Synthesis of a New Series of N,N’-Dimethyltetrahydrosalen (H2 [H2Me]salen) Ligands by the Reductive Ring-Opening of 3,3'-Ethylene-bis(3,4-dihydro-6-substituted-2H-1,3-benzoxazines)
Abstract
:1. Introduction
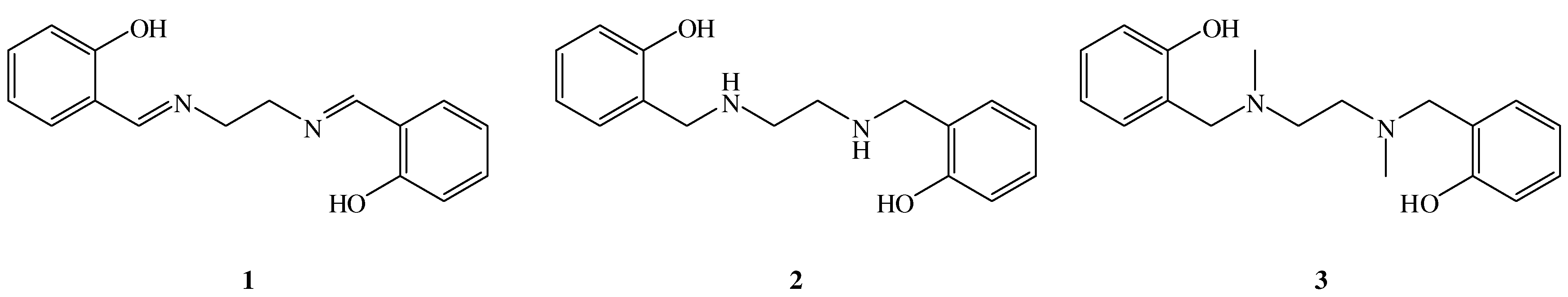
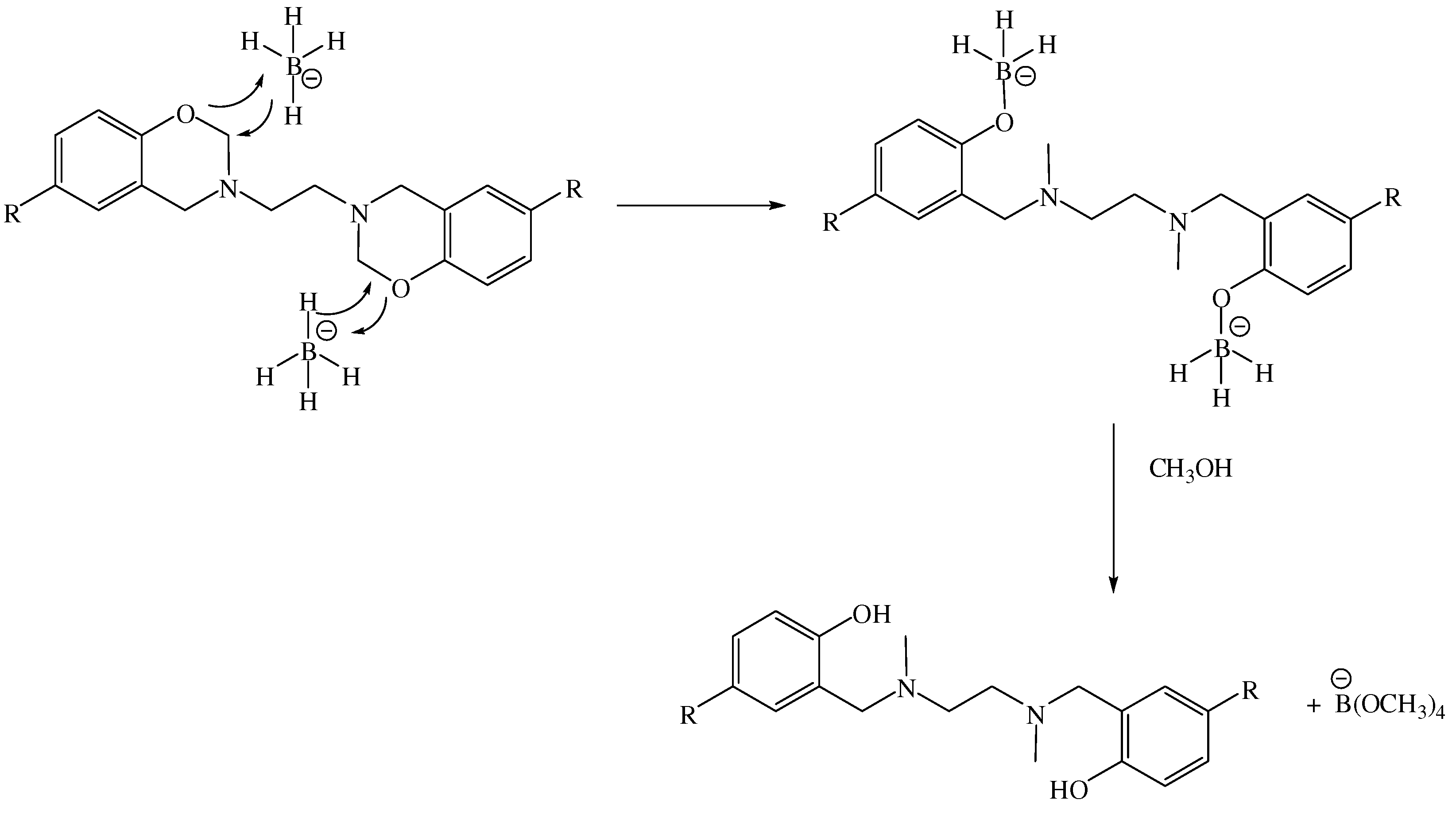
2. Results and Discussion
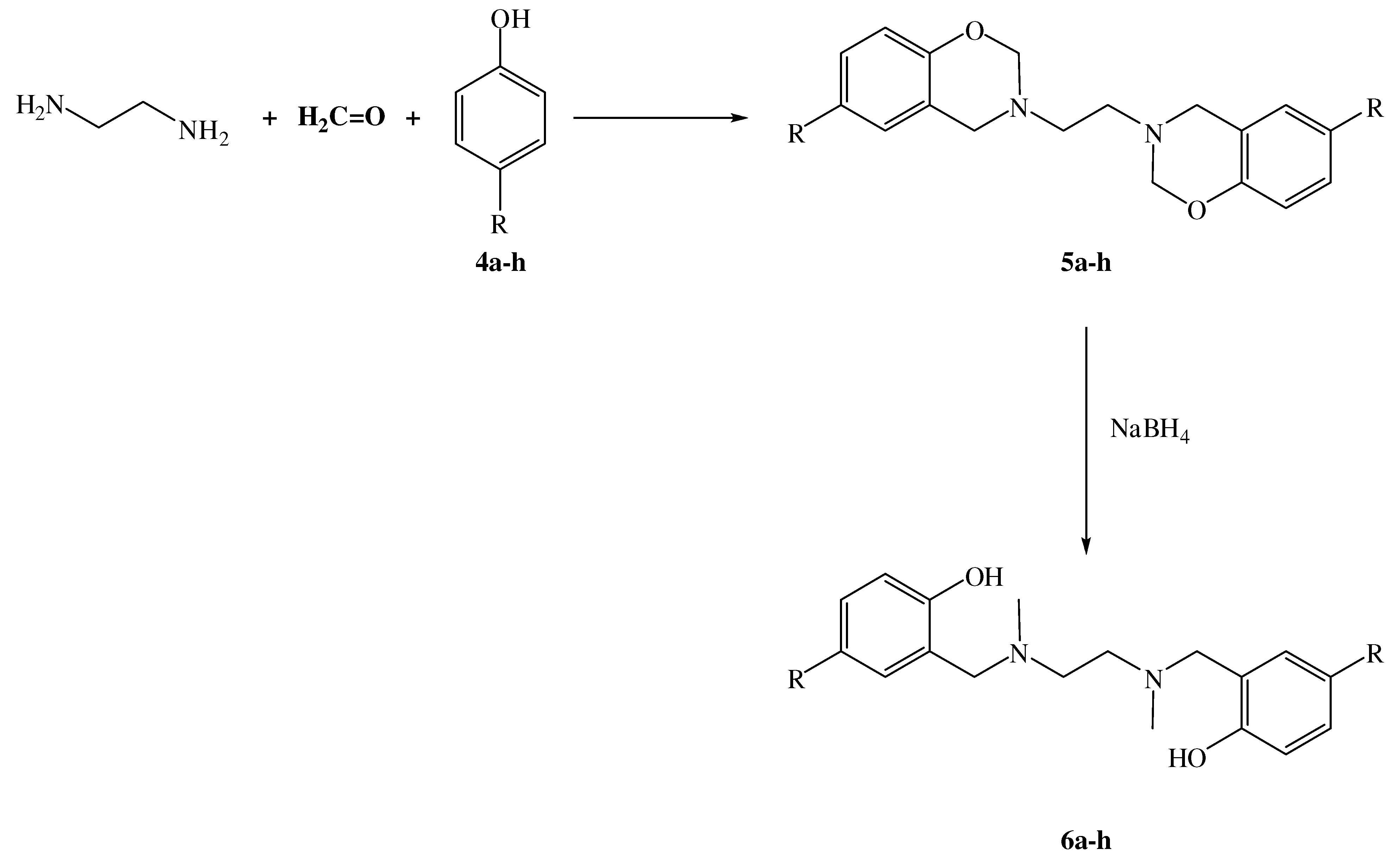
| Entry | Compound | R | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 5a | F | 6a | 38 |
| 2 | 5b | Cl | 6b | 61 |
| 3 | 5c | Br | 6c | 40 |
| 4 | 5d | I | 6d | 59 |
| 5 | 5e | COOMe | 6e | 36 |
| 6 | 5f | COOEt | 6f | 66 |
| 7 | 5g | COOPr | 6g | 70 |
| 8 | 5h | COOBu | 6h | 68 |
3. Experimental
3.1. General
3.2. General procedure for synthesis of BISBOAs
3.3. General procedure for reduction of BISBOAs
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 5a-f and 6a-f are available from the authors.
References and Notes
- Atwood, D.A.; Remington, M.P.; Rutherford, D. Use of the salan ligands to form bimetallic Aluminum Complexes. Organometallics 1996, 15, 4763–4769. [Google Scholar] [CrossRef]
- Wei, P.; Atwood, D.A. Syntheses and structures of products resulting from oxygen insertion and hydrolysis of salan-Al compounds. Polyhedron 1999, 18, 641–646. [Google Scholar]
- Atwood, D.A. Salan complexes of the group 12, 13 and 14 elements. Coord. Chem. Rev. 1997, 165, 267–296. [Google Scholar]
- Tshuva, E.Y.; Goldberg, I.; Kol, M. Isospecific living polymerization of 1-hexene by a readily available nonmetallocene C2-symmetrical Zirconium catalyst. J. Am. Chem. Soc. 2000, 122, 10706–10707. [Google Scholar]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Remarkable stereocontrol in the polymerization of racemic lactide using aluminum initiators supported by tetradentate aminophenoxide ligands. J. Am. Chem. Soc. 2004, 126, 2688–2689. [Google Scholar]
