Composition of Sulla (Hedysarum coronarium L.) Honey Solvent Extractives Determined by GC/MS: Norisoprenoids and Other Volatile Organic Compounds
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Honey Samples
3.2. Ultrasonic Solvent Extraction (USE)
3.3. Gas Chromatography and Mass Spectrometry (GC, GC/MS)
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Jerković, I.; Mastelić, J.; Marijanović, Z.; Klein, Ž.; Jelić, M. Comparison of hydrodistillation and ultrasonic solvent extraction for the isolation of volatile compounds from two unifloral honeys of Robinia pseudoacacia L. and Castanea sativa L. Ultrason. Sonochem. 2007, 14, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Evaluation of four isolation techniques for honey aroma compounds. J. Sci. Food Agric. 2005, 85, 91–97. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Pérez-Coello, M.S.; Cabezudo, M.D. Analysis of volatile compounds of rosemary honey. Comparison of different extraction techniques. Chromatographia 2003, 57, 227–233. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- D’Arcy, B.R.; Rintoul, G.B.; Rowland, C.Y.; Blackman, A.J. Composition of Australian honey extractives. 1. Norisoprenoids, monoterpenes, and other natural volatiles from blue gum (Eucalyptus leucoxylon) and yellow box (Eucalyptus melliodora) honeys. J. Agric. Food Chem. 1997, 45, 1834–1843. [Google Scholar] [CrossRef]
- Rowland, C.Y.; Blackman, A.J.; D’Arcy, B.R.; Rintoul, G.B. Comparison of organic extractives found in leatherwood (Eucryphia lucida) honey and leatherwood flowers and leaves. J. Agric. Food Chem. 1995, 43, 753–763. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Tan, S.-T.; Molan, P.C. Extractable organic substances from New Zealand unifloral vipers bugloss (Echium vulgare) honey. J. Apic. Res. 1995, 34, 73–78. [Google Scholar] [CrossRef]
- Tan, S.-T.; Wilkins, A.L.; Holland, P.T.; McGhie, T.K. Extractives from New Zealand unifloral honeys. 2. Degraded carotenoids and other substances from heather honey. J. Agric. Food Chem. 1989, 37, 1217–1221. [Google Scholar] [CrossRef]
