Synthesis and Biological Evaluation of a γ-Cyclodextrin-based Formulation of the Anticancer Agent 5,6,11,12,17,18,23,24- Octahydrocyclododeca[1,2-b:4,5-b’:7,8-b’’:10,11-b’’’]tetraindole (CTet)
Abstract
:1. Introduction
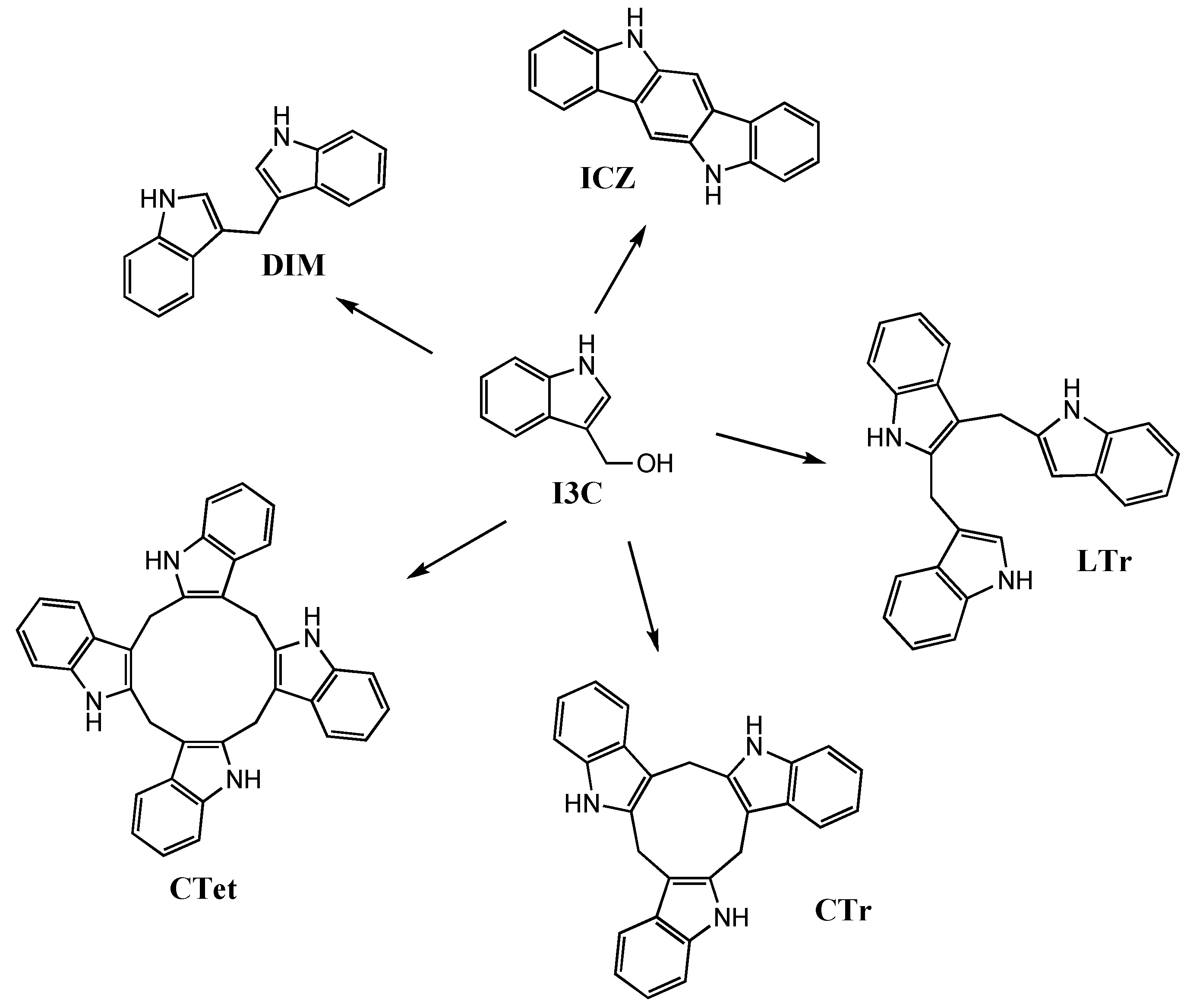
2. Results and Discussion
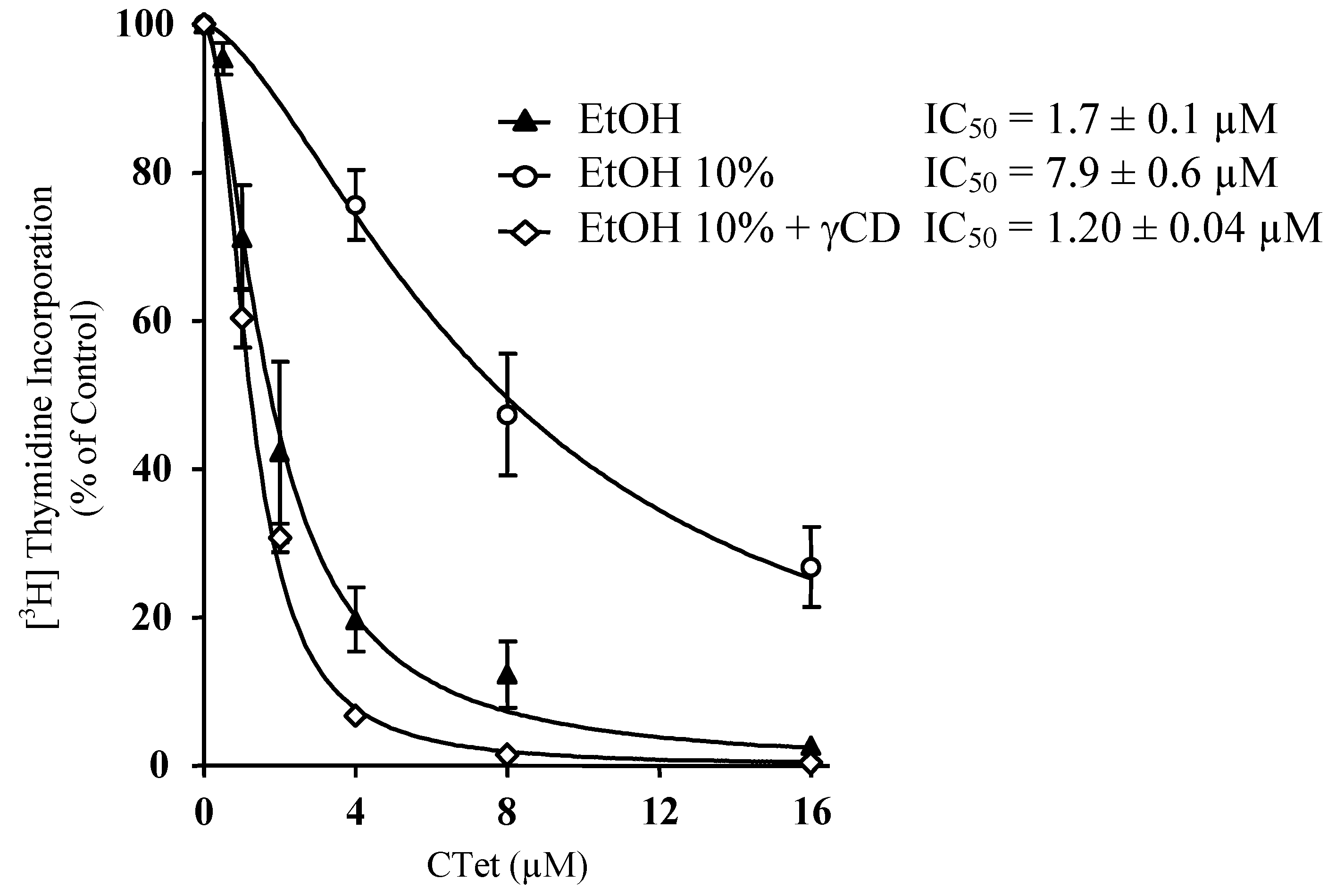
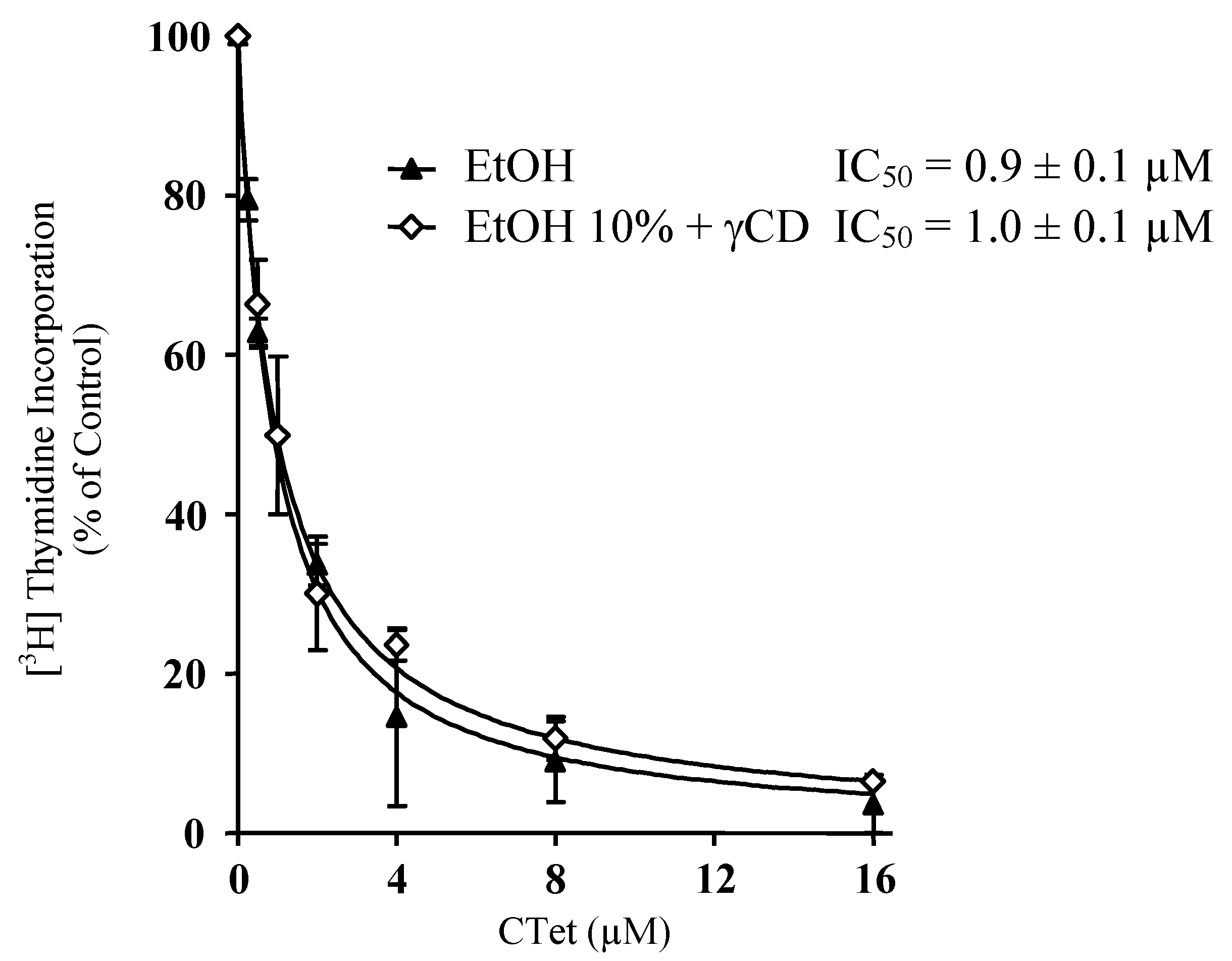
3. Experimental
