Triazolobithiophene Light Absorbing Self-Assembled Monolayers: Synthesis and Mass Spectrometry Applications
Abstract
:1. Introduction
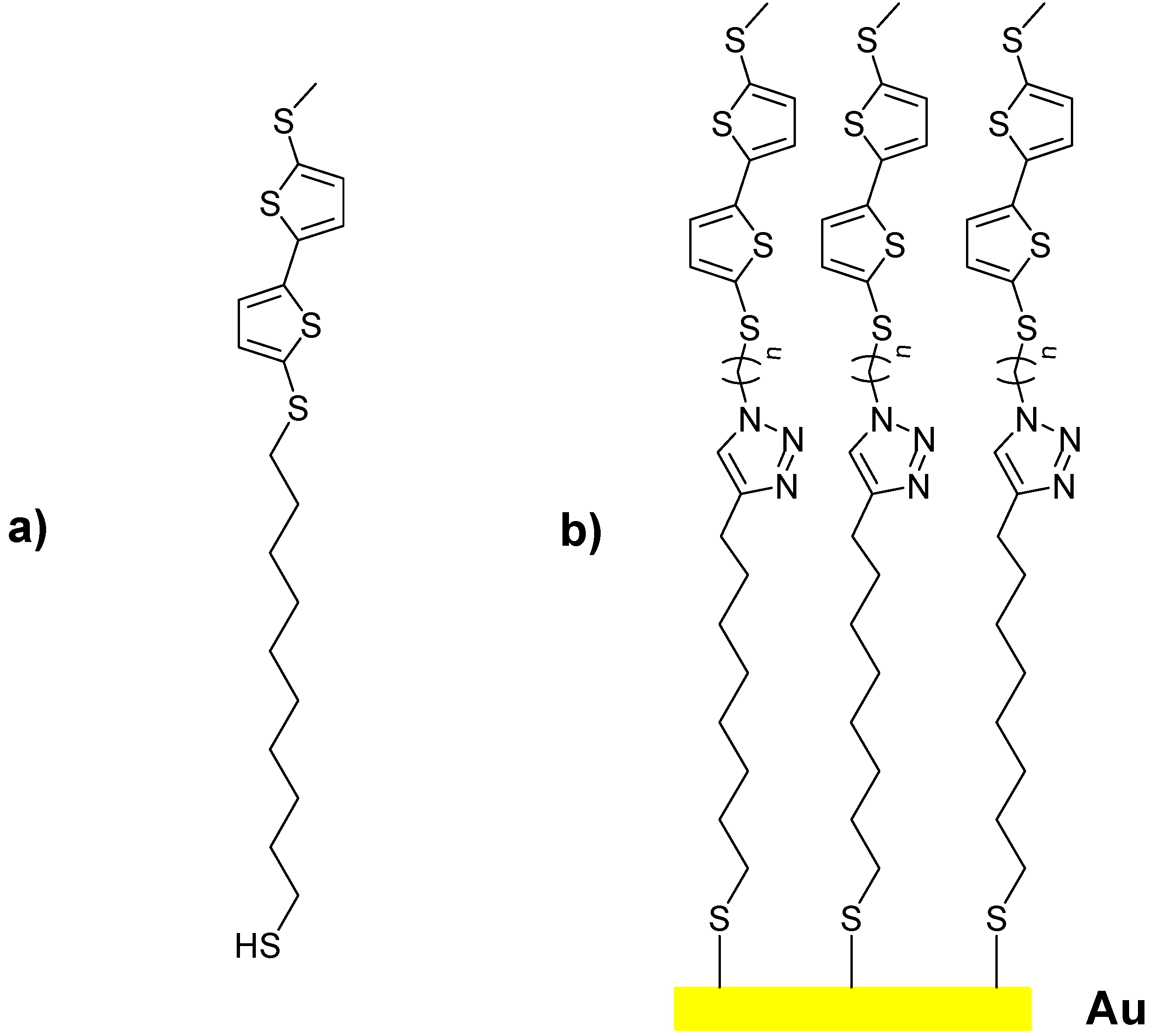
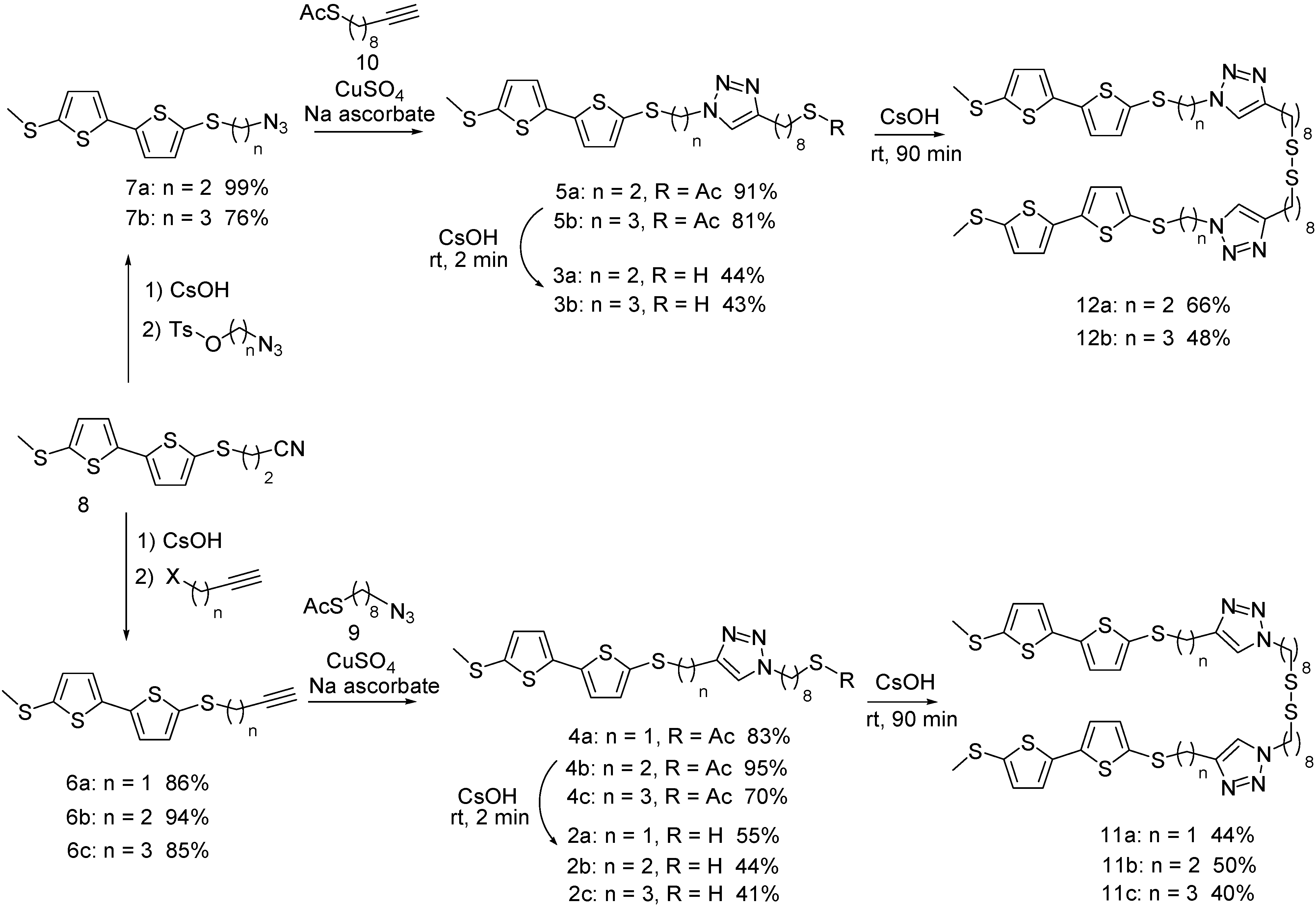
2. Results and Discussion
2.1. Formation of Free Thiols and Disulfides
2.2. Monolayer Formation and Properties
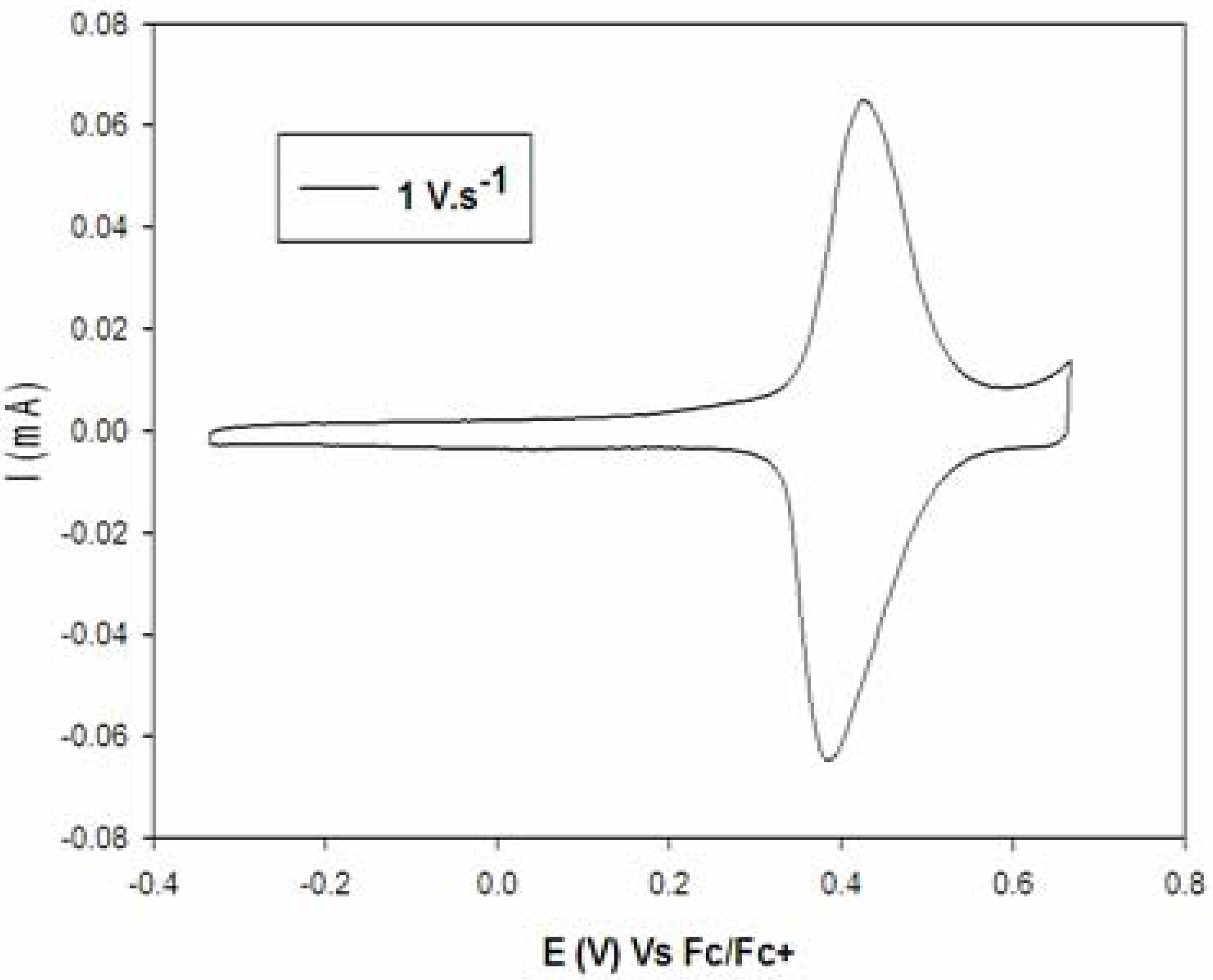
| Compounds | E1ox | Γ.10−10 |
|---|---|---|
| 11a | 0.52 | Not stable |
| 11b | 0.45 | 3.8 ± 0.1 |
| 11c | 0.41 | 3.6 ± 0.1 |
| 12a | 0.50 | 3.8 ± 0.3 |
| 12b | 0.41 | 3.8 ± 0.2 |
2.3. MS Application
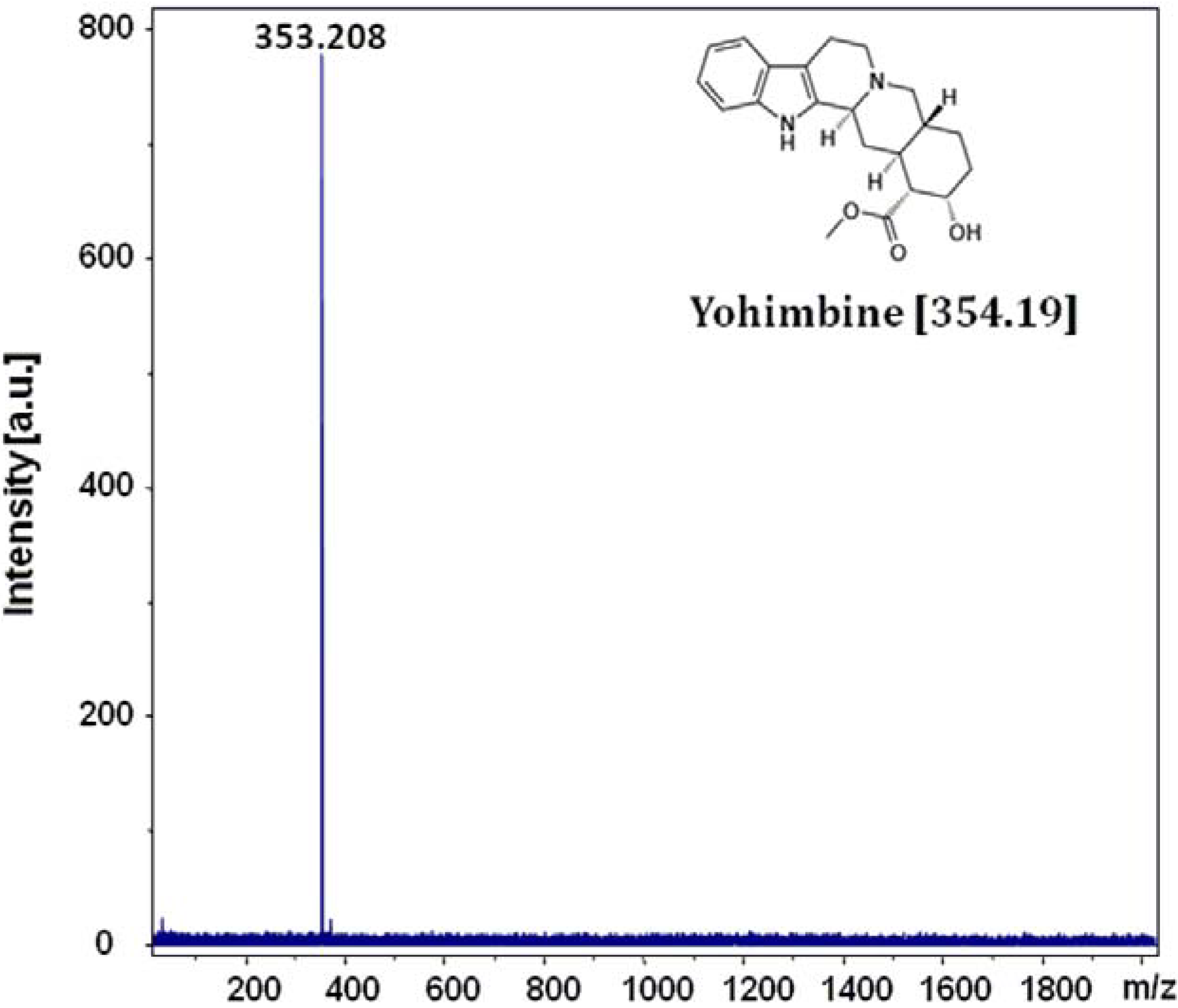
3. Experimental
3.1. General
3.2. Electrochemistry
3.3. Synthesis
3.3.1. Alkynylbithiophenes 6a–c
3.3.2. Azidobithiophenes 7a–b
3.3.3. 8-Azidooctyl Thioacetate (9)
3.3.4. Dec-9-ynyl Thioacetate (10)
3.3.5. Triazolobithiophenes 4a–c
3.3.6. Triazolobithiophenes 5a–b
3.3.7. Thiols 2a–c and 3a,b
3.3.8. Disulfides 11a–c, 12a,b
4. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
References and Notes
- Plunkett, K.N.; Mohraz, A.; Haasch, R.T.; Lewis, J.A.; Moore, J.S. Light-regulated electrostatic interactions in colloidal suspensions. J. Am. Chem. Soc. 2005, 127, 14574–14575. [Google Scholar] [CrossRef]
- Bounichou, M.; Sanguinet, L.; Elouarzaki, K.; Alévêque, O.; Dias, M.; Levillain, E.; Rondeau, D. Evaluation of a new matrix-free laser desorption/ionization method through statistic studies: Comparison of the DIAMS (desorption/ionization on self-assembled monolayer surface) method with the MALDI and TGFA-LDI techniques. J. Mass. Spectrom. 2008, 43, 1618–1626. [Google Scholar] [CrossRef]
- Sanguinet, L.; Alévêque, O.; Blanchard, P.; Dias, M.; Levillain, E.; Rondeau, D. Desorption/ionization on self-assembled monolayer surfaces (DIAMS). J. Mass. Spectrom. 2006, 41, 830–833. [Google Scholar] [CrossRef]
