Palladium Catalyzed Allylic C-H Alkylation: A Mechanistic Perspective
Abstract
:1. Background
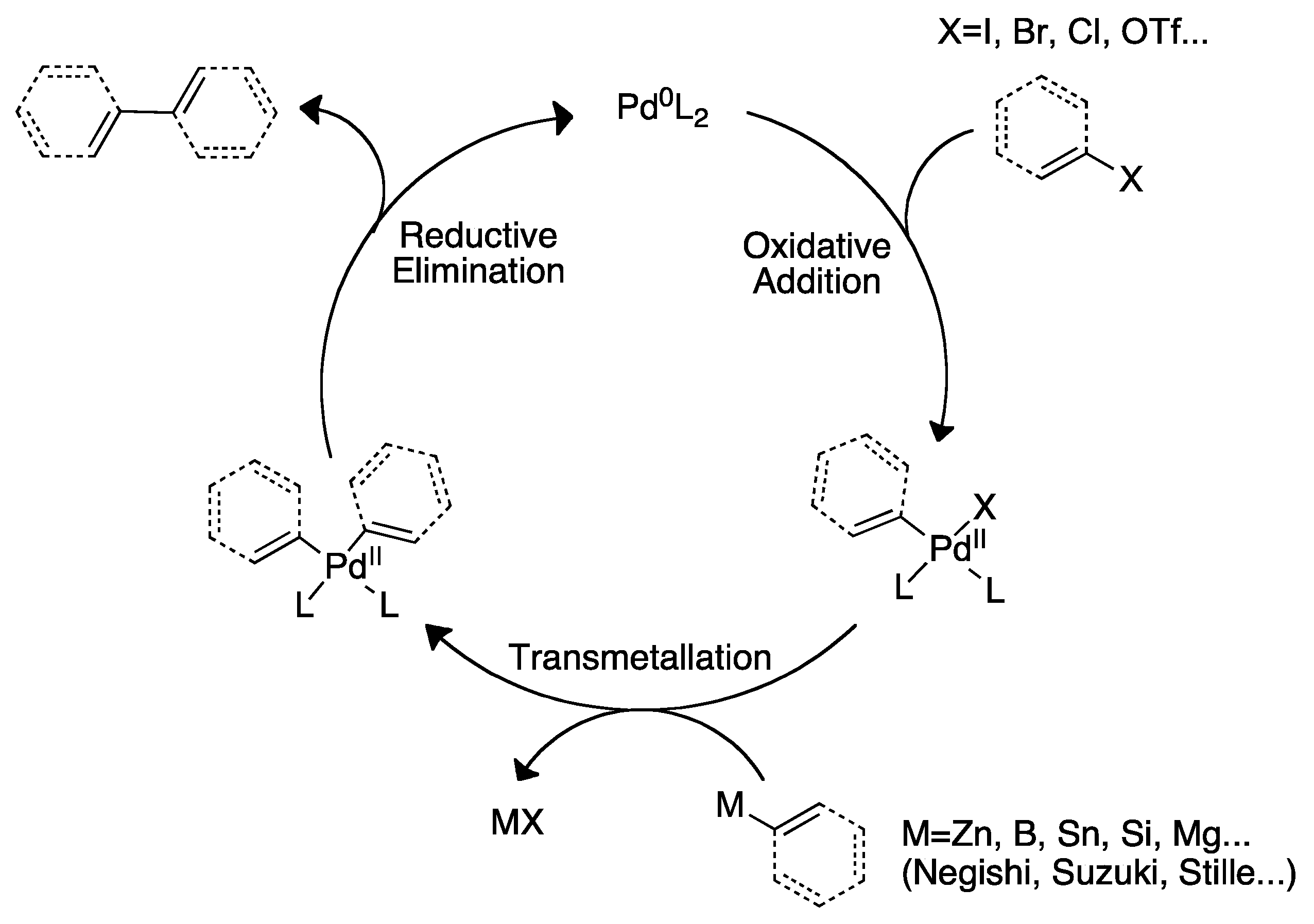
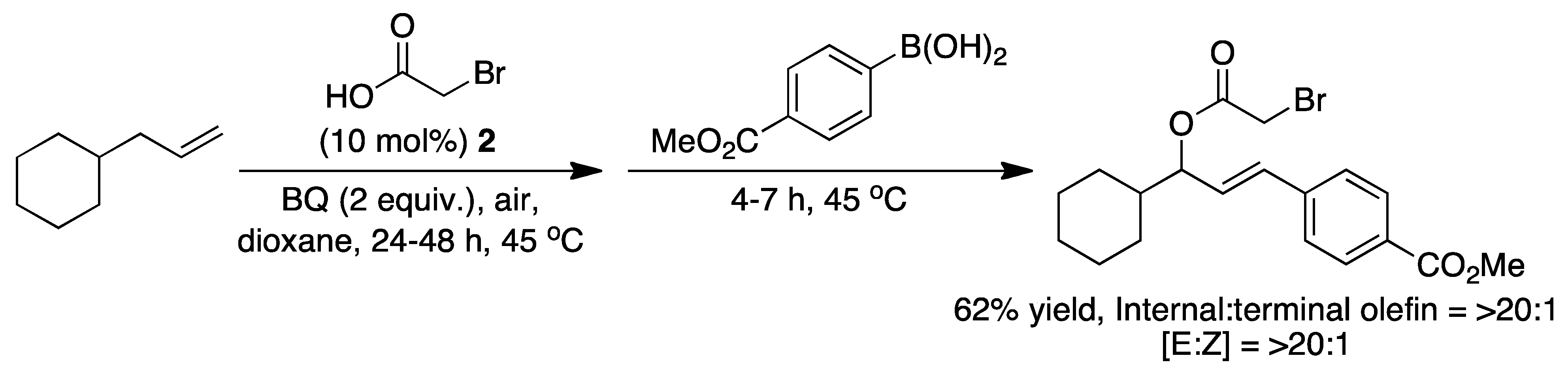
2. The Palladium Catalyzed Allylic C-H Alkylation
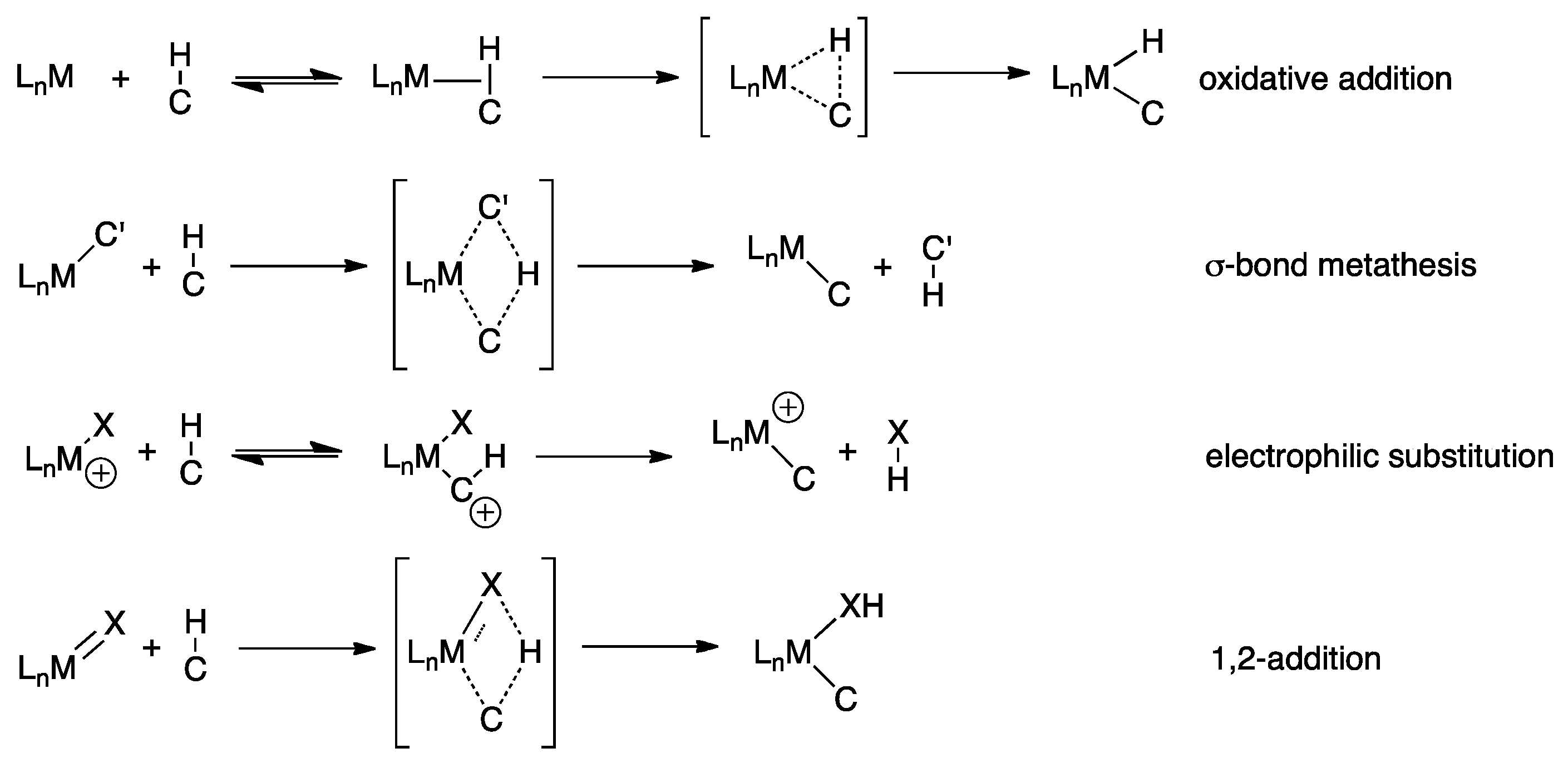
3. Elucidating the Mechanism
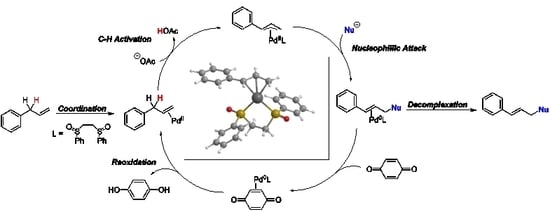
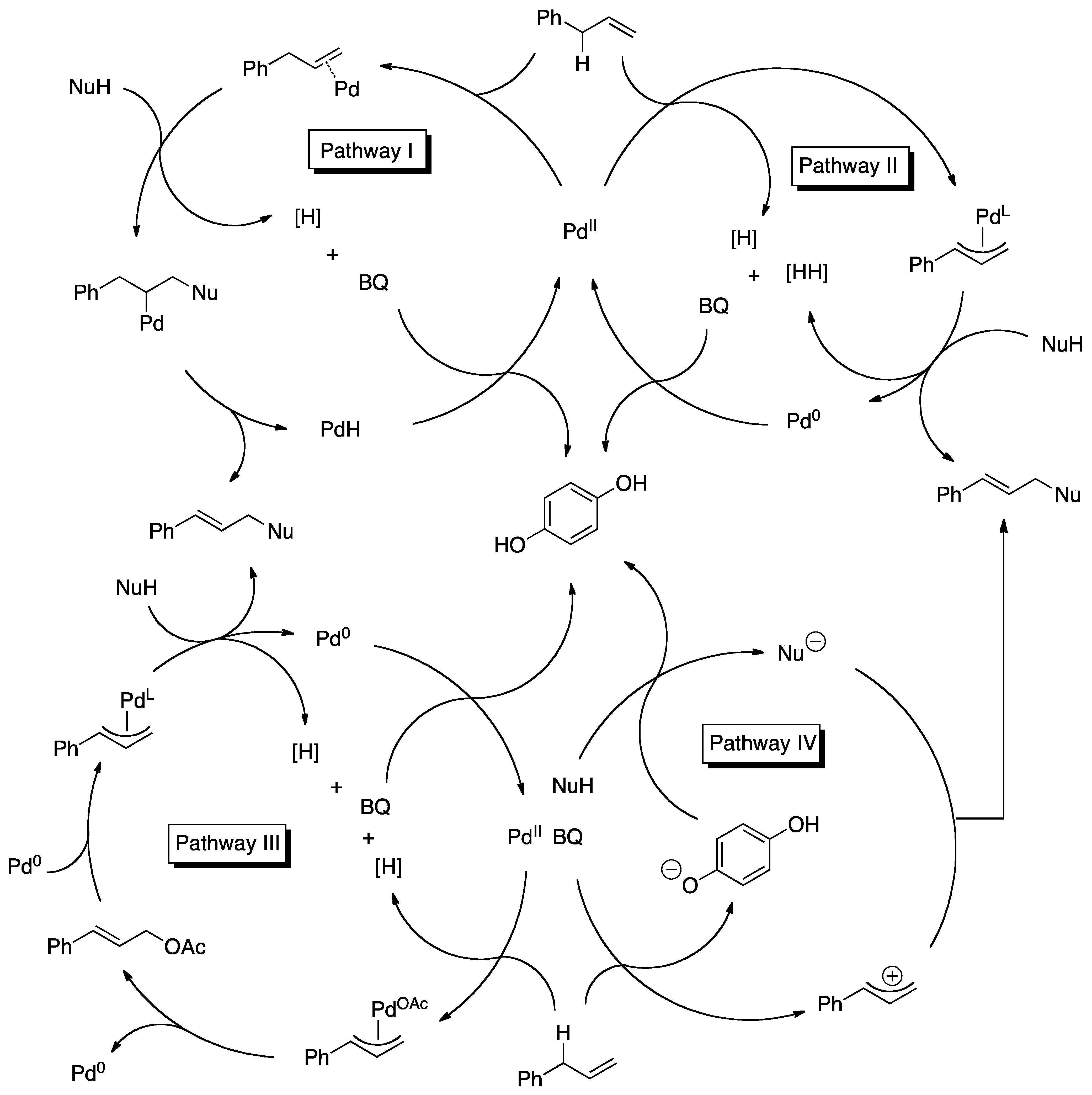
4. Detailed Mechanistic Proposals
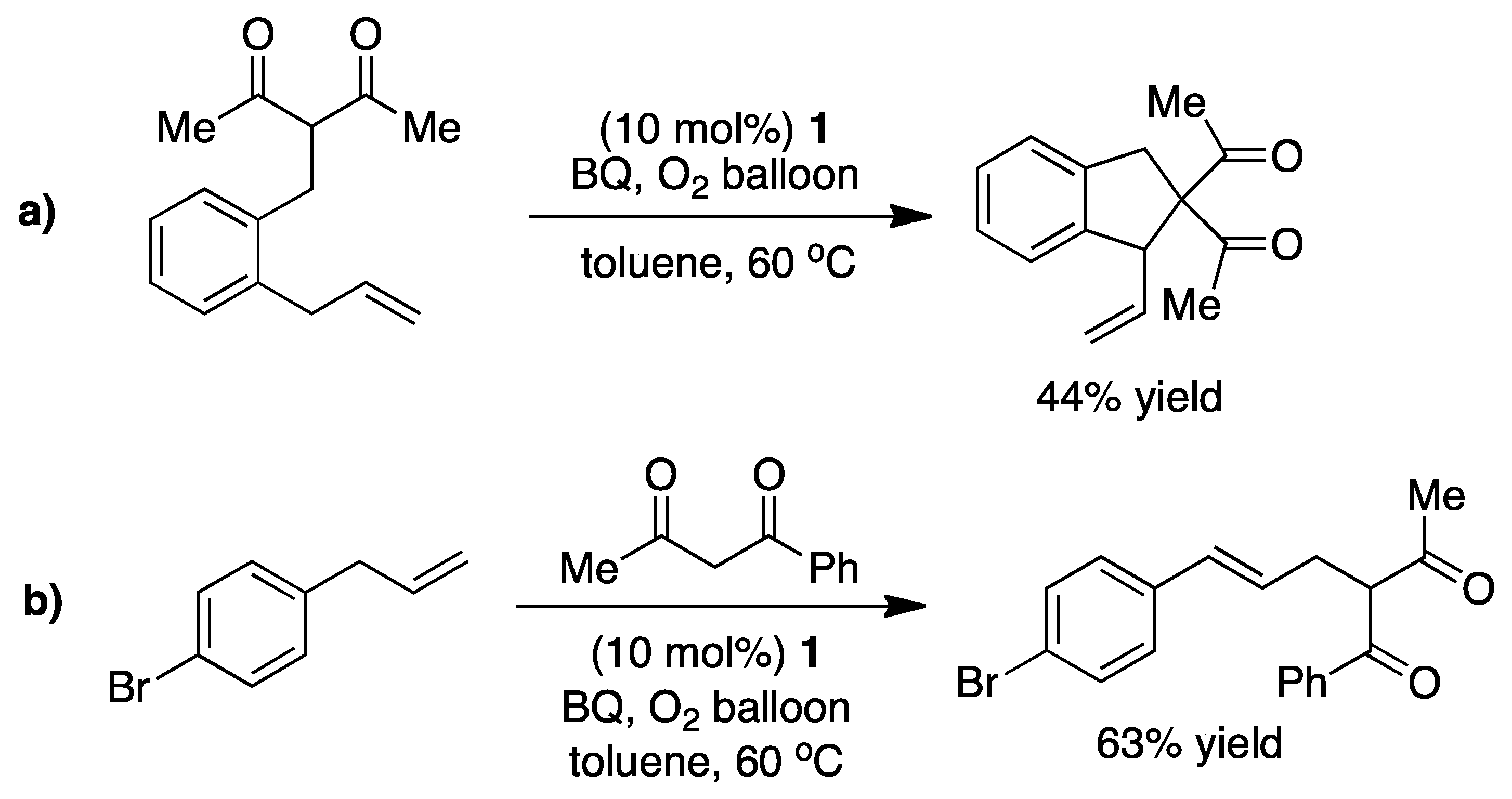
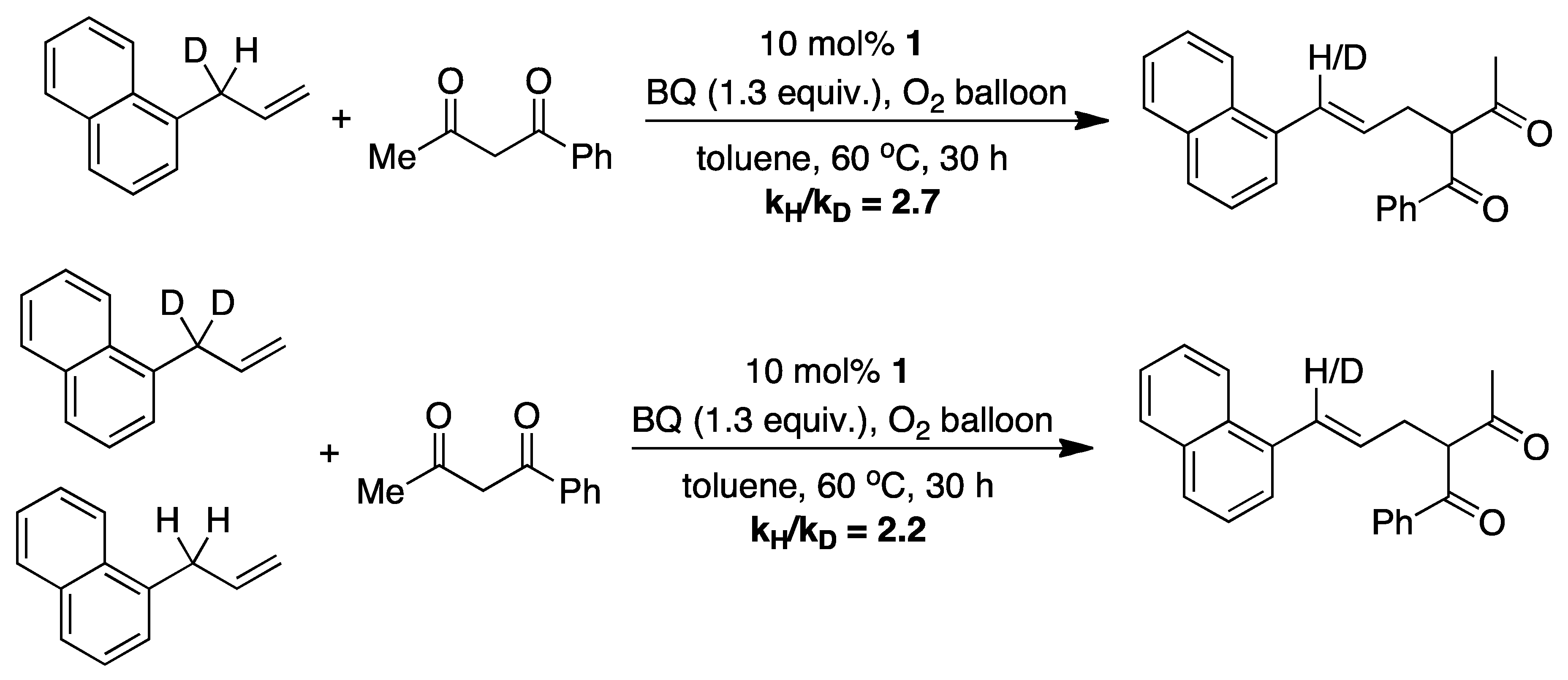
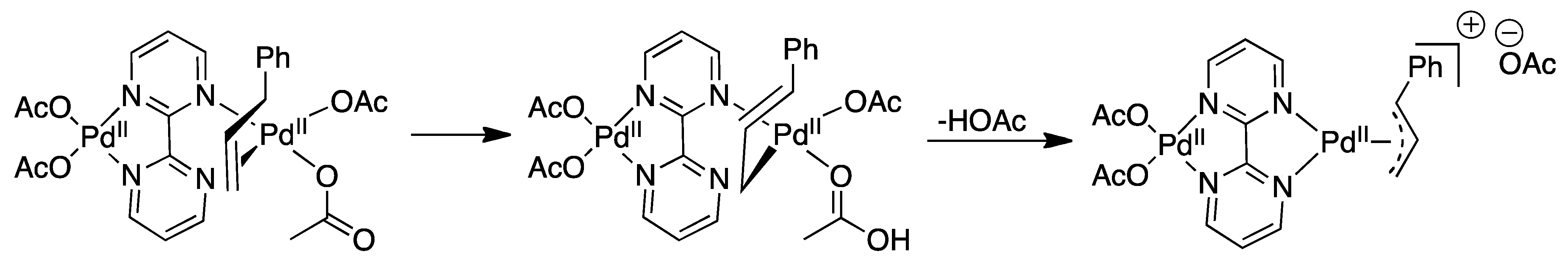
4.1. C-H Activation
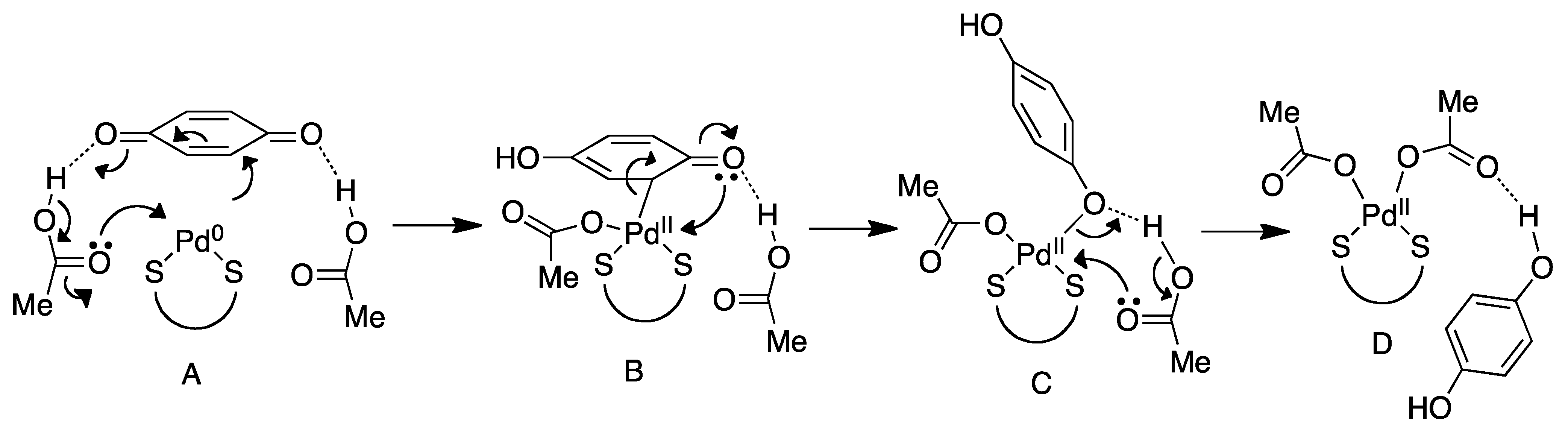
4.2. Nucleophilic Attack
4.3. Reoxidation
5. Conclusions and Outlook
Acknowledgments
References and Notes
- Available online: http://nobelprize.org/nobel_prizes/chemistry/laureates/2010/press.html (accessed on 21 January 2011).
- De Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; pp. 1–40. [Google Scholar]
- Tsuji, J.; Takahashi, H.; Morikawa, M. Organic syntheses by means of noble metal compounds .17. Reaction of pi-allylpalladium chloride with nucleophiles. Tetrahedron. Lett. 1965, 6, 4387–4388. [Google Scholar] [CrossRef]
- Trost, B.M. New rules of selectivity - allylic alkylations catalyzed by palladium. Acc. Chem. Res. 1980, 13, 385–393. [Google Scholar] [CrossRef]
- Trost, B.M.; Crawley, M.L. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2943. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Designing a Receptor for Molecular Recognition in a Catalytic Synthetic Reaction: Allylic Alkylation. Acc. Chem. Res. 1996, 29, 355–364. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D. Regio- and Enantioselective Allylic Alkylation of an Unsymmetrical Substrate: A Working Model. J. Am. Chem. Soc. 1999, 121, 4545–4554. [Google Scholar] [CrossRef]
- Trost, B.M.; Machacek, M.R.; Aponick, A. Predicting the Stereochemistry of Diphenylphosphino Benzoic Acid (DPPBA)-Based Palladium-Catalyzed Asymmetric Allylic Alkylation Reactions: A Working Model. Acc. Chem. Res. 2006, 39, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.P.; Filali, E.; Lloyd-Jones, G.C.; Norrby, P.-O.; Sale, D.A.; Schramm, Y. Structure-based rationale for selectivity in the asymmetric allylic alkylation of cycloalkenyl esters employing the Trost ’Standard Ligand’ (TSL): Isolation, Analysis and alkylation of the monomeric form of the cationic η3-cyclohexenyl complex [(η3-c-C6H9)Pd(TSL)]+. J. Am. Chem. Soc. 2009, 131, 9945–9957. [Google Scholar]