3.1. General
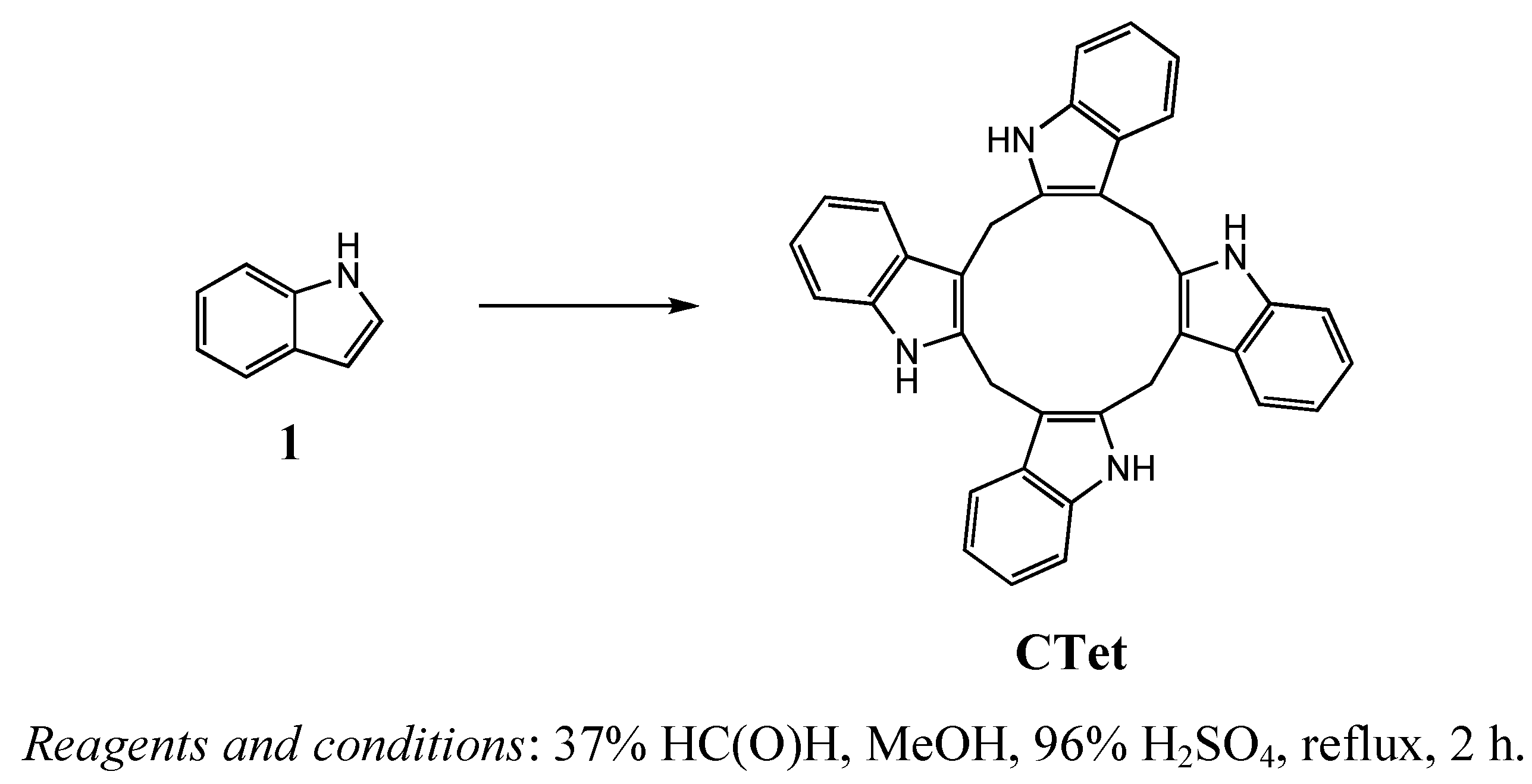
3.2. Synthesis of 5,6,11,12,17,18,23,24-octahydrocyclododeca[1,2-b:4,5-b’:7,8-b’’:10,11-b’’’]tetraindole (CTet)
3.3. CTet formulations
3.4. Cell cultures and antiproliferative assay
3.5. Statistical analyses
4. Conclusions
Acknowledgements
- Sample Availability: A sample of the compound is available from the authors.