- Nayak, R.; Knapp, D.R. Matrix-free LDI mass spectrometry platform using patterned nanostructured gold thin film. Anal. Chem. 2010, 82, 7772–7778. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, N.; Li, Y.; Liu, H.; Lu, F.; Zhuang, J.; Zhu, D. Self-assembled monolayers of C60-perylenetetracarboxylic diimide-C60 triad on indium tin oxide surface. Carbon 2005, 43, 1968–1975. [Google Scholar] [CrossRef]
- Nie, H.-Y.; McIntyre, N.S.; Lau, W.M.; Feng, J.M. Optical properties of an octadecylphosphonic acid self-assembled monolayer on a silicon wafer. Thin Solid Films 2008, 517, 814–818. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lin, J.T.; Chen, I.W.; Chen, C.H. Monolayers of diphenyldiacetylene derivatives: Tuning molecular tilt angles and photopolymerization efficiency via electrodeposited Ag interlayer on Au. J. Phys. Chem. B 2005, 109, 19161–19168. [Google Scholar] [CrossRef]
- Neilands, O.; Kirichenko, N.; Muzikante, I.; Fonavs, E.; Gerca, L.; Jursenas, S.; Valiokas, R.; Karpicz, R.; Valkunas, L. Detection of blue light by self-assembled monolayer of dipolar molecules. In UV Solid-State Light Emitters and Detectors; Shur, M.S., Zukauskas, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; NATO Science Series II Mathematics Physics and Chemistry; Volume 144, pp. 261–269. [Google Scholar]
- Kondo, M.; Nakamura, Y.; Fujii, K.; Nagata, M.; Suemori, Y.; Dewa, T.; Lida, K.; Gardiner, A.T.; Cogdell, R.J.; Nango, M. Self-assembled monolayer of light-harvesting core complexes from photosynthetic bacteria on a gold electrode modified with alkanethiols. Biomacromolecules 2007, 8, 2457–2463. [Google Scholar]
- Imahori, H.; Hasobe, T.; Yamada, H.; Nishimura, Y.; Yamazaki, I.; Fukuzumi, S. Concentration effects of porphyrin monolayers on the structure and photoelectrochemical properties of mixed self-assembled monolayers of porphyrin and alkanethiol on gold electrodes. Langmuir 2001, 17, 4925–4931. [Google Scholar]