- Trost, B.M. The atom economy - a search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Jensen, T.; Fristrup, P. Toward Efficient Palladium-Catalyzed Allylic C-H Alkylation. Chem. Eur. J. 2009, 15, 9632–9636. [Google Scholar] [CrossRef]
- Balcells, D.; Clot, E.; Eisenstein, O. For a review on the mechanistic aspects of C-H activation, C-H Bond Activation in Transition Metal Species from a Computational Perspective. Chem. Rev. 2010, 110, 749–823. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation of C−H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef]
- Young, A.J.; White, M.C. Catalytic Intermolecular Allylic C-H Alkylation. J. Am. Chem. Soc. 2008, 130, 14090–14091. [Google Scholar] [CrossRef]
- Lin, S.; Song, C.-X.; Cai, G.-X.; Wang, W.-H.; Shi, Z.-J. Intra/intermolecular direct allylic alkylation via Pd(II)-catalyzed allylic C-H activation. J. Am. Chem. Soc. 2008, 130, 12901–12903. [Google Scholar] [CrossRef] [PubMed]
- Pfaltz, A.; Lautens, M. Allylic substitution reactions. In Comprehensive Asymmetric Catalysis, 2nd ed.; Jacobsen, E.N., Pfaltz, A., Yamamoto, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 2, pp. 833–887. [Google Scholar]
- Trost, B.M.; Fullerto, T.J. New synthetic reactions—Allylic alkylation. J. Am. Chem. Soc. 1973, 95, 292–294. [Google Scholar] [CrossRef]
- Chen, M.S.; White, M.C. A sulfoxide-promoted, catalytic method for the regioselective synthesis of allylic acetates from monosubstituted olefins via C-H oxidation. J. Am. Chem. Soc. 2004, 126, 1346–1347. [Google Scholar] [CrossRef] [PubMed]
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. C-C, C-O, C-N bond formation on sp(2) carbon by Pd(II)-catalyzed reactions involving oxidant agents. Chem. Rev. 2007, 107, 5318–5365. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.T.; White, M.C. Allylic C-H amination for the preparation of syn-1,3-amino alcohol motifs. J. Am. Chem. Soc. 2009, 131, 11707–11711. [Google Scholar] [CrossRef]
- Vermeulen, N.A.; Delcamp, J.H.; White, M.C. Synthesis of complex allylic esters via C-H oxidation vs C-C bond formation. J. Am. Chem. Soc. 2010, 132, 11323–11328. [Google Scholar] [CrossRef]
- Rakshit, S.; Patureau, F.W.; Glorius, F. Pyrrole synthesis via allylic sp3 C-H activation of enamines followed by intermolecular coupling with unactivated alkynes. J. Am. Chem. Soc. 2010, 132, 9585–9587. [Google Scholar] [CrossRef]