- Tan, S.-T.; Holland, P.T.; Wilkins, A.L.; Molan, P.C. Extractives from New Zealand honeys. 1. White clover, manuka, and manuka unifloral honeys. J. Agric. Food Chem. 1988, 36, 453–460. [Google Scholar] [CrossRef]
- De Maria, C.A.B.; Moreira, R.F.A. Volatile compounds in floral honeys. Quim. Nova 2003, 26, 90–96. [Google Scholar] [CrossRef]
- de la Fuente, E.; Valencia-Barrera, R.M.; Martïnez-Castro, I.; Sanz, J. Occurrence of 2-hydroxy-5-methyl-3-hexanone and 3-hydroxy-5-methyl-2-hexanone as indicators of botanic origin in eucalyptus honeys. Food Chem. 2007, 103, 1176–1180. [Google Scholar] [CrossRef]
- Guyot, C.; Bouseta, A.; Scheirman, V.; Collin, S. Floral origin markers of chestnut and lime tree honeys. J. Agric. Food Chem. 1998, 46, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Guyot, C.; Scheirman, V.; Collin, S. Floral origin markers of heather honeys: Calluna vulgaris and Erica arborea. Food Chem. 1999, 64, 3–11. [Google Scholar] [CrossRef]
- Sulas, L.; Re, G.A.; Ledda, L.; Caredda, S. The effect of utilization frequency on the forage production of sulla (Hedysarum coronarium L.). Ital. J. Agron. 1997, 2, 89–94. [Google Scholar]
- Bonvehí, J.S.; Torrento, M.S.; Muntané Raich, J. Invertase activity in fresh and processed honeys. J. Sci. Food Agric. 2000, 80, 507–512. [Google Scholar] [CrossRef]
- Marini, F.; Magrì, A.L.; Balestrieri, F.; Fabretti, F.; Marini, D. Supervised pattern recognition applied to the discrimination of the floral origin of six types of Italian honey samples. Anal. Chim. Acta 2004, 515, 117–125. [Google Scholar] [CrossRef]
- Winterhalter, P.; Russell, L. Carotenoid-derived aroma compounds; American Chemical Society: Washington DC, USA, 2002; pp. 1–17. [Google Scholar]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Careri, M.; Musci, M. Volatile norisoprenoids as markers of botanical origin of Sardinian strawberry-tree (Arbutus unedo L.) honey: Characterisation of aroma compounds by dynamic headspace extraction and gas chromatography-mass spectrometry. Food Chem. 2005, 89, 527–532. [Google Scholar] [CrossRef]