References
- National Research Council, Diet, Nutrition and Cancer; Peter, F.M. (Ed.) National Academy Press: Washington, DC, USA, 1982; pp. 358–370.
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 269, 153–163. [Google Scholar]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis(3’-indolyl)methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef]
- Virtanen, A.I. Studies on organic sulphur compounds and other labile substances in plants. Pythochemistry 1965, 4, 207–228. [Google Scholar] [CrossRef]
- Wattenberg, L.W.; Loub, W.D. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978, 38, 1410–1413. [Google Scholar]
- Bjeldanes, L.F.; Kim, J.-Y.; Grose, K.R.; Bartholomew, J.C.; Bradfield, C.A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA 1991, 88, 9543–9547. [Google Scholar]
- D'Argy, R.; Bergman, J.; Dencker, L. Effects of immunosuppressive chemicals on lymphoid development in fetal thymus organ cultures. Pharmacol. Toxicol. 1989, 64, 33–38. [Google Scholar] [CrossRef]
- De Kruif, C.A.; Marsman, J.W.; Venekamp, J.C.; Falke, H.E.; Noordhoek, J.; Blaauboer, B.J.; Wortelboer, H.M. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem.-Biol. Interact. 1991, 80, 303–315. [Google Scholar]
- Chang, Y.-C.; Riby, J.; Chang, G.H.-F.; Peng, B.C.; Firestone, G.; Bjeldanes, L.F. Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3,3’-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochem. Pharmacol. 1999, 58, 825–834. [Google Scholar]
- Riby, J.E.; Feng, C.; Chang, Y.-C.; Schaldach, C.M.; Firestone, G.L.; Bjeldanes, L.F. The major cyclic trimeric product of indole-3-carbinol is a strong agonist of the estrogen receptor signaling pathway. Biochemistry 2000, 39, 910–918. [Google Scholar]
- Grose, K.R.; Bjeldanes, L.F. Oligomerization of indole-3-carbinol in aqueous acid. Chem. Res. Toxicol. 1992, 5, 188–193. [Google Scholar]
- Brandi, G.; Paiardini, M.; Cervasi, B.; Fiorucci, C.; Filippone, P.; De Marco, C.; Zaffaroni, N.; Magnani, M. A new indole-3-carbinol tetrameric derivative inhibits cyclin-dependent kinase 6 expression, and induces G1 cell cycle arrest in both estrogen-dependent and estrogen-independent breast cancer cell lines. Cancer Res. 2003, 63, 4028–4036. [Google Scholar]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Wang, D.; Lin, C.-H.; Yang, H.-C.; Ma, Y.; Sargeant, A.; Chiu, C.-F.; Tsai, M.-H.; Chen, C.-S. A potent indole-3-carbinol–derived antitumor agent with pleiotropic effects on multiple signaling patways in prostate cancer cells. Cancer Res. 2007, 67, 7815–7824. [Google Scholar]
- Jump, S.M.; Kung, J.; Staub, R.; Kinset, M.A.; Cram, E.J.; Yudina, L.N.; Preobrazhenskaya, M.N.; Bjeldanes, L.F.; Firestone, G.L. N-Alkoxy derivatization of indole-3-carbinol increases the efficacy of the G1 cell cycle arrest and of I3C-specific regulation of cell cycle gene transcription and activity in human breast cancer cells. Biochem. Pharmacol. 2008, 75, 713–724. [Google Scholar]
- Guo, W.; Wu, S.; Liu, J.; Fang, B. Identification of a small molecule with synthetic lethality for K-Ras and protein kinase C iota. Cancer Res. 2008, 68, 7403–7408. [Google Scholar] [CrossRef]
- Ramamoorthy, K.; Navo, M.; Gupta, M.S.; Safe, S.H. AhR-mediated antiestrogenicity of diindolylmethane and analogs in vivo and in vitro. Organohalogen Compd. 1998, 37, 321–324. [Google Scholar]