- Busico, V.; Cipullo, R.; Friederichs, N.; Ronca, S.; Talarico, G.; Togrou, M.; Wang, B. Block copolymers of highly isotactic polypropylene via controlled Ziegler−Natta polymerization. Macromolecules 2004, 37, 8201–8203. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, F.; Huang, X.; Wen, Y.; Feng, X. A new copper(I)–tetrahydrosalen-catalyzed asymmetric Henry reaction and its extension to the synthesis of (S)-norphenylephrine. Chem. Eur. J. 2007, 13, 829–833. [Google Scholar]
- MacMillan, S.N.; Jung, C.F.; Shalumova, T.; Tanski, J.M. Chiral-at-metal tetrahydrosalen complexes of resolved titanium(IV) sec-butoxides: Ligand wrapping and multiple asymmetric catalytic induction. Inorg. Chim. Acta 2009, 362, 3134–3146. [Google Scholar] [CrossRef]
- Huang, J.; Yu, J.F.; Wu, G.M.; Sun, W.L.; Shen, Z.Q. In situ generated, tetrahydrosalen stabilized yttrium borohydride complex: Efficient initiator for the ring-opening polymerization of ε-caprolactone. Chin. Chem. Lett. 2009, 20, 1357–1360. [Google Scholar]
- Matsumoto, K.; Sawada, Y.; Katsuki, T. Asymmetric epoxidation of olefins catalyzed by Ti(salan) complexes using aqueous hydrogen peroxide as the oxidant. Pure Appl. Chem. 2008, 80, 1071–1077. [Google Scholar] [CrossRef]
- Mitra, A.; Harvey, M.J.; Proffitt, M.K.; DePue L.J.; Parkin, S.; Atwood, A.D. Binuclear salan borate compounds with three-coordinate boron atoms. J. Organomet. Chem. 2006, 691, 523–528. [Google Scholar]
