In Vitro Antiophidian Properties of Dipteryx alata Vogel Bark Extracts
Abstract
:1. Introduction
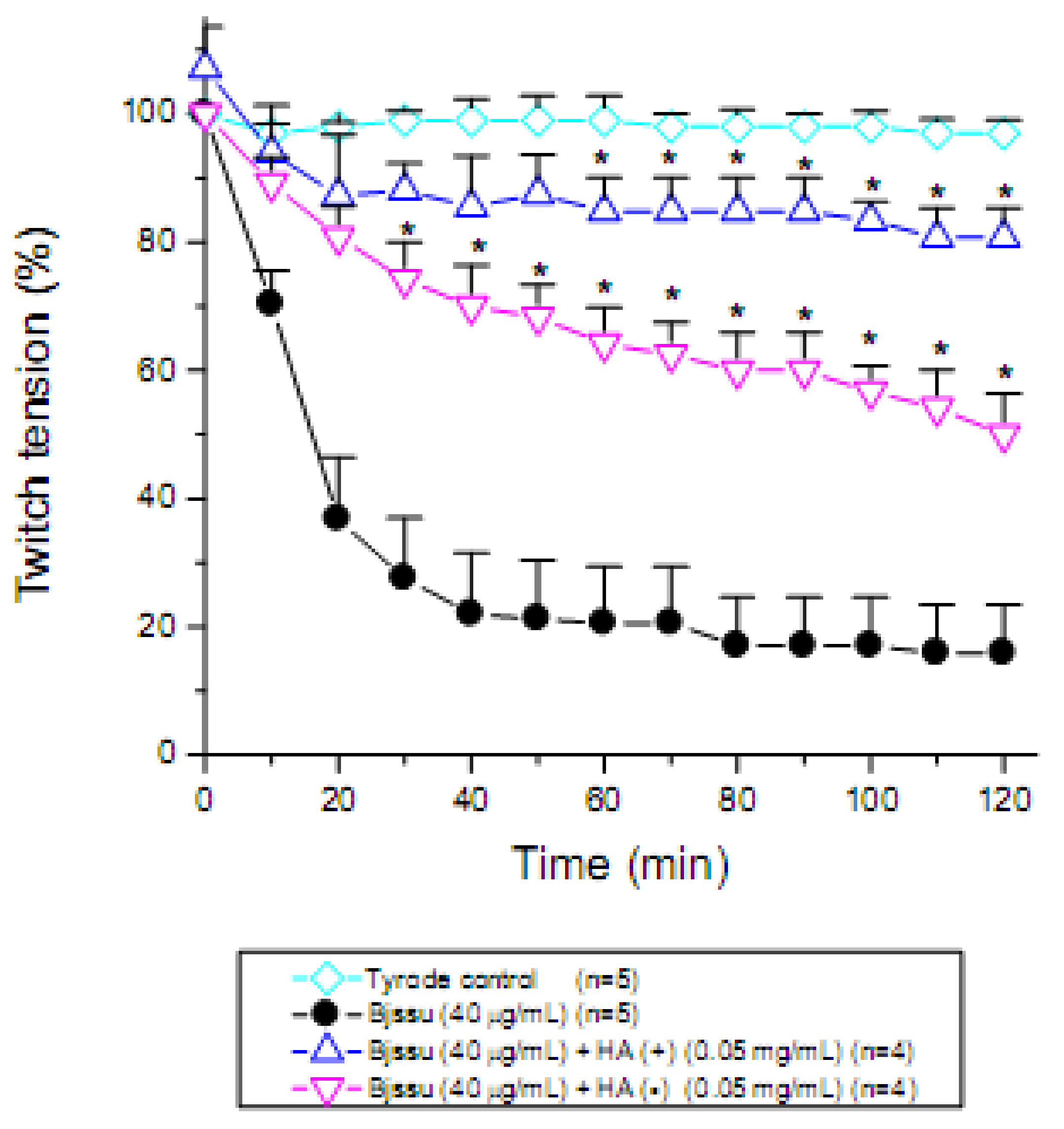
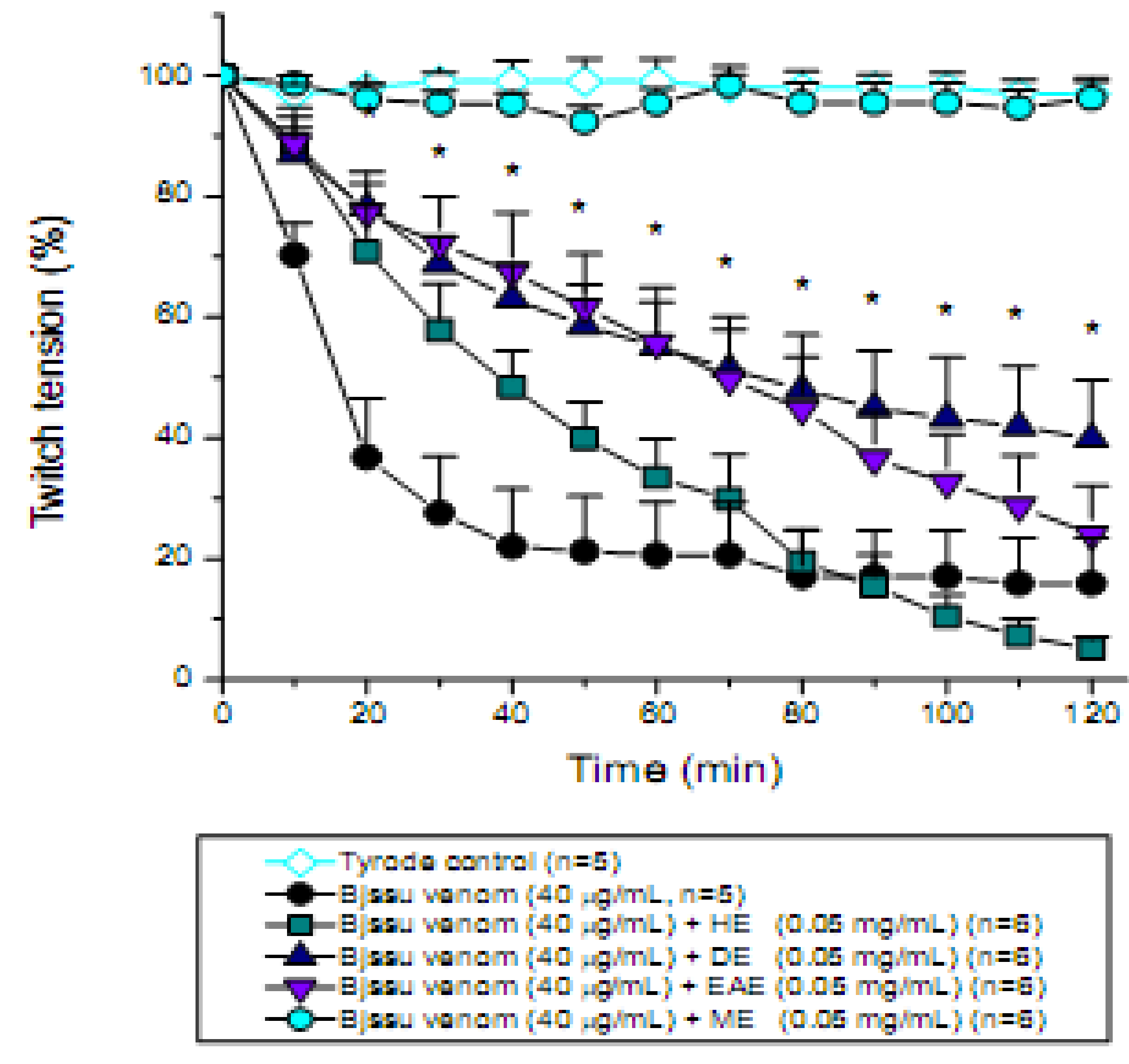
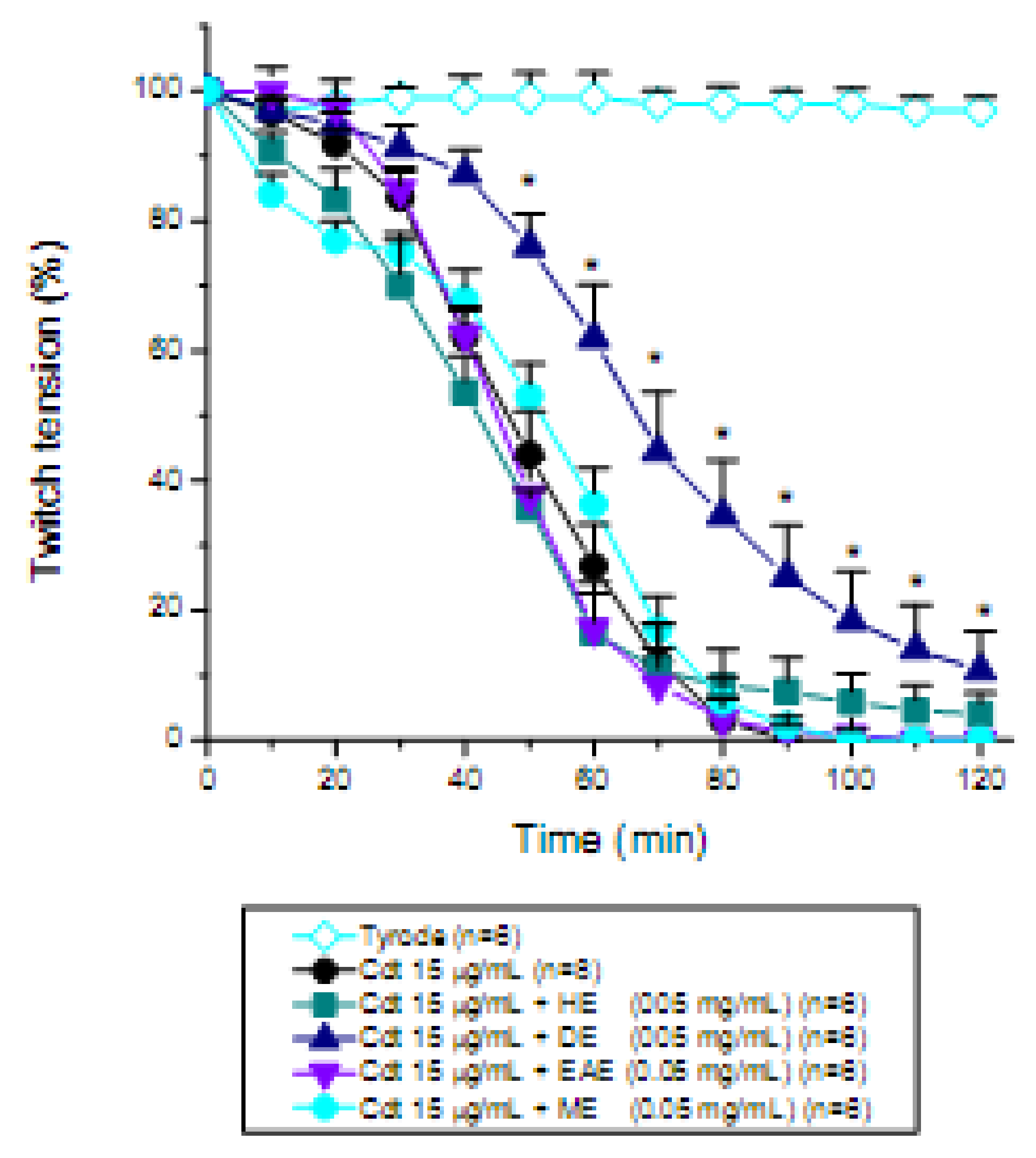
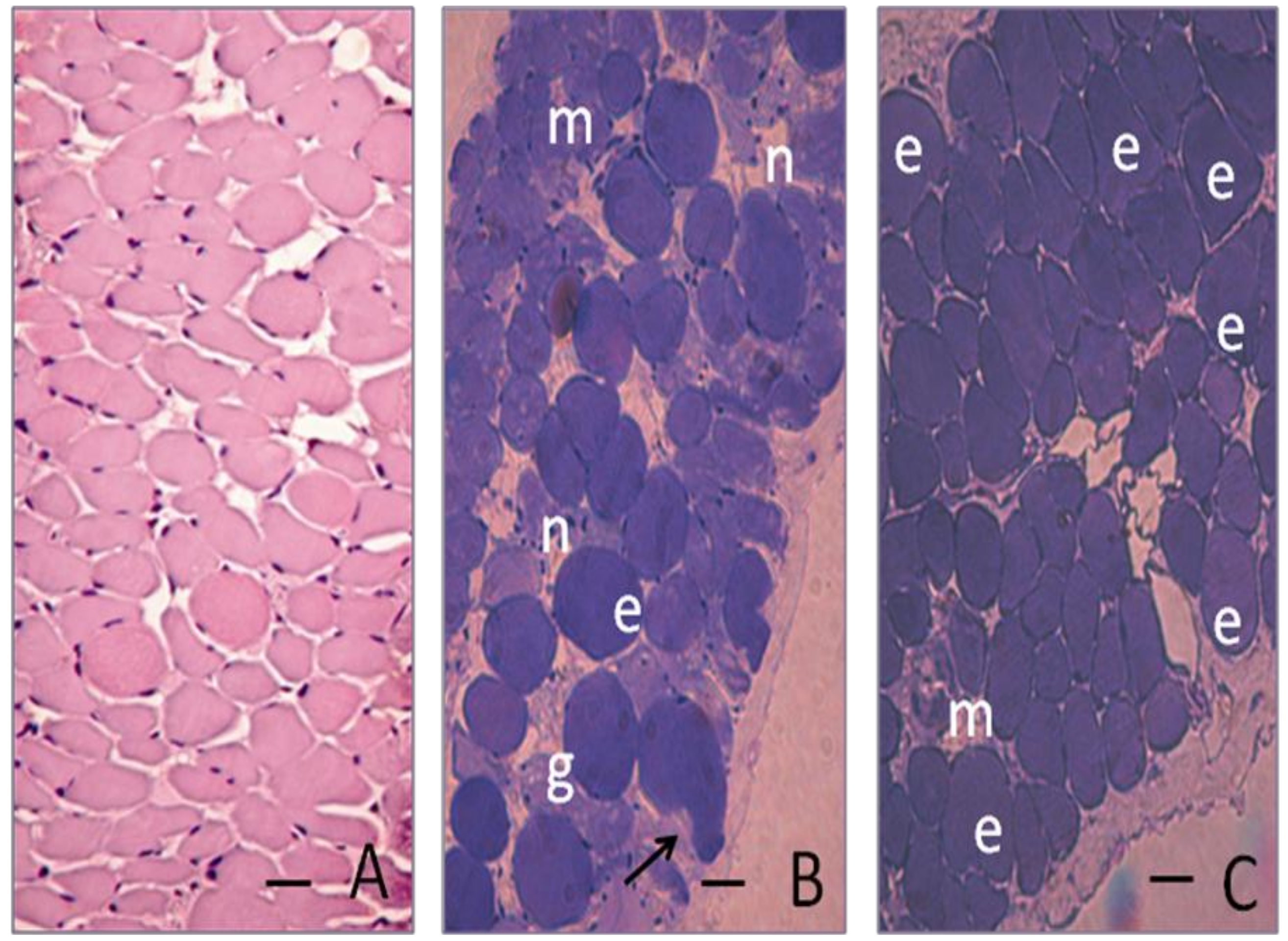
2. Results and Discussion
3. Experimental
3.1. Animals
3.2. Venoms
3.3. Dipteryx alata Vogel extracts
3.4. Protein precipitation assay and tannins determination
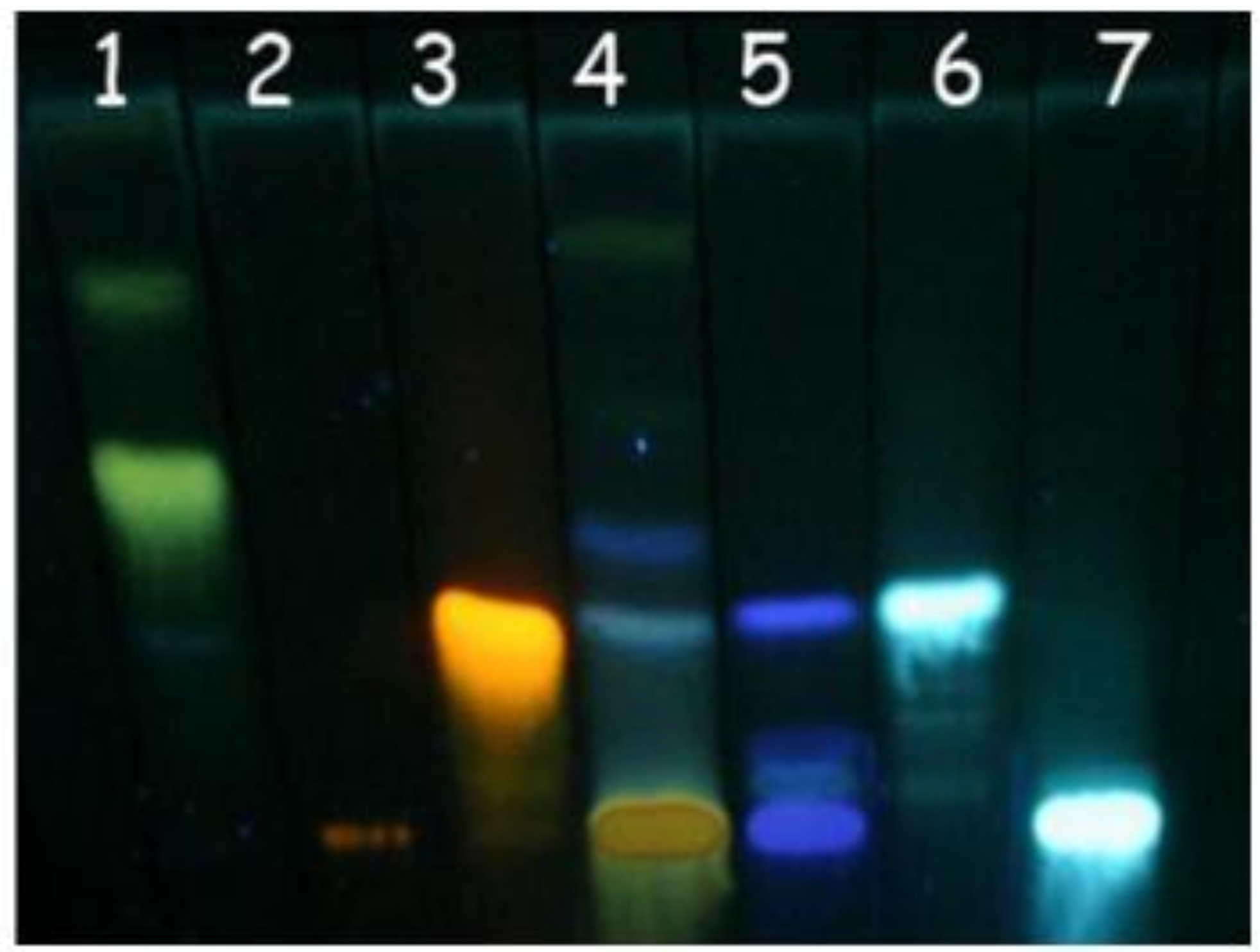
3.5. Thin layer chromatography
3.6. Mouse phrenic nerve-diaphragm muscle (PND) preparation
3.7. Histological and quantitative study
3.8. Statistical analysis
4. Conclusions
Acknowledgments
References
- Mirshafiey, A. Venom therapy in multiple sclerosis. Neuropharmacology 2007, 53, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.H.; Soares, A.M.; Rodrigues, V.M.; Oliveira, F.; Fransheschi, A.M.; Rucavado, A.; Giglio, J.R.; Homsi-Brandeburgo, M.I. Neutralization of proteases from Bothrops snake venoms by the aqueous extract from Casearia sylvestris (Flacourtiaceae). Toxicon 2001, 39, 1863–1869. [Google Scholar] [CrossRef]
- Da Silva, J.O.; Coppede, J.S.; Fernandes, V.C.; Sant’Ana, C.D.; Ticli, F.K.; Mazzi, M.V.; Giglio, J.R.; Pereira, O.S.; Soares, A.M.; Sampaio, S.V. Antihemorrhagic, antinucleolytic and other antiophidian properties of the aqueous extract from Pentaclethra macroloba. J. Ethnopharmacol. 