- Collins, D.J.; Jackson, W.R.; Timms, R.N. Stereospecific 6-beta-functionalization of 3-oxo-4-ene steroids via pi-allylpalladium complexes. Terahedron. Lett. 1976, 17, 495–496. [Google Scholar] [CrossRef]
- Fraunhoffer, K.J.; White, M.C. Syn-1,2-amino alcohols via diastereoselective allylic C-H amination. J. Am. Chem. Soc. 2007, 129, 7274–7276. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Wang, X.; Widenhoefer, R.A. Palladium-catalyzed intramolecular oxidative alkylation of unactivated olefins. J. Am. Chem. Soc. 2003, 125, 648–649. [Google Scholar]
- Cheng, D.P.; Bao, W.L. Highly efficient metal-free cross-coupling by C-H activation between allylic and active methylenic compounds promoted by DDQ. Adv. Synth. Catal. 2008, 350, 1263–1266. [Google Scholar] [CrossRef]
- Bäckvall, J.; Grennberg, H. Mechanism of palladium-catalyzed allylic acetoxylation of cyclohexene. Chem. Eur. J. 1998, 4, 1083–1089. [Google Scholar]
- Reed, S.A.; Mazzotti, A.; White, M. A catalytic, Brønsted base strategy for intermolecular allylic C-H amination. J. Am. Chem. Soc. 2009, 131, 11701–11706. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. Alkane C-H activation and functionalization with homogeneous transition metal catalysts: A century of progress-a new millennium in prospect. J. Chem. Soc. Dalton Trans. 2001, 2437–2450. [Google Scholar] [CrossRef]
- Recently, an entire issue of Chemical Reviews was devoted to C-H activation, see introduction by Prof. Crabtree: Crabtree, R. H. Chem. Rev. 2010, 110, 575.
- Baik, M.-H.; Newcomb, M.; Friesner, R.A.; Lippard, S.J. Mechanistic studies on the hydroxylation of methane by Methane Monooxygenase. Chem. Rev. 2003, 103, 2385–2419. [Google Scholar] [CrossRef]
- Periana, R.A.; Mironov, O.; Taube, D.; Bhalla, G.; Jones, C.J. Homogeneous, catalytic, oxidative coupling of methane to acetic acid in one step. Top. Catal. 2005, 32, 169–174. [Google Scholar] [CrossRef]
- Neufeldt, S.R.; Sanford, M.S. O-acetyl oximes as transformable directing groups for Pd-catalyzed C-H bond functionalization. Org. Lett. 2010, 12, 532–535. [Google Scholar] [CrossRef]
- Desai, L.V.; Hull, K.L.; Sanford, M.S. Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds. J. Am. Chem. Soc. 2004, 126, 9542–9543. [Google Scholar] [CrossRef]