- Soto-Garrido, G.; Salas-Reyes, V. Salen and tetrahydrosalen nickel(II) and copper(II) complexes. Transit. Metal Chem. 2000, 25, 192–195. [Google Scholar] [CrossRef]
- Rivera, A.; Quevedo, R.; Navarro, M.A.; Maldonado, M. Efficient tetrahydrosalen synthesis from Mannich type bases. Synth. Commun. 2004, 34, 2479–2485. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Gendeziuk, N.; Kol, M. Single-step synthesis of salans and substituted salans by Mannich condensation. Tetrahedron Lett. 2001, 42, 6405–6407. [Google Scholar] [CrossRef]
- Cohen, A.; Yeori, A.; Kopilov, J.; Goldberg, I.; Kol, M. Construction of C1-symmetric Zirconium complexes by the design of new salan ligands. Coordination chemistry and preliminary polymerisation catalysis studies. Chem. Commun. 2008, 2149–2151. [Google Scholar]
- Klement, R.; Stock, F.; Elias, H.; Paulus, H.; Pelikán, P.; Valko, M.; Mazúr, M. Copper(II) complexes with derivatives of salen and tetrahydrosalen: A spectroscopic, electrochemical and structural study. Polyhedron 1999, 18, 3617–3628. [Google Scholar] [CrossRef]
- Elias, H.; Stock, F.; Röhr, C.A. Dioxomolybdenum(VI) Complex with a new enantiomerically pure tetrahydrosalen ligand. Acta Cryst. 1997, C53, 862–864. [Google Scholar]
- Balsells, J.; Carroll, P.J.; Walsh, P.J. Achiral tetrahydrosalen ligands for the synthesis of C2-symmetric Titanium complexes: A structure and diastereoselectivity study. Inorg. Chem. 2001, 40, 5568–5574. [Google Scholar] [CrossRef]
- Hinshaw, C.J.; Peng, G.; Singh, R.; Spence, J.T.; Enemark, J.H.; Bruck, M.; Kristofzski, J.; Merbs, S.L.; Ortega, R.B.; Wexler, P.A. Molybdenum (VI)-dioxo complexes with linear and tripodal tetradentate ligands: models for the molybdenum(VI/V) centers of the molybdenum hydroxylases and related enzymes. 1. Syntheses and structures. Inorg. Chem. 1989, 28, 4483–4491. [Google Scholar]