2005, 100, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, C.M.; Rodrigues, C.M.; Silva, M.A.; Lopes-Ferreira, M.; Vilegas, W.; Hiruma-Lima, C.A. Anti-hemorrhagic activity of four Brazilian vegetable species against Bothrops jararaca venom. Molecules 2009, 14, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. Inhibition of Naja naja venom hyaluronidase by plant-derived bioactive components and polysaccharides. Biochemistry (Moscow). 2005, 70, 948–952. [Google Scholar] [CrossRef]
- Oliveira, C.Z.; Maiorano, V.A.; Marcussi, S.; Sant’Ana, C.D.; Januário, A.H.; Lourenço, M.V.; Sampaio, S.V.; França, S.C.; Pereira, O.S.; Soares, A.M. Anticoagulant and antifibrinogenolytic properties of the aqueous extract from Bauhinia forficata against snake venoms. J. Ethnopharmacol. 2005, 98, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Aguiyi, J.C.; Guerranti, R.; Pagani, R.; Marinello, E. Blood chemistry of rats pretreated with Mucuna pruriens seed aqueous extract MP101UJ after Echis carinatus venom challenge. Phytother. Res. 2001, 15, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Mors, W.B.; do Nascimento, M.C.; Parente, J.P.; da Silva, M.H.; Melo, P.A.; Suarez-Kurtz, G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae). Toxicon 1989, 27, 1003–1009. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Cherdchu, C.; Chudapongse, P. Studies on the cobra neurotoxin inhibiting activity in an extract of Curcuma sp. (Zingiberaceae) rhizome. Southeast Asian J. Trop. Med. Public Health 1993, 24, 178–185. [Google Scholar] [PubMed]