- Giri, R.; Maugel, N.; Foxman, B.M.; Yu, J.-Q. Dehydrogenation of inert alkyl groups via remote C-H activation: Converting a propyl group into a π-allylic complex. Organometallics 2008, 27, 1667–1670. [Google Scholar] [CrossRef]
- Lafrance, M.; Gorelsky, S.I.; Fagnou, K. High-yielding palladium-catalyzed intramolecular alkane arylation: Reaction development and Mechanistic studies. J. Am. Chem. Soc. 2007, 129, 14570–14571. [Google Scholar] [CrossRef] [PubMed]
- Liegault, B.; Fagnou, K. Palladium-catalyzed intramolecular coupling of arenes and unactivated alkanes in air. Organometallics 2008, 27, 4841–4843. [Google Scholar] [CrossRef]
- Rousseaux, S.; Davi, M.; Sofack-Kreutzer, J.; Pierre, C.; Kefalidis, C.E.; Clot, E.; Fagnou, K.; Baudoin, O. Intramolecular palladium-catalyzed alkane C-H arylation from aryl chlorides. J. Am. Chem. Soc. 2010, 132, 10706–10716. [Google Scholar] [CrossRef]
- Chaumontet, M.; Piccardi, R.; Audic, N.; Hitce, J.; Peglion, J.-L.; Clot, E.; Baudoin, O. Synthesis of cyclobutenes by palladium-catalyzed C-H activation of methyl groups: Method and mechanistic study. J. Am. Chem. Soc. 2008, 130, 15157–15166. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; White, M.C. Catalytic intermolecular linear allylic C-H amination via heterobimetallic catalysis. J. Am. Chem. Soc. 2008, 130, 3316–3318. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yuan, W.; Zhao, B.; Shi, Y. A Pd(0)-catalyzed diamination of terminal olefins at allylic and homoallylic carbons via formal C-H activation under solvent-free conditions. J. Am. Chem. Soc. 2007, 129, 7496–7497. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qiu, S.; Liu, G. Brønsted base-modulated regioselective Pd-catalyzed intramolecular aerobic oxidative amination of alkenes: Formation of seven-membered amides and evidence for allylic C-H activation. Org. Lett. 2009, 11, 2707–2710. [Google Scholar] [CrossRef]
- Speziali, M.G.; Costa, V.V.; Robles-Dutenhefner, P.A.; Gusevskaya, E.V. Aerobic palladium(II)/copper(II)-catalyzed oxidation of olefins under chloride-free nonacidic conditions. Organometallics 2009, 28, 3186–3192. [Google Scholar] [CrossRef]