- Wong, Y.-L.; Yan, Y.; Chan, E.S.H.; Yang, Q.; Mak, T.C.W.; Ng, D.K.P. Cis-Dioxo-tungsten(VI) and -molybdenum(VI) complexes with N2O2 tetradentate ligands: synthesis, structure, electrochemistry and oxo-transfer properties. J. Chem. Soc., Dalton Trans. 1 1998, 3057–3064. [Google Scholar]
- Rivera, A.; Aguilar, Z.; Clavijo, D.; Joseph-Nathan, P. Synthesis and characterization of two new 6-halosubstituted bis benzoxazines. Anal. Quim. 1989, 85, 9–10. [Google Scholar]
- Rivera, A.; Gallo, G.I.; Gayón, M.E.; Joseph-Nathan, P. 1,3-bis-(2´-hydroxybenzil)imidazolidines as novel precursors of 3,3´-ethylene-bis)-3,4-dihydro-2H-1,3-benzoxazine. Synth. Commun. 1994, 24, 2081–2089. [Google Scholar] [CrossRef]
- Rivera, A.; Sánchez, A.; Ospina, E.; Joseph-Nathan, P. Síntesis de nuevas bis-benzoxazinas del tipo 3,3´-etilen-bis-(3,4-dihidro-6-R-2H-1,3-benzoxazina), elucidación de sus estructuras y comparación de sus espectros de RMN-1H con espectros teóricamente calculados (parte II). Rev. Colomb. Quim. 1985, 14, 77–80. [Google Scholar]
- García-Zarracino, R.; Ramos-Quiñones, J.; Höpfl, H. Preparation and structural characterization of six new diorganotin(IV) complexes of the R2Sn(SaleanH2) and R2Sn(SalceanH2) type (R = Me, n-Bu, Ph). J. Organomet. Chem. 2002, 664, 188–200. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Ciccarella, G.; Naso, F.; Perna, F.; Tortorella, P. Use of readily available chiral compounds related to the Betti base in the enantioselective addition of ethylzinc to aryl aldehydes. Tetrahedron 1999, 55, 14685–14692. [Google Scholar]