- Maiorano, V.A.; Marcussi, S.; Daher, M.A.F.; Oliveira, C.Z.; Couto, L.B.; Gomes, A.O.; França, S.C.; Soares, A.M.; Pereira, O.S. Antiophidian properties of the aqueous extract of Mikania glomerata. J. Ethnopharmacol. 2005, 102, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Sévenet, T. Looking for new drugs: what criteria? J. Ethnopharmacol. 1991, 32, 83–90. [Google Scholar] [CrossRef]
- Bezerra, J.A.; Campos, A.C.; Vasconcelos, P.R.; Nicareta, J.R.; Ribeiro, E.R.; Sebastião, A.P.; Urdiales, A.I.; Moreira, M.; Borges, A.M. Extract of Passiflora edulis in the healing of colonic anastomosis in rats: tensiometric and morphologic study. Acta Cir. Bras. 2006, 21, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.; Ticli, F.K.; Marcussi, S.; Lourenço, M.V.; Januário, A.H.; Sampaio, S.V.; Giglio, J.R.; Lomonte, B.; Pereira, P.S. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005, 12, 2625–2641. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Ahmad, M.; Jabeen, A.; Zafar, M.; Nadeem, S. Pharmacognostic studies of some indigenous medicinal plants of Pakistan. Ethnobotanical Leaflets 2005, 9, 1–7. [Google Scholar]