- Delcamp, J.H.; White, M.C. Sequential hydrocarbon functionalization: Allylic C-H oxidation/vinylic C-H arylation. J. Am. Chem. Soc. 2006, 128, 15076–15077. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira reaction: A Booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Scarborough, C.C.; McDonald, R.I.; Hartmann, C.; Sazama, G.T.; Bergant, A.; Stahl, S.S. Steric modulation of chiral biaryl diamines via Pd-catalyzed directed C-H arylation. J. Org. Chem. 2009, 74, 2613–2615. [Google Scholar] [CrossRef]
- Martin-Matute, B.; Mateo, C.; Cardenas, D.J.; Echavarren, A.M. Intramolecular C-H activation by alkylpalladium(II) complexes: Insights into the mechanism of the palladium-catalyzed arylation reaction. Chem. Eur. J. 2001, 7, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Kalyani, D.; Deprez, N.R.; Desai, L.V.; Sanford, M.S. Oxidative C-H activation/C-C bond forming reactions: Synthetic scope and mechanistic insights. J. Am. Chem. Soc. 2005, 127, 7330–7331. [Google Scholar] [CrossRef] [PubMed]
- Hull, K.L.; Lanni, E.L.; Sanford, M.S. Highly regioselective catalytic oxidative coupling reactions: Synthetic and mechanistic investigations. J. Am. Chem. Soc. 2006, 128, 14047–14049. [Google Scholar] [CrossRef] [PubMed]
- Desai, L.V.; Malik, H.A.; Sanford, M.S. Oxone as an inexpensive, safe, and environmentally benign oxidant for C-H bond oxygenation. Org. Lett. 2006, 8, 1141–1144. [Google Scholar] [CrossRef]
- Arnold, P.L.; Sanford, M.S.; Pearson, S.M. Chelating N-heterocyclic carbene alkoxide as a supporting ligand for PdII/IV C-H bond functionalization catalysis. J. Am. Chem. Soc. 2009, 131, 13912–13913. [Google Scholar] [CrossRef]
- Hull, K.L.; Sanford, M.S. Catalytic and highly regioselective cross-coupling of aromatic C-H substrates. J. Am. Chem. Soc. 2007, 129, 11904–11905. [Google Scholar] [CrossRef]
- Hull, K.L.; Sanford, M.S. Mechanism of benzoquinone-promoted palladium-catalyzed oxidative cross-coupling reactions. J. Am. Chem. Soc. 2009, 131, 9651–9653. [Google Scholar] [CrossRef]
- Garcia-Rubia, A.; Urones, B.; Arrayas, R.G.; Carretero, J.C. Pd(II)-catalyzed C-H functionalisation of indoles and pyrroles assisted by the removable N-(2-pyridyl)sulfonyl group: C2-alkenylation and dehydrogenative homocoupling. Chem. Eur. J. 2010, 16, 9676–9685. [Google Scholar] [CrossRef]
- Deprez, N.R.; Kalyani, D.; Krause, A.; Sanford, M.S. Room temperature palladium-catalyzed 2-arylation of indoles. J. Am. Chem. Soc. 2006, 128, 4972–4973. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Stoltz, B.M. Catalytic C-H bond functionalization with palladium(II): Aerobic oxidative annulations of indoles. J. Am. Chem. Soc. 2003, 125, 9578–9579. [Google Scholar] [CrossRef]