- Cimarelli, C.; Palmieri, G.; Volpini, E. Ready N-alkylation of enantiopure aminophenols: Synthesis of aminotertiary aminophenols. Tetrahedron 2001, 57, 6089–6096. [Google Scholar]
© 2010 by the authors;
Share and Cite
Rivera, A.; Rojas, J.J.; Salazar-Barrios, J.; Maldonado, M.; Ríos-Motta, J. Synthesis of a New Series of N,N’-Dimethyltetrahydrosalen (H2 [H2Me]salen) Ligands by the Reductive Ring-Opening of 3,3'-Ethylene-bis(3,4-dihydro-6-substituted-2H-1,3-benzoxazines). Molecules 2010, 15, 4102-4110. https://doi.org/10.3390/molecules15064102
Rivera A, Rojas JJ, Salazar-Barrios J, Maldonado M, Ríos-Motta J. Synthesis of a New Series of N,N’-Dimethyltetrahydrosalen (H2 [H2Me]salen) Ligands by the Reductive Ring-Opening of 3,3'-Ethylene-bis(3,4-dihydro-6-substituted-2H-1,3-benzoxazines). Molecules. 2010; 15(6):4102-4110. https://doi.org/10.3390/molecules15064102
Chicago/Turabian StyleRivera, Augusto, Jicli José Rojas, Jairo Salazar-Barrios, Mauricio Maldonado, and Jaime Ríos-Motta. 2010. "Synthesis of a New Series of N,N’-Dimethyltetrahydrosalen (H2 [H2Me]salen) Ligands by the Reductive Ring-Opening of 3,3'-Ethylene-bis(3,4-dihydro-6-substituted-2H-1,3-benzoxazines)" Molecules 15, no. 6: 4102-4110. https://doi.org/10.3390/molecules15064102
APA StyleRivera, A., Rojas, J. J., Salazar-Barrios, J., Maldonado, M., & Ríos-Motta, J. (2010). Synthesis of a New Series of N,N’-Dimethyltetrahydrosalen (H2 [H2Me]salen) Ligands by the Reductive Ring-Opening of 3,3'-Ethylene-bis(3,4-dihydro-6-substituted-2H-1,3-benzoxazines). Molecules, 15(6), 4102-4110. https://doi.org/10.3390/molecules15064102