- Cintra-Francischinelli, M.; Silva, M.G.; Andréo-Filho, N.; Gerenutti, M.; Cintra, A.C.O.; Giglio, J.R.; Leite, G.B.; Cruz-Höfling, M.A.; Rodrigues-Simioni, L.; Oshima-Franco, Y. Antibothropic action of Casearia sylvestris Sw (Flacourtiaceae) extracts. Phytother. Res. 2008, 22, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S.; Farrapo, N.M.; Rocha-Junior, D.S.; Silva, M.G.; Cogo, J.C.; Dal Belo, C.A.; Rodrigues-Simioni, L.; Groppo, F.C.; Oshima-Franco, Y. Antiophidian mechanisms of medicinal plants. In Flavonoids: Biosynthesis, Biological Effects and Dietary Sources; Keller, R.B., Ed.; Nova Science: New York, NY, USA, 2009; pp. 249–262. [Google Scholar]
- Cintra-Francischinelli, M.; Pizzo, P.; Rodrigues-Simioni, L.; Ponce-Soto, L.A.; Rossetto, O.; Lomonte, B.; Gutiérrez, J.M.; Pozzan, T.; Montecucco, C. Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of acceptors. Cell Mol. Life Sci. 2009, 66, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde do Brasil. Manual de Diagnóstico e Tratamento de Acidentes Por Animais Peçonhentos; Fundação Nacional da Saúde: Brasília, Brazil, 2001. [Google Scholar]
- Oshima-Franco, Y.; Hyslop, S.; Prado-Franceschi, J.; Cruz-Höfling, M.A.; Rodrigues-Simioni, L. Neutralizing capacity of antisera raised in horses and rabbits against Crotalus durissus terrificus (South American rattlesnake) venom and its main toxin, crotoxin. Toxicon 1999, 37, 1341–1357. [Google Scholar] [CrossRef]
