8-Hydroxyquinoline Dansylates Modified with PAMAM Dendrimer as Fluorescent Fe3+ Sensors
Abstract
:1. Introduction
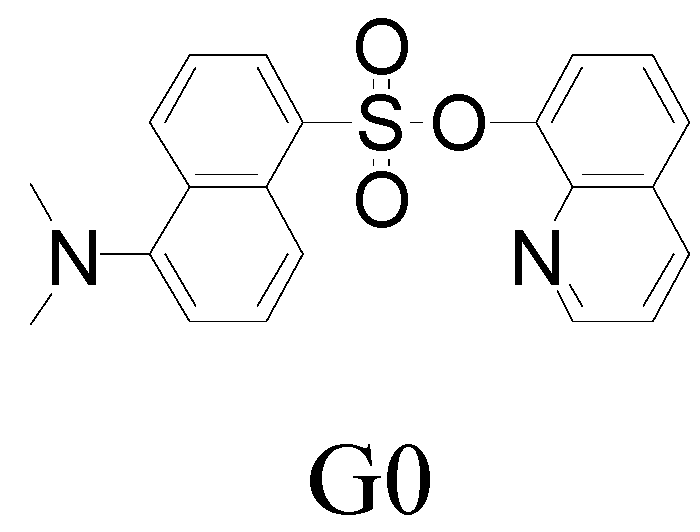
