.; Yokawa, T.; Shinohara, H. Lanthanoid endohedral metallofullerenols for MRI contrast agents. J. Am. Chem. Soc. 2003, 125, 4391–4397. [Google Scholar] [CrossRef]
- Sitharaman, B.; Bolskar, R.D.; Rusakova, I.; Wilson, L.J. Gd@C60[C(COOH)2]10 and Gd@C60(OH)x: Nanoscale aggregation studies of two metallofullerene MRI contrast agents in aqueous solution. Nano Lett. 2004, 4, 2373–2378. [Google Scholar] [CrossRef]
- Toth, E.; Bolskar, R.D.; Borel, A.; Gonzalez, G.; Helm, L.; Merbach, A.E.; Sitharaman, B.; Wilson, L.J. Water soluble gadofullerenes: Toward high-relaxivity, pH-responsive MRI contrast agents. J. Am. Chem. Soc. 2005, 127, 799–805. [Google Scholar]
- Shu, C.Y.; Zhang, E.Y.; Xiang, J.F.; Zhu, C.F.; Wang, C.R.; Pei, X.L.; Han, H.B. Aggregation studies of the water-soluble gadofullerene magnetic resonance imaging contrast agent: [Gd@C82O6(OH)16(NHCH2CH2COOH)8]x. J. Phys. Chem. B 2006, 110, 15597–15601. [Google Scholar]
- Shu, C.Y.; Gan, L.H.; Wang, C.R.; Pei, X.L.; Han, H.B. Synthesis and characterization of a new water soluble endohedral metallofullerene for MRI contrast agents. Carbon 2006, 44, 496–500. [Google Scholar] [CrossRef]
- Fatouros, P.P.; Corwin, F.D.; Chen, Z.-J.; Broaddus, W.C.; Tatum, J.L.; Kettenmann, B.; Ge, Z.; Gibson, H.W.; Russ, J.L.; Leonard, A.P.; Duchamp, J.C.; Dorn, H.C. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology 2006, 240, 756–764. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Shu, C.Y.; Feng, L.; Wang, C.R. Preparation and characterization of two new water-soluble endohedral metallofullerenes as magnetic resonance imaging contrast agents. J. Phys. Chem. B 2007, 111, 14223–14226. [Google Scholar] [CrossRef]
- MacFarland, D.K.; Walker, K.L.; Lenk, R.P.; Wilson, S.R.; Kumar, K.; Kepley, C.L.; Garbow, J.R. Hydrochalarones: A novel endohedral metallofullerene platform for enhancing magnetic resonance imaging contrast. J. Med. Chem. 2008, 51, 3681–3683. [Google Scholar]
- Shu, C.Y.; Wang, C.R.; Zhang, J.F.; Gibson, H.W.; Dorn, H.C.; Corwin, F.D.; Fatouros, P.P.; Dennis, T.J.S. Organophosphonate functionalized Gd@C82 as a magnetic resonance imaging contrast agent. Chem. Mater. 2008, 20, 2106–2109. [Google Scholar] [CrossRef]
- Shu, C.Y.; Ma, X.Y.; Zhang, J.F.; Corwin, F.D.; Sim, J.H.; Zhang, E.Y.; Dorn, H.C.; Gibson, H.W.; Fatouros, P.P.; Wang, C.R.; Fang, X.H. Conjugation of a water-soluble gadolinium endohedral fulleride with an antibody as a magnetic resonance imaging contrast agent. Bioconjugate Chem. 2008, 19, 651–655. [Google Scholar] [CrossRef]
- Shu, C.Y.; Corwin, F.D.; Zhang, J.F.; Chen, Z.J.; Reid, J.E.; Sun, M.H.; Xu, W.; Sim, J.H.; Wang, C.R.; Fatouros, P.P.; Esker, A.R.; Gibson, H.W.; Dorn, H.C. Facile preparation of a new gadofullerene-based magnetic resonance imaging contrast agent with high 1H relaxivity. Bioconjugate Chem. 2009, 20, 1186–1193. [Google Scholar] [CrossRef]
- Zhang, J.; Fatouros, P.P.; Shu, C.; Reid, J.; Owens, L.S.; Cai, T.; Gibson, H.W.; Long, G.L.; Corwin, F.D.; Chen, Z.-J.; Dorn, H.C. High relaxivity trimetallic nitride (Gd3N) metallofullerene MRI contrast agents with optimized functionality. Bioconjugate Chem. 2010, 21, 610–615. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef]
- Maeda, Y.; Matsunaga, Y.; Wakahara, T.; Takahashi, S.; Tsuchiya, T.; Ishitsuka, M.O.; Hasegawa, T.; Akasaka, T.; Liu, M.T.H.; Kokura, K.; et al. Isolation and characterization of a carbene derivative of La@C82. J. Am. Chem. Soc. 2004, 126, 6858–6859. [Google Scholar]
- Yamada, M.; Someya, C.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Yoza, K.; Horn, E.; Liu, M.T.H.; Mizorogi, N.; Nagase, S. Metal atoms collinear with the spiro carbon of 6,6-open adducts, M2@C80(Ad) (M = La and Ce, Ad = adamantylidene). J. Am. Chem. Soc. 2008, 130, 1171–1176. [Google Scholar]
- Ishitsuka, M.O.; Enoki, H.; Tsuchiya, T.; Slanina, Z.; Mizorogi, N.; Nagase, S.; Liu, M.T.H.; Akasaka, T. Chemical modification of endohedral metallofullerene La@C82 with 3-chloro-3-phenyldiazirine. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1124–1130. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Sano, S.; Enoki, H.; Sato, S.; Nikawa, H.; Tsuchiya, T.; Slanina, Z.; Mizorogi, N.; Liu, M.T.H.; Akasaka, T.; Nagase, S. Regioselective bis-functionalization of endohedral dimetallofullerene, La2@C80: External La-La distance. J. Am. Chem. Soc. 2011, 133, 7128–7134. [Google Scholar]