- Oshima-Franco, Y.; Leite, G.B.; Silva, G.H.; Cardoso, D.F.; Hyslop, S.; Giglio, J.R.; da Cruz-Höfling, M.A.; Rodrigues-Simioni, L. Neutralization of the pharmacological effects of bothropstoxin-I from Bothrops jararacussu (jararacuçu) venom by crotoxin antiserum and heparin. Toxicon 2001, 39, 1477–1485. [Google Scholar] [CrossRef]
- Dos Santos, M.G.; Lolis, S.F.; Dal Belo, C.A. Ethnobotanic Survey of Two Remaining Communities of Black-Africans of Jalapão Region Tocantins State; Daliana Editora: Belo Horizonte, Brazil, 2006; pp. 29–49. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Plantarum: Nova Odessa, Brazil, 1992. [Google Scholar]
- Togashi, M.; Sgarbieri, V.C. Avaliação nutricional da proteína e do óleo de semente de baru (Dipteryx alata Vog.). Ciênc. Technol. Aliment. 1995, 15, 66–69. [Google Scholar]
- Guerranti, R.; Aguiyi, J.C.; Néri, S.; Leoncini, R.; Pagani, R.; Marinello, E. Proteins from Mucuna pruriens and enzymes from Echis carinatus venom: characterization and cross-reactions. J. Biol. Chem. 2002, 277, 17072–17078. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plants Analysis. Chapman & Hall: London, UK, 1998. [Google Scholar]
- Mors, W.B.; do Nascimento, M.C.; Parente, J.P.; da Silva, M.H.; Melo, P.A.; Suarez-Kurtz, G. Plant natural products active against sanke bite – the molecular approach. Phytochemistry 2000, 55, 627–642. [Google Scholar] [CrossRef]