- Li, Y.; Li, Y.; Liu, H.; Wang, S.; Wang, N.; Zhuang, J.; Li, X.; He, X.; Zhu, D. Self-assembled monolayers of porphyrin perylenetetracarboxylic diimide [60] fullerene on indium tin oxide electrodes: Enhancement of light harvesting in the visible light region. Nanotechnology 2005, 16, 1899–1904. [Google Scholar] [CrossRef]
- Mrksich, M. Mass spectroscopy of self-assembled monolayers: A new tool for molecular surface science. ACS Nano 2008, 2, 7–18. [Google Scholar] [CrossRef]
- Patrie, S.M.; Mrksich, M. Self-assembled monolayers for MALDI-TOF mass spectrometry for immunoassays of human protein antigens. Anal. Chem. 2007, 79, 5878–5887. [Google Scholar] [CrossRef]
- Plutowski, U.; Richert, C. A direct glimpse of cross-hybridization: background-passified microarrays that allowmass-spectrometric detection of captured oligonucleotides. Angew. Chem. Int. Ed. 2005, 44, 621–625. [Google Scholar] [CrossRef]
- Zotti, G.; Vercelli, B.; Berlin, A. Monolayers and multilayers of conjugated polymers as nanosized electronic components. Acc. Chem. Res. 2008, 41, 1098–1109. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, H.; Harima, Y.; Yamashita, K.; Aso, Y.; Otsubo, T. Enhanced hole injection in organic light-emitting diodes consisting of self-assembled monolayer of tripod-shaped π-conjugated thiols. J. Mater. Chem. 2002, 12, 2250–2254. [Google Scholar]
- De Boer, B.; Meng, H.; Perepichka, D.F.; Zheng, J.; Frank, M.M.; Chabal, Y.J.; Bao, Z. Synthesis and characterization of conjugated mono- and dithiol oligomers and characterization of their self-assembled monolayers. Langmuir 2003, 19, 4272–4284. [Google Scholar] [CrossRef]
- Tour, J.M.; Leroy, J.; Pearson, D.L.; Lamba, J.J.S.; Burgin, T.P.; Whitesides, G.M.; Allara, D.L.; Parikh, A.T.; Atre, S.V. Self-assembled monolayers and multilayers of conjugated thiols, α,ω-dithiols, and thioacetyl-containing adsorbate. Understanding attachments between potential molecular wires and gold surfaces. J. Am. Chem. Soc. 1995, 117, 9529–9534. [Google Scholar]