- Miyasaka, M.; Hirano, K.; Satoh, T.; Miura, M. Palladium-catalyzed direct oxidative alkenylation of azoles. J. Org. Chem. 2010, 75, 5421–5424. [Google Scholar] [CrossRef]
- Thiery, E.; Aouf, C.; Belloy, J.; Harakat, D.; Le Bras, J.; Muzart, J. Palladium-catalyzed allylic acyloxylation of terminal alkenes in the presence of a base. J. Org. Chem. 2010, 75, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.H.; Check, C.T.; Proust, N.; Stambuli, J.P. Allylic oxidations of terminal olefins using a palladium thioether catalyst. Org. Lett. 2010, 12, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-L.; Labinger, J.A.; Bercaw, J.E. Mechanistic investigations of bipyrimidine-promoted palladium-catalyzed allylic acetoxylation of olefins. Can. J. Chem. 2008, 87, 264–271. [Google Scholar] [CrossRef]
- Campeau, L.-C.; Schipper, D.J.; Fagnou, K. Site-selective sp2 and benzylic sp3 palladium-catalyzed direct arylation. J. Am. Chem. Soc. 2008, 130, 3266–3267. [Google Scholar] [CrossRef]
- Hull, K.L.; Anani, W.Q.; Sanford, M.S. Palladium-catalyzed fluorination of carbon-hydrogen bonds. J. Am. Chem. Soc. 2006, 128, 7134–7135. [Google Scholar] [CrossRef]
- Chen, M.S.; Prabagaran, N.; Labenz, N.A.; White, M.C. Serial ligand catalysis: A highly slective allylic C-H oxidation. J. Am. Chem. Soc. 2005, 127, 6970. [Google Scholar] [CrossRef]
- Nahra, F.; Liron, F.; Prestat, G.; Mealli, C.; Messaoudi, A.; Poli, G. Striking AcOH acceleration in direct intramolecular allylic amination reactions. Chem. Eur. J. 2009, 15, 11078–11082. [Google Scholar] [CrossRef]
- Boutadla, Y.; Davies, D.L.; Macgregor, S.A.; Poblador-Bahamonde, A.I. Mechanisms of C-H bond activation: Rich synergy between computation and experiment. Dalton Trans. 2009, 5820–5831. [Google Scholar] [CrossRef]
- Yin, G.; Wu, Y.; Liu, G. Scope and mechanism of allylic C-H amination of terminal alkenes by the palladium/PhI(OPiv)2 catalyst system: Insights into the effect of naphtoquinone. J. Am. Chem. Soc. 2010, 132, 11978–11987. [Google Scholar] [CrossRef] [PubMed]
- 68 Hartwig, J.F. Allylic substitution. In Organotransition Metal Chemistry: From Bonding to Catalysis, 1st ed.; Murdzek, J., Ed.; University Science Books: Herndon, VA, USA, 2010; pp. 967–1014. [Google Scholar]