- Kuppusamy, U.R.; Das, N.P. Protective effects of tannic acid and related natural compounds on Crotalus adamanteus subcutaneous poisoning in mice. Pharmacol. Toxicol. 1993, 72, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pithayanukul, P.; Ruenraroengsak, P.; Bavovada, R.; Pakmanee, N.; Suttisri, R. In vitro investigation of the protective effects of tannic acid against the activities of Naja kaouthia venom. Pharm. Biol. 2007, 45, 94–97. [Google Scholar] [CrossRef]
- Haslam, E. Vegetable tannins-lessons of a phytochemical lifetime. Phytochemistry 2007, 68, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.T.; dos Santos, A.T.; Pimenta, F.C. Atividade antimicrobiana das plantas. Rev. Patol. Trop. 2005, 34, 113–122. [Google Scholar]
- Dos Santos, S.C.; Krueger, C.L.; Steil, A.A.; Kreuger, M.R.; Biavatti, M.W.; Wisniewski, A., Jr. LC characterisation of guaco medicinal extracts, Mikania laevigata and M. glomerata, and their effects on allergic pneumonits. Planta Med. 2006, 72, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Bon, C. Multicomponent neurotoxic phospholipases A2. In Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism; Kini, R.M., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 269–285. [Google Scholar]
- Chang, C.C.; Lee, J.D. Crotoxin, the neurotoxin of South American rattlesnake venom, is a presynaptic toxin acting like beta-bungarotoxin. Naunyn Schmiedebergs Arch. Pharmacol. 1977, 296, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Slotta, K.H.; Fraenkel-Conrat, H. Schlangengiffe, III: Mitteilung Reiningung und crystallization des klappershclangengiffes. Ber. Dtsch. Chem. Ges. 1938, 71, 1076–1081. [Google Scholar] [CrossRef]