- Berlin, A.; Zotti, G.; Schiavon, G.; Zecchin, S. Adsorption of carboxyl-terminated dithiophene and terthiophene molecules on ITO electrodes and their electrochemical coupling to polymer layers. The influence of molecular geometry. J. Am. Chem. Soc. 1998, 120, 13453–13460. [Google Scholar] [CrossRef]
- Clegg, R.S.; Hutchison, J.E. Control of monolayer assembly structure by hydrogen bonding rather than by adsorbate-substrate templating. J. Am. Chem. Soc. 1999, 121, 5319–5327. [Google Scholar] [CrossRef]
- Valiokas, R.; Svedhem, S.; Svensson, S.C.T.; Liedberg, B. Self-assembled monolayers of oligo(ethylene glycol)-terminated and amide group containing alkanethiolates on gold. Langmuir 1999, 15, 3390–3394. [Google Scholar] [CrossRef]
- Valiokas, R.; Malysheva, L.; Onipko, A.; Lee, H-H.; Ruželė, Ž.; Svedhem, S.; Svensson, S.C.T.; Gelius, U.; Liedberg, B. On the quality and structural characteristics of oligo(ethylene glycol) assemblies on gold: an experimental and theoretical study. J. Electron. Spectr. Relat. Phenom. 2009, 172, 9–20. [Google Scholar] [CrossRef]
- Evans, S.D.; Goppert-Berarducci, K.E.; Urankar, E.; Gerenser, L.J.; Ulman, A. Monolayers having large in-plane dipole moments: Characterization of sulfone-containing self-assembled monolayers of alkanethiols on gold by Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy and wetting. Langmuir 1991, 7, 2700–2709. [Google Scholar] [CrossRef]
- Evans, S.D.; Urankar, E.; Ulman, A.; Ferris, N. Self-assembled monolayers of alkanethiols containing a polar aromatic group: Effects of the dipole position on molecular packing, orientation, and surface wetting propertie. J. Am. Chem. Soc. 1991, 113, 4121–4131. [Google Scholar]
- Kang, J.F.; Ulman, A.; Liao, S.; Jordan, R. Mixed self-assembled monolayers of highly polar rigid biphenyl thiols. Langmuir 1999, 15, 2095–2098. [Google Scholar] [CrossRef]