- Graddon, A.D.; Morrison, J.D.; Smith, J.F. Volatile constituents of some unifloral Australian honeys. J. Agric. Food Chem. 1979, 27, 832–837. [Google Scholar] [CrossRef]
- Jerković, I.; Hegić, G.; Marijanović, Z.; Bubalo, D. Organic extractives from Mentha spp. honey and the bee-stomach: methyl syringate, vomifoliol, terpenediol I, hotrienol and other compounds. Molecules 2010, 15, 2911–2924. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Slessor, K.N.; Higo, H.A.; Winston, M.L. Isolation and identification of new components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc. Nat. Acad. Sci. USA 2003, 100, 4486–4491. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Bifulco, E.; Jerković, I.; Caboni, P.; Cabras, P.; Floris, I. Methyl syringate: a chemical marker of asphodel (Asphodelus microcarpus Salzm. et Viv.) monofloral honey J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Marijanović, Z.; Ljubičić, I.; Gugić, M. Contribution of the bees and combs to honey volatiles: blank-trial probe for honey biodiversity chemical profiling. Chem. Biodivers. 2010, 7, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 39–153. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The pherobase: database of insect pheromones and semiochemicals. http://www.pherobase.com/ (accessed 21 June 2010).
Sample Availability: Contact the corresponding author. |
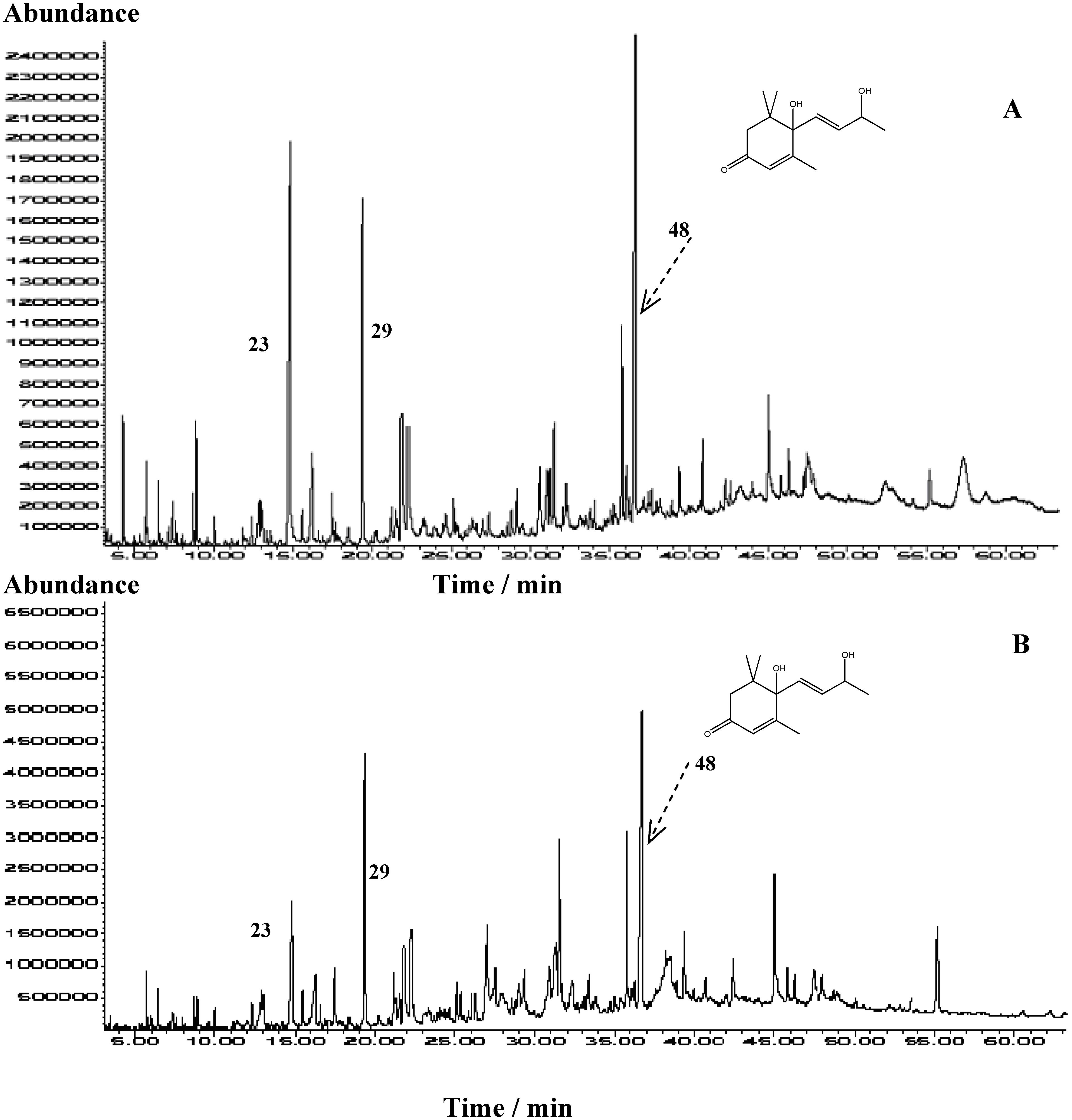
| No. | Compound | RI | Area percentage (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent A | Solvent B | ||||||||||
| Min. | Max. | Av. | SD. | Min. | Max. | Av. | SD. | ||||
| 1. | 2-Furanmethanol | < 900 | 0.0 | 0.1 | 0.05 | 0.06 | - | - | - | - | |
| 2. | 2-Methylbutanoic acid | < 900 | 0.0 | 0.1 | 0.05 | 0.06 | - | - | - | - | |
| 3. | Hexan-1-ol | < 900 | 0.0 | 0.1 | 0.08 | 0.05 | - | - | - | - | |
| 4. | 1,4-Dimethylbenzene** | < 900 | 0.0 | 0.2 | 0.10 | 0.08 | - | - | - | - | |