- Homsi-Brandeburgo, M.I.; Queiroz, L.S.; Santo-Neto, H.; Rodrigues-Simioni, L.; Giglio, J.R. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of bothropstoxin. Toxicon 1988, 26, 615–627. [Google Scholar] [CrossRef]
- Cavalcante, W.L.G.; Campos, T.O.; Dal Pai-Silva, M.D.; Pereira, O.S.; Oliveira, C.Z.; Soares, A.M.; Gallaci, M. Neutralization of snake venom phospholipase A2 toxins by aqueous extract of Casearia sylvestris (Flacourtiaceae) in mouse neuromuscular preparation. J. Ethnopharmacol. 2008, 112, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Portuguese Pharmacopoeia Committee. Portuguese Pharmacopoeia; Infarmed Editors: Lisboa, Portugal, 2002. [Google Scholar]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Choosing appropriate methods and standards for assaying tannins. J. Chem. Ecol. 1989, 15, 1795–1810. [Google Scholar] [CrossRef] [PubMed]
- Bülbring, E. Observation on the isolated phrenic nerve diaphragm preparation of the rat. Br. J. Pharmacol. 1946, 1, 38–61. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds D. alata are available from the authors. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nazato, V.S.; Rubem-Mauro, L.; Vieira, N.A.G.; Dos Santos Rocha, D., Júnior; Glauzer Silva, M.; Santos Lopes, P.; Dal-Belo, C.A.; Cogo, J.C.; Dos Santos, M.G.; Da Cruz-Höfling, M.A.; et al. In Vitro Antiophidian Properties of Dipteryx alata Vogel Bark Extracts. Molecules 2010, 15, 5956-5970. https://doi.org/10.3390/molecules15095956
Nazato VS, Rubem-Mauro L, Vieira NAG, Dos Santos Rocha D Júnior, Glauzer Silva M, Santos Lopes P, Dal-Belo CA, Cogo JC, Dos Santos MG, Da Cruz-Höfling MA, et al. In Vitro Antiophidian Properties of Dipteryx alata Vogel Bark Extracts. Molecules. 2010; 15(9):5956-5970. https://doi.org/10.3390/molecules15095956
Chicago/Turabian StyleNazato, Virgínia Sbrugnera, Leandro Rubem-Mauro, Nathalia Aparecida Gatto Vieira, Dimas Dos Santos Rocha, Júnior, Magali Glauzer Silva, Patricia Santos Lopes, Cháriston André Dal-Belo, Jose Carlos Cogo, Marcio Galdino Dos Santos, Maria Alice Da Cruz-Höfling, and et al. 2010. "In Vitro Antiophidian Properties of Dipteryx alata Vogel Bark Extracts" Molecules 15, no. 9: 5956-5970. https://doi.org/10.3390/molecules15095956
APA StyleNazato, V. S., Rubem-Mauro, L., Vieira, N. A. G., Dos Santos Rocha, D., Júnior, Glauzer Silva, M., Santos Lopes, P., Dal-Belo, C. A., Cogo, J. C., Dos Santos, M. G., Da Cruz-Höfling, M. A., & Oshima-Franco, Y. (2010). In Vitro Antiophidian Properties of Dipteryx alata Vogel Bark Extracts. Molecules, 15(9), 5956-5970. https://doi.org/10.3390/molecules15095956