- Negishi, E.-i.; Chatterjee, S. Highly regioselective generation of thermodynamic enolates and their direct characterization by NMR. Tetrahedron Lett. 1983, 24, 1341–1344. [Google Scholar] [CrossRef]
- Tsuji, J.; Minami, I.; Shimizu, I. Palladium-catalyzed allylation of ketones and aldehydes with allylic carbonates via silyl enol ethers under neutral conditions. Chem. Lett. 1983, 12, 1325–1326. [Google Scholar] [CrossRef]
- Tsuda, T.; Chujo, Y.; Nishi, S.; Tawara, K.; Saegusa, T. Facile generation of a reactive palladium(II) enolate intermediate by the decarboxylation of palladium(II) beta-ketocarboxylate and its utilization in allylic acylation. J. Am. Chem. Soc. 1980, 102, 6381–6384. [Google Scholar] [CrossRef]
- Pilarski, L.T.; Selander, N.; Böse, D.; Szabo, K.J. Catalytic allylic C-H acetoxylation and benzoyloxylation via suggested (η3-allyl)palladium(IV) intermediates. Org. Lett. 2009, 11, 5518–5521. [Google Scholar] [CrossRef]
- Liu, G.; Yin, G.; Wu, L. Palladium-catalyzed intermolecular aerobic oxidative amination of terminal alkenes: Efficient synthesis of linear allylamine derivatives. Angew. Chem. Int. Ed. 2008, 47, 4733–4736. [Google Scholar] [CrossRef]
- Campbell, A.N.; White, P.B.; Guzei, I.A.; Stahl, S.S. Allylic C-H acetoxylation with a 4,5-diazafluorenone-Ligated palladium catalyst: A ligand-based strategy to achieve aerobic catalytic turnover. J. Am. Chem. Soc. 2010, 132, 15116–15119. [Google Scholar] [CrossRef]
- Covell, D.J.; White, M.C. A chiral lewis acid strategy for enantioselective allylic C-H oxidation. Angew. Chem. Int. Ed. 2008, 47, 6448–6451. [Google Scholar] [CrossRef]
- Stahl, S.S. Palladium oxidase catalysis: Selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Lu, J.; Loh, T.-P. Direct cross-coupling reaction of simple alkenes with acrylates catalyzed by palladium catalyst. J. Am. Chem. Soc. 2009, 131, 1372–1373. [Google Scholar] [CrossRef]
- Hansson, S.; Heumann, A.; Rein, T.; Åkermark, B. Preparation of allylic acetates from simple alkenes by palladium(II)-catalyzed acetoxylation. J. Org. Chem. 1990, 55, 975–984. [Google Scholar] [CrossRef]
- Shimizu, Y.; Obora, Y.; Ishii, Y. Intermolecular Aerobic oxidative allylic amination of simple alkenes with diarylamines catalyzed by the Pd(OCOCF3)2/NPMoV/O2 system. Org. Lett. 2010, 12, 1372–1374. [Google Scholar] [CrossRef]