| 5. | Benzaldehyde | 965 | 0.0 | 0.1 | 0.05 | 0.06 | 0.1 | 0.2 | 0.13 | 0.05 | |
| 6. | 2,4-Dimethyl-3,6-dihydro-2H-pyran | 973 | 0.0 | 0.1 | 0.05 | 0.06 | - | - | - | - | |
| 7. | 2-Methylfuran* | 988 | - | - | - | - | 0.2 | 2.3 | 0.98 | 0.93 | |
| 8. | 2-Hydroxy-3-methylcyclopent-2-en-1-one | 1036 | - | - | - | - | 0.0 | 0.1 | 0.05 | 0.06 | |
| 9. | Benzyl alcohol | 1037 | 0.1 | 0.3 | 0.18 | 0.09 | 0.0 | 0.3 | 0.15 | 0.13 | |
| 10. | Dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone (Pantolactone) | 1044 | 0.1 | 0.2 | 0.15 | 0.06 | 0.3 | 0.6 | 0.43 | 0.15 | |
| 11. | Phenylacetaladehyde | 1048 | 0.0 | 0.2 | 0.13 | 0.10 | 0.3 | 0.5 | 0.28 | 0.17 | |
| 12. | 4,5-Dimethyl-2-formylfuran | 1078 | 0.4 | 0.8 | 0.40 | 0.29 | 0.3 | 0.7 | 0.26 | 0.26 | |
| 13. | Methyl 2-furoate | 1084 | 0.0 | 0.4 | 0.18 | 0.21 | 0.0 | 1.5 | 0.55 | 0.71 | |
| 14. | 1-(2-Furanyl)-2-hydroxyethanone | 1088 | 0.2 | 0.7 | 0.45 | 0.21 | 0.8 | 2.2 | 1.23 | 0.66 | |
| 15. | 2-Phenylethanol | 1116 | 0.2 | 0.4 | 0.30 | 0.12 | 0.2 | 0.5 | 0.35 | 0.13 | |
| 16. | 3,5,5-Trimethyl-cyclohex-3-en-1-one (α-Isophorone) | 1124 | 0.0 | 0.2 | 0.08 | 0.10 | 0.0 | 0.2 | 0.10 | 0.08 | |
| 17. | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 1145 | 0.3 | 0.6 | 0.43 | 0.15 | 0.2 | 0.9 | 0.48 | 0.31 | |
| 18. | 3,5,5-Trimethyl-cyclohex-2-ene-1,4-dione (4-Ketoisophorone) | 1147 | 0.0 | 0.2 | 0.10 | 0.08 | 0.0 | 0.2 | 0.10 | 0.08 | |
| 19.. | Benzoic acid | 1162 | 0.4 | 4.6 | 1.73 | 1.95 | 0.4 | 3.9 | 1.48 | 1.67 | |
| 20. | 2,4-Dimethylphenol** | 1181 | 0.3 | 0.4 | 0.33 | 0.10 | 0.3 | 0.5 | 0.33 | 0.13 | |
| 21. | (E)-3,7-Dimethyl-octa-1,5-diene-3,7-diol | 1191 | 0.0 | 0.4 | 0.20 | 0.23 | 0.3 | 0.6 | 0.45 | 0.13 | |
| 22. | Decanal | 1207 | 0.0 | 0.2 | 0.08 | 0.10 | 0.0 | 0.1 | 0.05 | 0.06 | |
| 23. | 5-Hydroxymethylfurfural | 1230 | 2.4 | 9.3 | 4.78 | 3.08 | 6.5 | 22.8 | 13.03 | 6.91 | |
| 24. | 1,3-Bis(1,1-dimethylethyl)benzene | 1261 | 0.0 | 0.7 | 0.23 | 0.33 | - | - | - | - | |
| 25. | Phenylacetic acid | 1269 | 1.1 | 5.8 | 2.90 | 2.02 | 1.0 | 4.8 | 2.53 | 1.62 | |