- Vasella, A.; Uhlmann, P.; Waldraff, C.A.A.; Diederich, F.; Thilgen, C. Fullerene sugars: Preparation of enantiomerically pure, spiro-linked C-glycosides of C60. Angew. Chem. Int. Ed. Engl. 1992, 31, 1388–1390. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Ivanov, V.G.; Mokeev, M.V.; Zgonnik, V.N. Water-soluble [60]fullerene compositions with carbohydrates. Mendeleev Commun. 2001, 11, 193–194. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Synthesis of [60]fulleropyrrolidine glycoconjugates using 1,3-dipolar cycloaddition with C-glycosyl azomethine ylides. Tetrahedron Lett. 2002, 43, 1649–1652. [Google Scholar] [CrossRef]
- Mikata, Y.; Takagi, S.; Tanahashi, M.; Ishii, S.; Obata, M.; Miyamoto, Y.; Wakita, K.; Nishisaka, T.; Hirano, T.; Ito, T.; Hoshino, M.; Ohtsuki, C.; Tanihara, M.; Yano, S. Detection of 1270 nm emission from singlet oxygen and photocytotoxic property of sugar-pendant [60] fullerenes. Bioorg. Med. Chem. Lett. 2003, 13, 3289–3292. [Google Scholar]
- Enes, R.F.; Tome, A.C.; Cavaleiro, J.A.S.; El-Agamey, A.; McGarvey, D.J. Synthesis and solvent dependence of the photophysical properties of [60]fullerene-sugar conjugates. Tetrahedron 2005, 61, 11873–11881. [Google Scholar] [CrossRef]
- Isobe, H.; Cho, K.; Solin, N.; Werz, D.B.; Seeberger, P.H.; Nakamura, E. Synthesis of fullerene glycoconjugates via a copper-catalyzed Huisgen cycloaddition reaction. Org. Lett. 2007, 9, 4611–4614. [Google Scholar]
- Tanimoto, S.; Sakai, S.; Matsumura, S.; Takahashi, D.; Toshima, K. Target-selective photo-degradation of HIV-1 protease by a fullerene-sugar hybrid. Chem. Commun. 2008, 5767–5769. [Google Scholar]
- Ishida, Y.; Tanimoto, S.; Takahashi, D.; Toshima, K. Photo-degradation of amyloid ® by a designed fullerene–sugar hybrid. Med. Chem. Commun. 2010, 1, 212–215. [Google Scholar] [CrossRef]
- Sanchez-Navarro, M.; Munoz, A.; Illescas, B.M.; Rojo, J.; Martin, N. [60]Fullerene as multivalent scaffold: Efficient molecular recognition of globular glycofullerenes by Concanavalin A. Chem. Eur. J. 2011, 17, 766–799. [Google Scholar]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.-F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH—Towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar]
- Beer, D.; Vasella, A. 239. Aldonhydroximo-lactones. Preparation and determination of configuration. Helv. Chim. Acta 1985, 68, 2254–2274. [Google Scholar] [CrossRef]
- Briner, K.; Vasella, A. 153. Glycosylidene carbenes. A new approach to glycoside synthesis. Part 1. Preparation of glycosylidene-derived diaziridines and diazirines. Helv. Chim. Acta 1989, 72, 1371–1382. [Google Scholar] [CrossRef]
- Tate, M.E.; Bishop, C.T. Preparation, thin layer chromatography, and ultraviolet spectra of some O-benzyl derivatives of D-glucos. Can. J. Chem. 1963, 41, 1801–1806. [Google Scholar] [CrossRef]
- Koto, S.; Morishima, N.; Miyata, Y.; Zen, S. Preparation of 2,3,4,6-tetra-O-benzyl-D-mannose. Bull. Chem. Soc. Jpn. 1976, 49, 2639–2640. [Google Scholar] [CrossRef]
- Aebischer, B.M.; Hanssen, H.W.; Vasella, A.T.; Schweizer, W.B. Oxidation of aldose oximes. Formation and structure of hydroxydiazene oxide acetals and preparation of hydroximolactones. X-ray crystal structure of 2,3:5,6-di-O-isopropylidene-α-D-mannofuranosyl-ONN-azoxy 2,3:5,6-di-O-isopropylidene-α-D-mannofuranoside. J. Chem. Soc. Perkin Trans. 1 1982, 2139-2147. [Google Scholar]
- Karplus, M.; Anderson, D.H. Valence-bond interpretation of electron-coupled nuclear spin interactions; application to methane. J. Chem. Phys. 1959, 30, 6–10. [Google Scholar] [CrossRef]
- Karplus, M. Contact electron-spin coupling of nuclear magnetic moments. J. Chem. Phys. 1959, 30, 11–15. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Suzuki, T.; Maruyama, Y.; Kato, T.; Kikuchi, K.; Nakao, Y.; Achiba, Y.; Kobayashi, K.; Nagase, S. Electrochemistry and ab initio study of the dimetallofullerene La2@C80. Angew. Chem. Int. Ed. Engl. 1995, 34, 1094–1096. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