- Barrena, E.; Palacios-Lidón, E.; Munuera, C.; Torrelles, X.; Ferrer, S.; Jonas, U.; Salmeron, M.; Ocal, C. The role of intermolecular and molecule-substrate interactions in the stability of alkanethiol nonsaturated phases on Au(111). J. Am. Chem. Soc. 2004, 126, 385–395. [Google Scholar]
- Lewis, P.A.; Smith, R.K.; Kelly, K.F.; Bumm, L.A.; Reed, S.M.; Clegg, R.S.; Gunderson, J.D.; Hutchison, J.E.; Weiss, P.S. The role of buried hydrogen bonds in self-assembled mixed composition thiols on Au(111). J. Phys. Chem. B 2001, 105, 10630–10636. [Google Scholar]
- Boal, A.K.; Rotello, V.M. Intra- and inter-monolayer hydrogen bonding in amide functionalized alkanethiol SAMs on gold nanoparticles. Langmuir 2000, 16, 9527–9532. [Google Scholar]
- Lenk, T.J.; Hallmark, V.M.; Hoffmann, C.L.; Rabolt, J.F.; Castner, D.G.; Erdelen, C.; Ringsdorf, H. Structural investigation of molecular organization in self-assembled monolayers of a semifluorinated amidethiol. Langmuir 1994, 10, 4610–4617. [Google Scholar]
- Tam-Chang, S.-W.; Biebuyck, H.A.; Whitesides, G.M.; Nooh, J.; Nuzzo, R.G. Self-assembled monolayers on gold generated from alkanethiols with the structure RNHCOCH2SH. Langmuir 1995, 11, 4371–4382. [Google Scholar]
- Sabapathy, R.C.; Bhattacharyya, S.; Leavy, M.C.; Cleland, W.E.; Hussey, C.L. Electrochemical and spectroscopic characterization of self-assembled monolayers of ferrocenylalkyl compounds with amide linkages. Langmuir 1998, 14, 124–136. [Google Scholar] [CrossRef]
- Clegg, R.S.; Hutchison, J.E. Hydrogen-bonding, self-assembled monolayers: Ordered molecular films for study of through-peptide electron transfer. Langmuir 1996, 12, 5239–5243. [Google Scholar] [CrossRef]
- Clegg, R.S.; Reed, S.M.; Hutchison, J.E. Self-assembled monolayers stabilized by three-dimensional networks of hydrogen bonds. J. Am. Chem. Soc. 1998, 120, 2486–2487. [Google Scholar] [CrossRef]