| 26. | Nonanoic acid | 1273 | 0.0 | 0.2 | 0.08 | 0.10 | - | - | - | - | |
| 27. | 1-Methoxy-4-propylbenzene | 1305 | 0.0 | 0.5 | 0.18 | 0.24 | 0.0 | 0.8 | 0.40 | 0.34 | |
| 28. | 2,4,6-Trimethylphenol** | 1332 | 0.0 | 0.6 | 0.28 | 0.32 | 0.0 | 0.4 | 0.13 | 0.19 | |
| 29. | 3-Hydroxy-4-phenylbutan-2-one | 1354 | 0.8 | 5.4 | 3.73 | 2.02 | 0.6 | 5.7 | 3.43 | 2.15 | |
| 30. | 1-(4-Methoxyphenyl)-ethanone (4-Methoxyacetophenone) | 1360 | 0.0 | 0.2 | 0.08 | 0.10 | - | - | - | - | |
| 31. | (E)-8-Hydroxylinalool | 1367 | 0.0 | 1.1 | 0.33 | 0.53 | 0.2 | 0.3 | 0.23 | 0.05 | |
| 32. | Tetradecane | 1400 | 0.0 | 0.8 | 0.28 | 0.38 | - | - | - | - | |
| 33. | 4-Hydroxyphenyl ethanol | 1445 | 0.0 | 1.2 | 0.80 | 0.57 | 0.0 | 0.4 | 0.18 | 0.21 | |
| 34. | 4-Methoxybenzoic acid | 1451 | 0.0 | 0.7 | 0.23 | 0.33 | 0.0 | 0.6 | 0.33 | 0.25 | |
| 35. | 3-Phenylprop-2-enoic acid** (Cinnamic acid) | 1454 | 0.0 | 0.9 | 0.40 | 0.38 | 0.0 | 0.6 | 0.33 | 0.25 | |
| 36. | 4-Methoxyphenylacetic acid | 1496 | 0.0 | 2.6 | 0.85 | 1.23 | 0.0 | 2.2 | 0.73 | 1.04 | |
| 37. | Pentadecane | 1500 | 0.0 | 0.5 | 0.20 | 0.25 | - | - | - | - | |
| 38. | 4-Methyl-2,6-bis(1,1-dimethylethyl)-phenol | 1514 | 1.1 | 3.0 | 1.83 | 0.82 | 0.0 | 0.1 | 0.05 | 0.06 | |
| 39. | α-Hydroxyphenylpropanoic acid | 1545 | 0.0 | 15.7 | 5.25 | 7.40 | 0.0 | 1.4 | 0.48 | 0.66 | |
| 40. | 2-Hydroxydecanoic acid | 1557 | 0.0 | 2.0 | 0.78 | 0.86 | 0.0 | 0.6 | 0.33 | 0.25 | |
| 41. | 4-Hydroxybenzoic acid | 1558 | 0.2 | 1.2 | 0.55 | 0.55 | - | - | - | - | |
| 42. | cis-p-Menth-8-ene* | 1632 | 0.0 | 0.9 | 0.38 | 0.45 | 0.0 | 1.7 | 0.68 | 0.73 | |
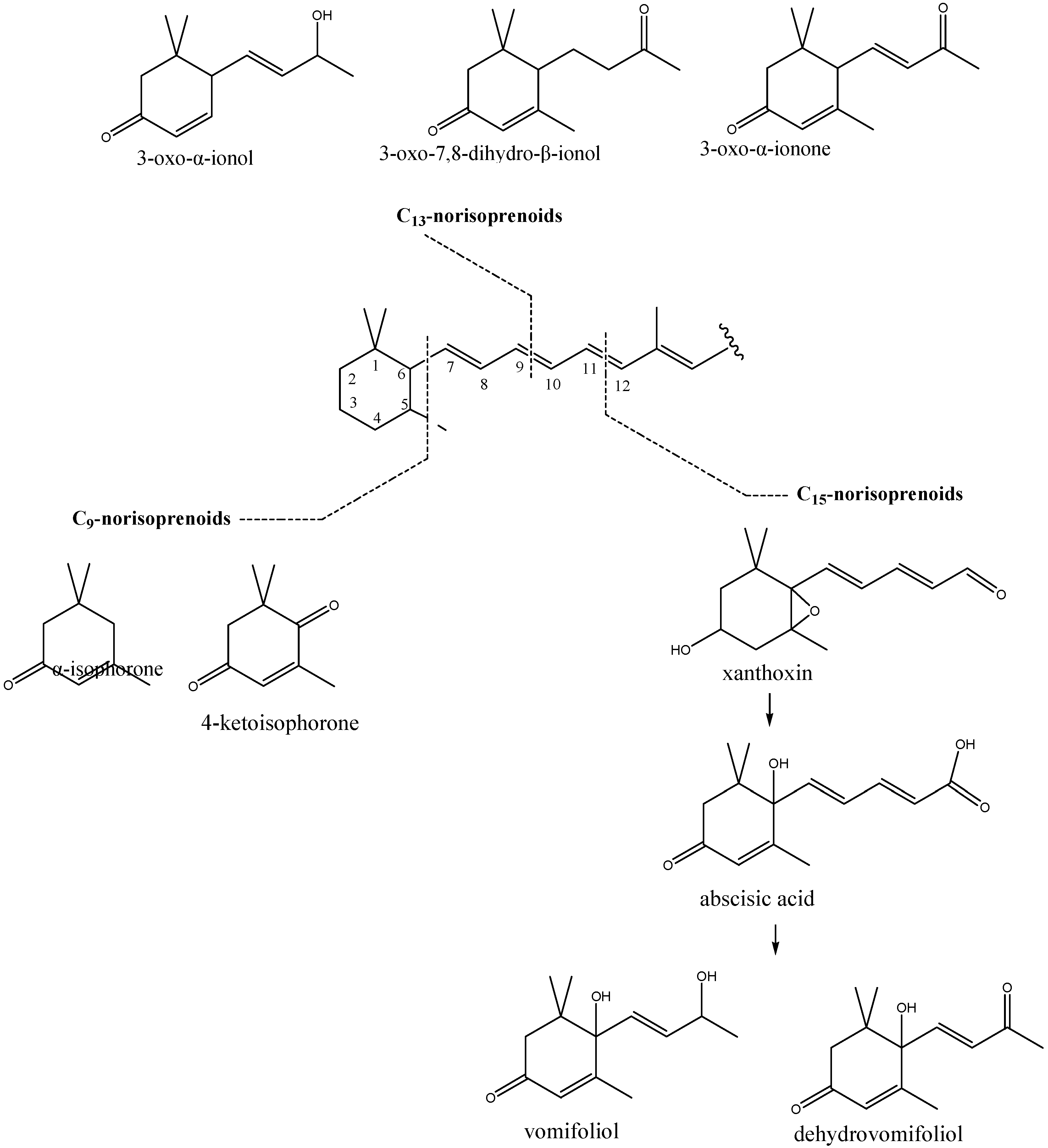
| 43. | 3-Oxo-α-ionol | 1660 | 0.2 | 1.5 | 0.83 | 0.56 | 1.0 | 1.2 | 1.08 | 0.10 | |
| 44. | 3-Oxo-α-ionone | 1665 | 0.0 | 2.9 | 1.90 | 1.32 | 1.1 | 1.9 | 1.60 | 0.38 | |
| 45. | 3-Oxo-7,8-dihydro-α-ionone | 1682 | 0.0 | 0.6 | 0.38 | 0.26 | - | - | - | - | |
| 46. | 6,7-Dehydro-7,8-dihydro-3-oxo-α-ionol | 1720 | 0.0 | 0.6 | 0.25 | 0.30 | - | - | - | - | |