2. Results and Discussion
2.1. Synthesis of dendrimers

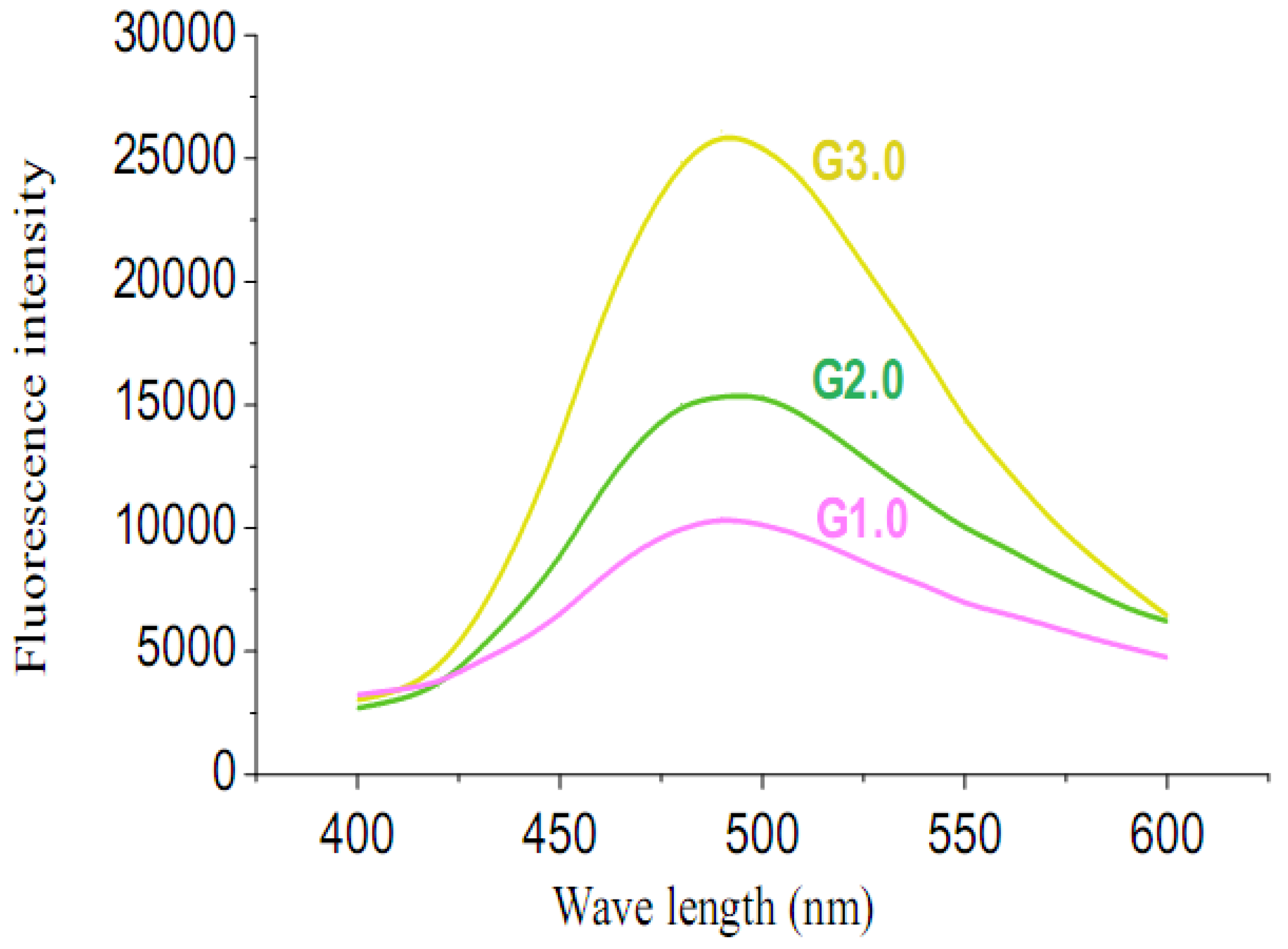
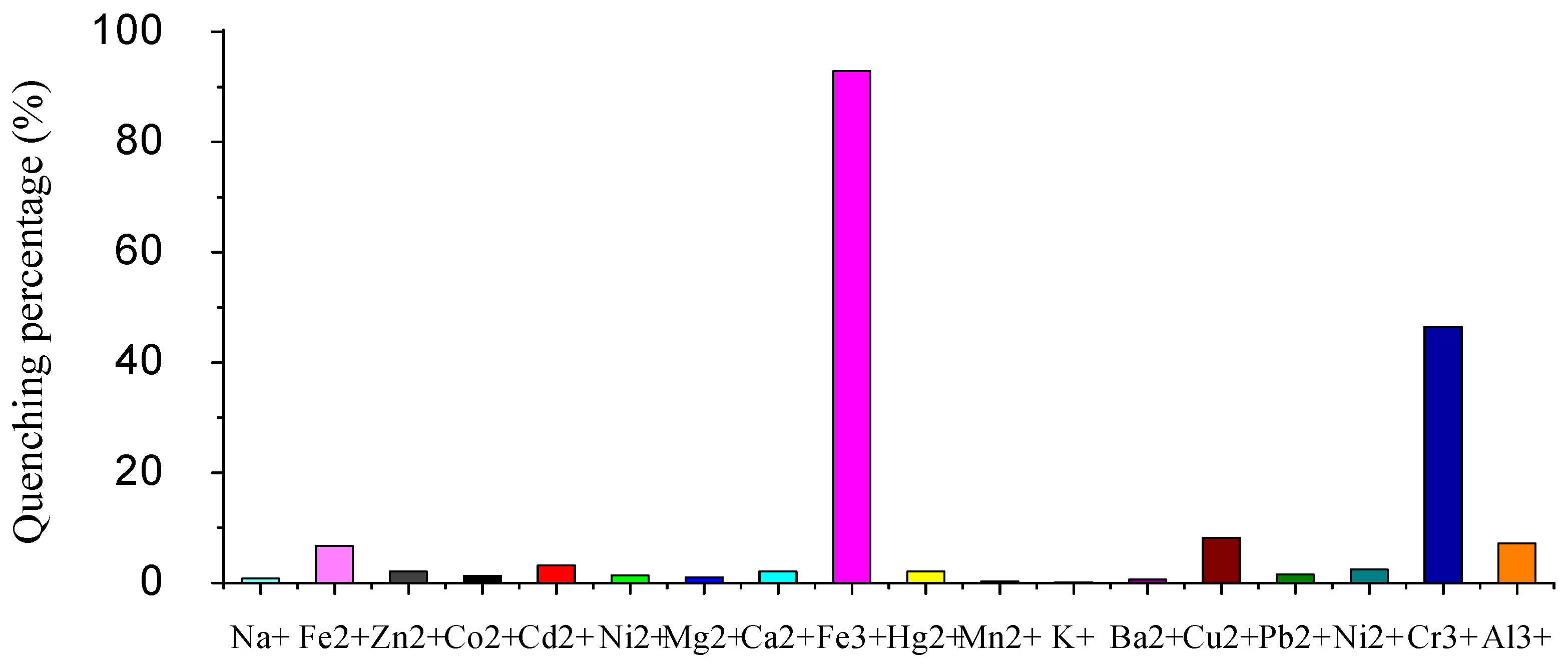