- Clegg, R.S.; Reed, S.M.; Smith, R.K.; Barron, B.L.; Rear, J.A.; Hutchison, J.E. The interplay of lateral and tiered interactions in stratified self-organized molecular assemblies. Langmuir 1999, 15, 8876–8883. [Google Scholar]
- Clegg, R.S.; Hutchison, J.E. Control of monolayer assembly structure by hydrogen bonding rather than by adsorbate-substrate templating. J. Am. Chem. Soc. 1999, 121, 5319–5327. [Google Scholar] [CrossRef]
- Dou, R.-F.; Ma, X.-C.; Xi, L.; Yip, H.L.; Wong, K.Y.; Lau, W.M.; Jia, J.-F.; Xue, Q.-K.; Yang, W.-S.; Ma, H.; Jen, A.K.-Y. Self-assembled monolayers of aromatic thiols stabilized by parallel-displaced π-π stacking interactions. Langmuir 2006, 22, 3049–3056. [Google Scholar]
- Yin, G.; Zhang, Y.; Li, B.; Zhang, Y. Syntheses, structures and luminescent properties of a dimer and an one-dimensional chain coordination polymer with the flexible bis(triazole) and hydroxybenzoate ligands. J. Mol. Struct. 2007, 837, 263–268. [Google Scholar] [CrossRef]
- Yu, S.; Chai, X.; Hu, H.; Yan, Y.; Guan, Z.; Zou, Y.; Sun, Q.; Wu, Q. Synthesis and antifungal evaluation of novel triazole derivatives as inhibitors of cytochrome P450 14α-demethylase. Eur. J. Med. Chem. 2010, 45, 4435–4445. [Google Scholar] [CrossRef]
- Chelmowski, R.; Käfer, D.; Köster, S.D.; Klasen, T.; Winkler, T.; Terfort, A.; Metzler-Nolte, N.; Wöll, C. Postformation modification of SAMs: Using click chemistry to functionalize organic surfaces. Langmuir 2009, 25, 11480–11485. [Google Scholar]
- Lee, J.K.; Chi, Y.S.; Choi, I.S. Reactivity of acetylenyl-terminated self-assembled monolayers on gold: Triazole formation. Langmuir 2004, 20, 3844–3847. [Google Scholar]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Blanchard, P.; Jousselme, B.; Frère, P.; Roncali, J. 3- and 3,4-bis(2-cyanoethylsulfanyl)thiophenes as building blocks for functionalized thiophene-based π-conjugated systems. J. Org. Chem. 2002, 67, 3961–3964. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: (1,2,3)-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar]