- Ramamoorthy, K.; McDougal, A.; Safe, S.H. Structure-Ah receptor agonist/binding activity relationships of various chlorine-substituted diindolylmethane compounds. Organohalogen Compd. 1999, 42, 363–367. [Google Scholar]
- McDougal, A.; Gupta, M.S.; Ramamoorthy, K.; Sun, G.; Safe, S.H. Inhibition of carcinogen-induced rat mammary tumor growth and other estrogen-dependent responses by symmetrical dihalo-substituted analogs of diindolylmethane. Cancer Lett. 2000, 151, 169–179. [Google Scholar] [CrossRef]
- McDougal, A.; Gupta, M.S.; Morrow, D.; Ramamoorthy, K.; Lee, J.-E.; Safe, S.H. Methyl-substituted diindolylmethanes as inhibitors of estrogen-induced growth of T47D cells and mammary tumors in rats. Breast Cancer Res. Treat. 2001, 66, 147–157. [Google Scholar] [CrossRef]
- Benabadji, S.H.; Wen, R.; Zheng, J.-B.; Dong, X.-C.; Yuan, S.-G. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacol.Sin. 2004, 25, 666–671. [Google Scholar]
- Pisano, C.; Kollar, P.; Gianní, M.; Kalac, Y.; Giordano, V.; Ferrara, F.F.; Tancredi, R.; Devoto, A.; Rinaldi, A.; Rambaldi, A.; Penco, S.; Marzi, M.; Moretti, G.; Vesci, L.; Tinti, O.; Carminati, P.; Terao, M.; Garattini, E. Bis-indols: a novel class of molecules enhancing the cytodifferentiating properties of retinoids in myeloid leukemia cells. Blood 2002, 100, 3719–3730. [Google Scholar]
- Giannini, G.; Marzi, M.; Moretti, G.P.; Penco, S.; Tinti, M.O.; Pesci, S.; Lazzaro, F.; De Angelis, F. Synthesis of cycloalkanoindoles by an unusual DAST-triggered rearrangement reaction. Eur. J. Org. Chem. 2004, 2411–2420. [Google Scholar]
- Maciejewska, D.; Rasztawicka, M.; Wolska, I.; Anuszewka, E.; Gruber, B. Novel 3,3’-diindolylmethane derivatives: Synthesis and cytotoxicity, structural characterization in solid state. Eur. J. Med. Chem. 2009, 44, 4136–4147. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Slobbe, L.; Lansbergen, G.W.A.; Safe, S.; van der Berg, M. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and diindolylmethanes differentially induce cytochrome P450 1A1, 1B1, and 19 in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 2001, 61, 40–48. [Google Scholar] [CrossRef]
- Qin, C.; Morrow, D.; Stewart, J.; Spencer, K.; Porter, W.; Smith, R., III; Phillips, T.; Abdelrahim, M.; Samudio, I.; Safe, S. A new class of peroxisome proliferator-activated receptor g (PPARg) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3’-indolyl)-1-(p-substituted phenyl)methanes. Mol. Cancer Ther. 2004, 3, 247–259. [Google Scholar]
- Contractor, R.; Samudio, I.J.; Estrov, Z.; Harris, D.; McCubrey, J.A.; Safe, S.H.; Andreeff, M.; Konopleva, M. A novel ring-substituted diindolylmethane, 1,1-bis[3’-(5-methoxyindolyl)]-1-(p-t-butylphenyl) methane, inhibits extracellular signal-regulated kinase activation and induces apoptosis in acute myelogenous leucemia. Cancer Res. 2005, 65, 2890–2898. [Google Scholar]
- Chintharlapalli, S.; Burghardt, R.; Papineni, S.; Ramaiah, S.; Yoon, K.; Safe, S. Activation of Nur77 by selected 1,1-bis(3’-indolyl)-1-(p-substitued phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem. 2005, 280, 24903–24914. [Google Scholar]