| 47. | Methyl syringate | 1744 | 3.0 | 5.7 | 4.38 | 1.15 | 2.2 | 4.1 | 3.28 | 0.79 | |
| 48. | 6-Hydroxy-3-oxo-α-ionol (Vomifoliol) | 1802 | 5.3 | 11.2 | 8.88 | 2.62 | 9.6 | 14.0 | 11.63 | 1.84 | |
| 49. | Hexadecan-1-ol | 1882 | 0.0 | 3.6 | 1.50 | 1.51 | 0.0 | 2.6 | 1.18 | 1.07 | |
| 50. | Nonadecane | 1900 | 0.0 | 0.7 | 0.33 | 0.38 | - | - | - | - | |
| 51. | Hexadecanoic acid | 1963 | 0.9 | 2.5 | 1.43 | 0.73 | 0.5 | 1.1 | 0.75 | 0.25 | |
| 52. | (Z)-Octadec-9-en-1-ol | 2060 | 1.0 | 4.9 | 2.55 | 1.95 | 1.5 | 3.6 | 2.70 | 0.98 | |
| 53. | Octadecan-1-ol | 2084 | 0.0 | 0.8 | 0.50 | 0.36 | - | - | - | - | |
| 54. | Heneicosane | 2100 | 0.0 | 0.6 | 0.20 | 0.28 | - | - | - | - | |
| 55. | (Z)-Octadec-9-enoic acid | 2147 | 0.0 | 2.4 | 1.30 | 1.10 | 0.0 | 1.2 | 0.58 | 0.53 | |
| 56. | Tetracosane | 2400 | 0.0 | 3.1 | 1.60 | 1.68 | 0.0 | 3.3 | 1.85 | 1.37 | |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jerković, I.; Tuberso, C.I.G.; Gugić, M.; Bubalo, D. Composition of Sulla (Hedysarum coronarium L.) Honey Solvent Extractives Determined by GC/MS: Norisoprenoids and Other Volatile Organic Compounds. Molecules 2010, 15, 6375-6385. https://doi.org/10.3390/molecules15096375
Jerković I, Tuberso CIG, Gugić M, Bubalo D. Composition of Sulla (Hedysarum coronarium L.) Honey Solvent Extractives Determined by GC/MS: Norisoprenoids and Other Volatile Organic Compounds. Molecules. 2010; 15(9):6375-6385. https://doi.org/10.3390/molecules15096375
Chicago/Turabian StyleJerković, Igor, Carlo I.G. Tuberso, Mirko Gugić, and Dragan Bubalo. 2010. "Composition of Sulla (Hedysarum coronarium L.) Honey Solvent Extractives Determined by GC/MS: Norisoprenoids and Other Volatile Organic Compounds" Molecules 15, no. 9: 6375-6385. https://doi.org/10.3390/molecules15096375
APA StyleJerković, I., Tuberso, C. I. G., Gugić, M., & Bubalo, D. (2010). Composition of Sulla (Hedysarum coronarium L.) Honey Solvent Extractives Determined by GC/MS: Norisoprenoids and Other Volatile Organic Compounds. Molecules, 15(9), 6375-6385. https://doi.org/10.3390/molecules15096375