- Nuzzo, R.G.; Allara, D.L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. [Google Scholar] [CrossRef]
- Noh, J.; Murase, T.; Nakajima, K.; Lee, H.; Hara, M. Nanoscopic investigation of the self-assembly processes of dialkyl disulfides and dialkyl sulfides on Au(111). J. Phys. Chem. B 2000, 104, 7411–7416. [Google Scholar]
- Biebuyck, H.A.; Bain, C.D.; Whitesides, G.M. Comparison of organic monolayers on polycrystalline gold spontaneously assembled from solutions containing dialkyl disulfides or alkanethiols. Langmuir 1994, 10, 1825–1831. [Google Scholar]
- Bain, C.D.; Biebuyck, H.A.; Whitesides, G.M. Comparison of self-assembled monolayers on gold: coadsorption of thiols and disulfides. Langmuir 1989, 5, 723–727. [Google Scholar] [CrossRef]
- Strong, L.; Whitesides, G.M. Structures of self-assembled monolayer films of organosulfur compounds adsorbed on gold single crystals: Electron diffraction studies. Langmuir 1988, 4, 546–558. [Google Scholar] [CrossRef]
- Guyard, L.; Hapiot, P.; Jouini, M.; Lacroix, J.-C.; Lagrost, C.; Neta, P. Oxidative coupling of small oligothiophenes and oligopyrroles in water in the presence of cyclodextrin pulse radiolysis investigations. J. Phys. Chem. A 1999, 103, 4009–4015. [Google Scholar]
- Bounichou, M.; Alévêque, O.; Breton, T.; Dias, M.; Sanguinet, L.; Levillain, E.; Rondeau, D. Self-assembled monolayer-assisted mass spectrometry. J. Mater. Chem. 2009, 19, 8032–8039. [Google Scholar]
- Zanolari, B.; Ndjoko, K.; Ioset, J.-R.; Marston, A.; Hostettmann, K. Qualitative and quantitative determination of yohimbine in authentic yohimbe bark and in commercial aphrodisiacs by HPLC-UV-API/MS methods. Phytochem. Anal. 2003, 14, 193–201. [Google Scholar] [CrossRef]