- Inamoto, T.; Papineni, S.; Chintharlapalli, S.; Cho, S.-D.; Safe, S.; Kamat, A.M. 1,1-Bis(3’-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol. Cancer Ther. 2008, 7, 3825–3833. [Google Scholar] [CrossRef]
- Noguchi-Yachide, T.; Tetsuhashi, M.; Aoyama, H.; Hashimoto, Y. Enhancement of chemically-induced HL-60 cell differentiation by 3,3’-diindolylmethane derivatives. Chem. Pharm. Bull. 2009, 57, 536–540. [Google Scholar]
- Chao, W.-R.; Yean, D.; Amin, K.; Green, C.; Jong, L. Computer-aided rational drug design: A novel agent (SR13668) designed to mimic the unique anticancer mechanisms of dietary indole-3-carbinol to block akt signaling. J. Med. Chem. 2007, 50, 3412–3415. [Google Scholar]
- Vincent, E.; Shirani, H.; Bergman, J.; Rannug, U.; Janosik, T. Synthesis and biological evaluation of fused thio- and selenopyrans as new indolocarbazole analogues with aryl hydrocarbon receptor affinity. Bioorg. Med. Chem. 2009, 17, 1648–1653. [Google Scholar]
- Xue, L.; Schaldach, C.M.; Janosik, T.; Bergman, J.; Bjeldanes, L.F. Effects of analogs of indole-3-carbinol cyclic trimerization product in human breast cancer cells. Chem.-Biol. Interact. 2005, 152, 119–129. [Google Scholar]
- Bergman, J.; Högberg, S.; Lindström, J.-O. Macrocyclic condensation products of indole and simple aldehydes. Tetrahedron 1970, 26, 3347–3352. [Google Scholar] [CrossRef]
- Staub, R.E.; Bjeldanes, L.F. Convenient synthesis of 5,6,11,12,17,18-hexahydrocyclononal[1,2-b:4,5-b’:7,8-b’’]triindole, a novel phytoestrogen. J. Org. Chem. 2003, 68, 167–169. [Google Scholar]
© 2010 by the authors;
Share and Cite
Lucarini, S.; De Santi, M.; Antonietti, F.; Brandi, G.; Diamantini, G.; Fraternale, A.; Paoletti, M.F.; Tontini, A.; Magnani, M.; Duranti, A. Synthesis and Biological Evaluation of a γ-Cyclodextrin-based Formulation of the Anticancer Agent 5,6,11,12,17,18,23,24- Octahydrocyclododeca[1,2-b:4,5-b’:7,8-b’’:10,11-b’’’]tetraindole (CTet). Molecules 2010, 15, 4085-4093. https://doi.org/10.3390/molecules15064085
Lucarini S, De Santi M, Antonietti F, Brandi G, Diamantini G, Fraternale A, Paoletti MF, Tontini A, Magnani M, Duranti A. Synthesis and Biological Evaluation of a γ-Cyclodextrin-based Formulation of the Anticancer Agent 5,6,11,12,17,18,23,24- Octahydrocyclododeca[1,2-b:4,5-b’:7,8-b’’:10,11-b’’’]tetraindole (CTet). Molecules. 2010; 15(6):4085-4093. https://doi.org/10.3390/molecules15064085
Chicago/Turabian StyleLucarini, Simone, Mauro De Santi, Francesca Antonietti, Giorgio Brandi, Giuseppe Diamantini, Alessandra Fraternale, Maria Filomena Paoletti, Andrea Tontini, Mauro Magnani, and Andrea Duranti. 2010. "Synthesis and Biological Evaluation of a γ-Cyclodextrin-based Formulation of the Anticancer Agent 5,6,11,12,17,18,23,24- Octahydrocyclododeca[1,2-b:4,5-b’:7,8-b’’:10,11-b’’’]tetraindole (CTet)" Molecules 15, no. 6: 4085-4093. https://doi.org/10.3390/molecules15064085
APA StyleLucarini, S., De Santi, M., Antonietti, F., Brandi, G., Diamantini, G., Fraternale, A., Paoletti, M. F., Tontini, A., Magnani, M., & Duranti, A. (2010). Synthesis and Biological Evaluation of a γ-Cyclodextrin-based Formulation of the Anticancer Agent 5,6,11,12,17,18,23,24- Octahydrocyclododeca[1,2-b:4,5-b’:7,8-b’’:10,11-b’’’]tetraindole (CTet). Molecules, 15(6), 4085-4093. https://doi.org/10.3390/molecules15064085