- Bäckvall, J.-E.; Hopkins, R.B.; Grennberg, H.; Mader, M.M.; Awasthi, A.K. Multistep electron transfer in palladium-catalyzed aerobic oxidations via a metal macrocycle-quinone system. J. Am. Chem. Soc. 1990, 112, 5160–5166. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Li, S.; Pan, D.; Ding, S.; Cui, Y.; Jiao, N. Indoles from simple anilines and alkynes: Palladium-catalyzed C-H activation using dioxygen as the oxidant. Angew. Chem. Int. Ed. 2009, 48, 4572–4576. [Google Scholar] [CrossRef]
- Ding, S.; Shi, Z.; Jiao, N. Pd(II)-catalyzed synthesis of carbolines by iminoannulation of internal alkynes via direct C-H bond cleavage using dioxygen as oxidant. Org. Lett. 2010, 12, 1540–1543. [Google Scholar] [CrossRef]
- Shi, Z.; Cui, Y.; Jiao, N. Synthesis of beta- and gamma-carbolinones via Pd-catalyzed direct dehydrogenative annulation (DDA) of indole-carboxamides with alkynes using air as the oxidant. Org. Lett. 2010, 12, 2908–2911. [Google Scholar] [CrossRef]
- Mariz, R.; Luan, X.; Gatti, M.; Linden, A.; Dorta, R. A chiral bis-sulfoxide ligand in late-transition metal catalysis; Rhodium-catalyzed asymmetric addition of arylboronic acids to electron-deficient olefins. J. Am. Chem. Soc. 2008, 130, 2172–2173. [Google Scholar] [CrossRef] [PubMed]
- Nishikata, T.; Abela, A.R.; Huang, S.; Lipshutz, B.H. Cationic palladium(II) catalysis: C-H activation/Suzuki-Miyaura couplings at room temperature. J. Am. Chem. Soc. 2010, 132, 4978–4979. [Google Scholar] [CrossRef] [PubMed]

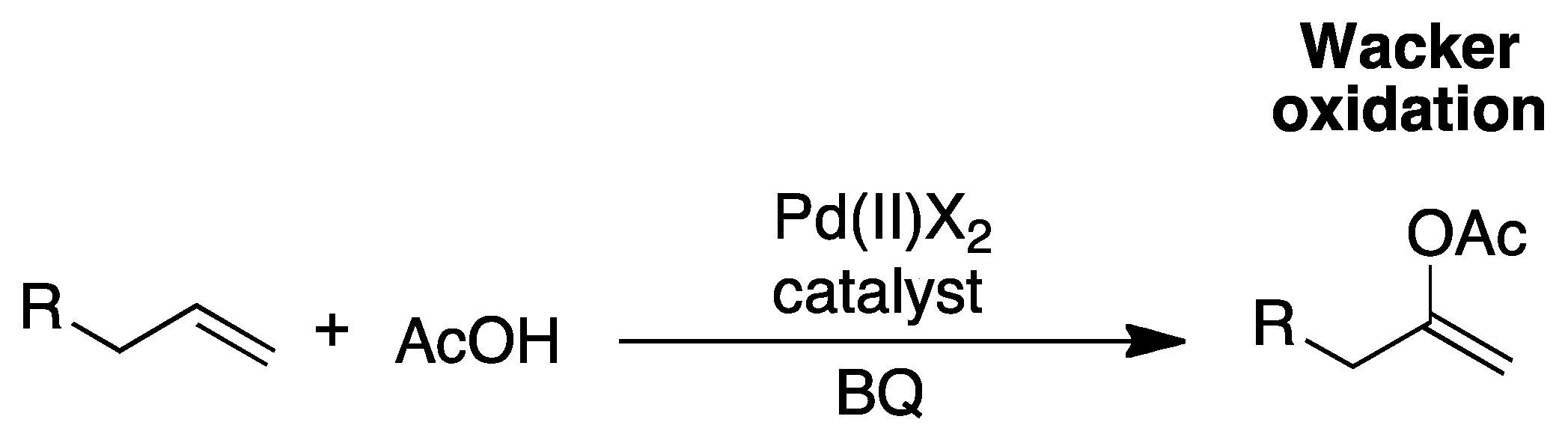



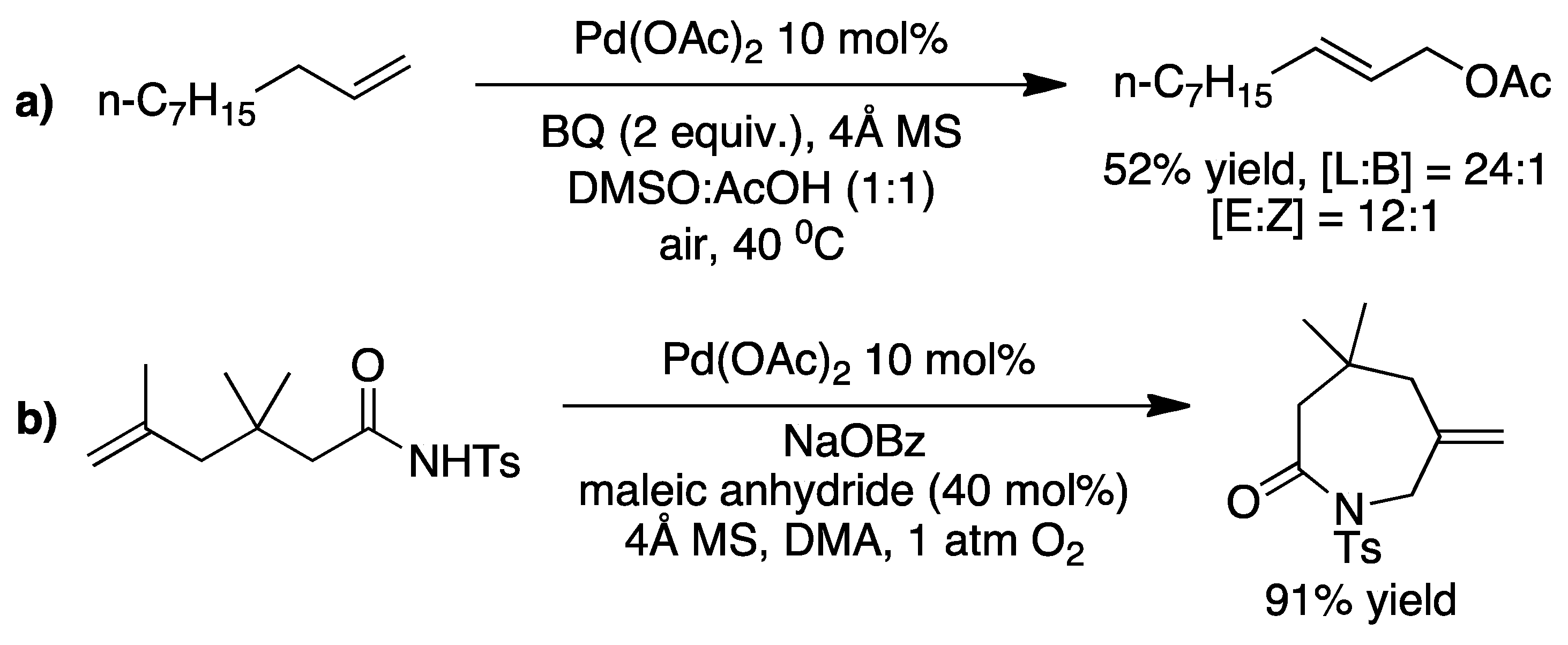

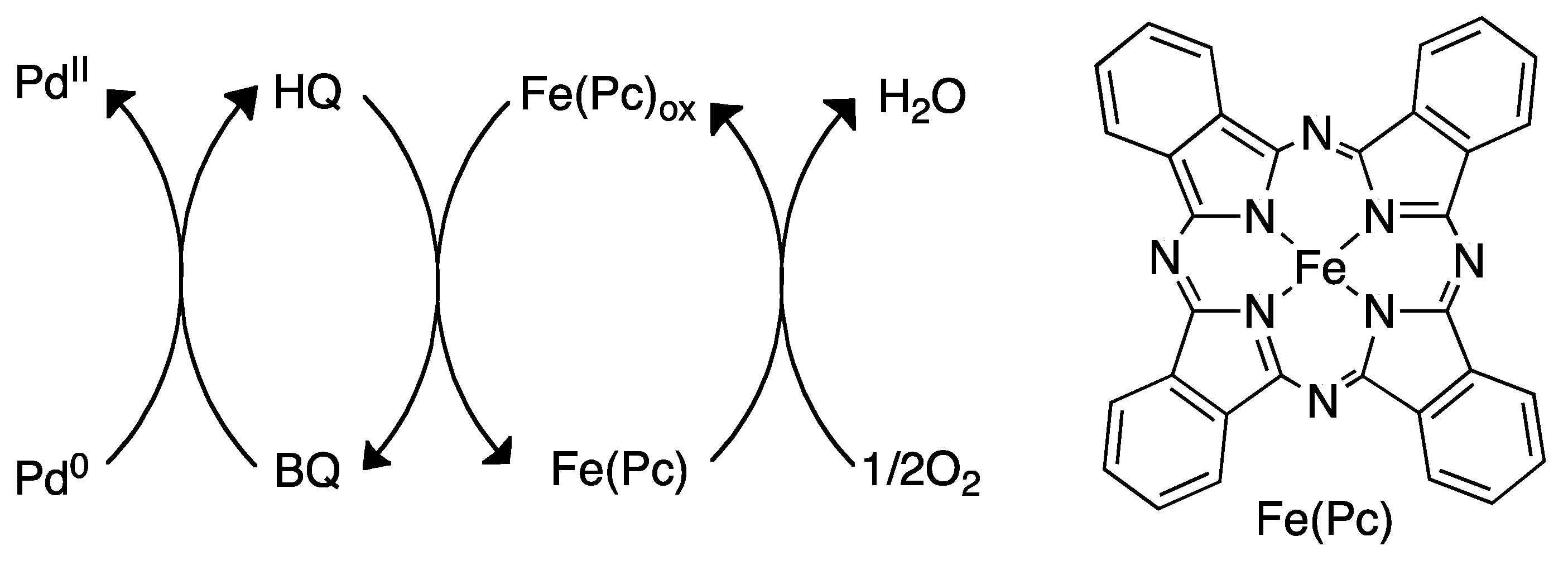
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/3.0/).
Share and Cite
Engelin, C.J.; Fristrup, P. Palladium Catalyzed Allylic C-H Alkylation: A Mechanistic Perspective. Molecules 2011, 16, 951-969. https://doi.org/10.3390/molecules16010951
Engelin CJ, Fristrup P. Palladium Catalyzed Allylic C-H Alkylation: A Mechanistic Perspective. Molecules. 2011; 16(1):951-969. https://doi.org/10.3390/molecules16010951
Chicago/Turabian StyleEngelin, Casper Junker, and Peter Fristrup. 2011. "Palladium Catalyzed Allylic C-H Alkylation: A Mechanistic Perspective" Molecules 16, no. 1: 951-969. https://doi.org/10.3390/molecules16010951
APA StyleEngelin, C. J., & Fristrup, P. (2011). Palladium Catalyzed Allylic C-H Alkylation: A Mechanistic Perspective. Molecules, 16(1), 951-969. https://doi.org/10.3390/molecules16010951