- Zenobie, R.; Knochenmuss, R. Ion Formation in MALDI Mass Spectrometry. Mass Spectrom. Rev. 1998, 17, 337–366. [Google Scholar] [CrossRef]
- Schinkovitz, A.; Tsague-Kenfack, G.; Levillain, E.; Dias, M.; Helesbeux, J.-J.; Derbré, S.; Séraphin, D.; Richomme, P. Free and immobilized matrix molecules: impairing ionization by quenching secondary ion formation in laser desorption MS. J. Mass. Spectrom. 2011, 46, 884–890. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kenfack, G.T.; Schinkovitz, A.; Babu, S.; Elouarzaki, K.; Dias, M.; Derbré, S.; Helesbeux, J.-J.; Levillain, E.; Richomme, P.; Séraphin, D. Triazolobithiophene Light Absorbing Self-Assembled Monolayers: Synthesis and Mass Spectrometry Applications. Molecules 2011, 16, 8758-8774. https://doi.org/10.3390/molecules16108758
Kenfack GT, Schinkovitz A, Babu S, Elouarzaki K, Dias M, Derbré S, Helesbeux J-J, Levillain E, Richomme P, Séraphin D. Triazolobithiophene Light Absorbing Self-Assembled Monolayers: Synthesis and Mass Spectrometry Applications. Molecules. 2011; 16(10):8758-8774. https://doi.org/10.3390/molecules16108758
Chicago/Turabian StyleKenfack, Ghislain Tsague, Andreas Schinkovitz, Suresh Babu, Kamal Elouarzaki, Marylène Dias, Séverine Derbré, Jean-Jacques Helesbeux, Eric Levillain, Pascal Richomme, and Denis Séraphin. 2011. "Triazolobithiophene Light Absorbing Self-Assembled Monolayers: Synthesis and Mass Spectrometry Applications" Molecules 16, no. 10: 8758-8774. https://doi.org/10.3390/molecules16108758
APA StyleKenfack, G. T., Schinkovitz, A., Babu, S., Elouarzaki, K., Dias, M., Derbré, S., Helesbeux, J.-J., Levillain, E., Richomme, P., & Séraphin, D. (2011). Triazolobithiophene Light Absorbing Self-Assembled Monolayers: Synthesis and Mass Spectrometry Applications. Molecules, 16(10), 8758-8774. https://doi.org/10.3390/molecules16108758