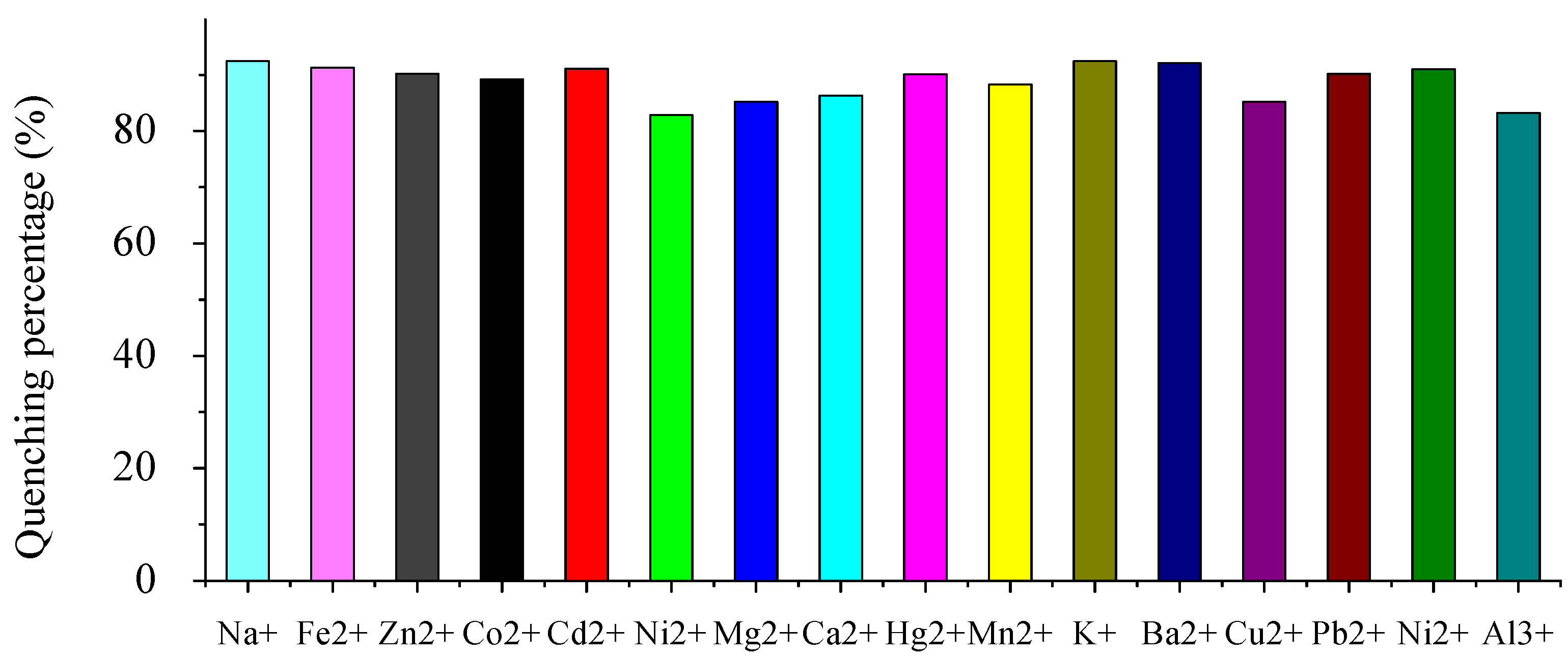
2.2. Fluorescence emission properties
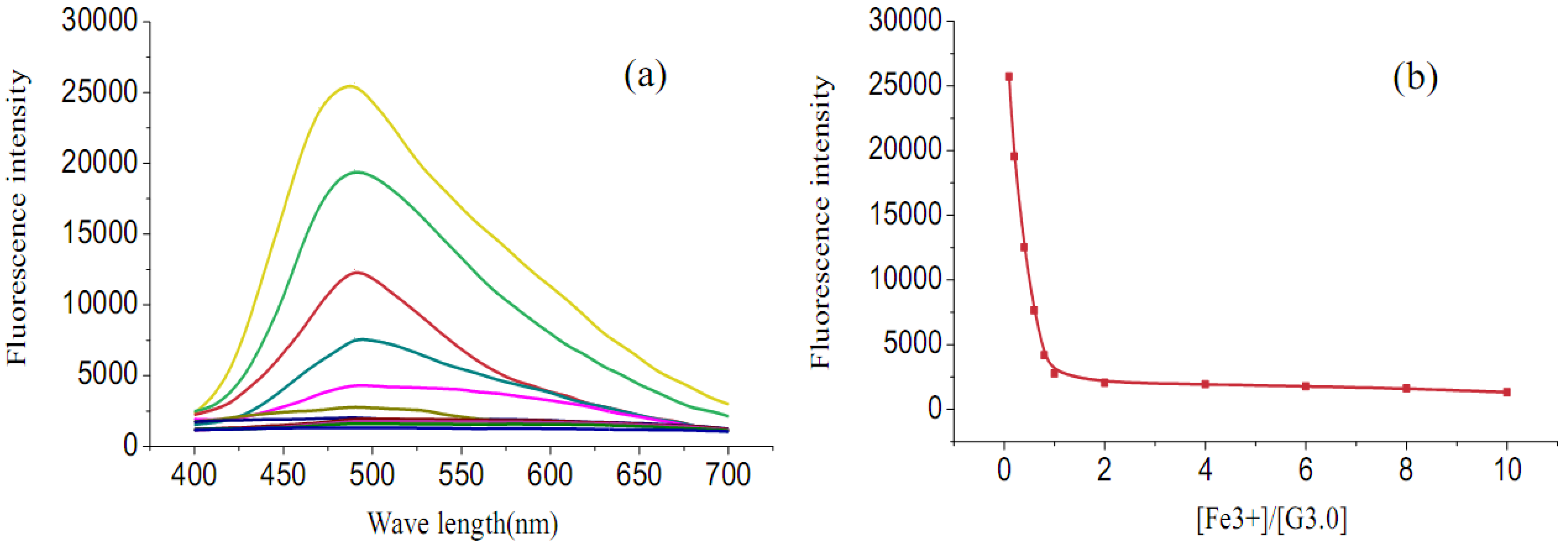
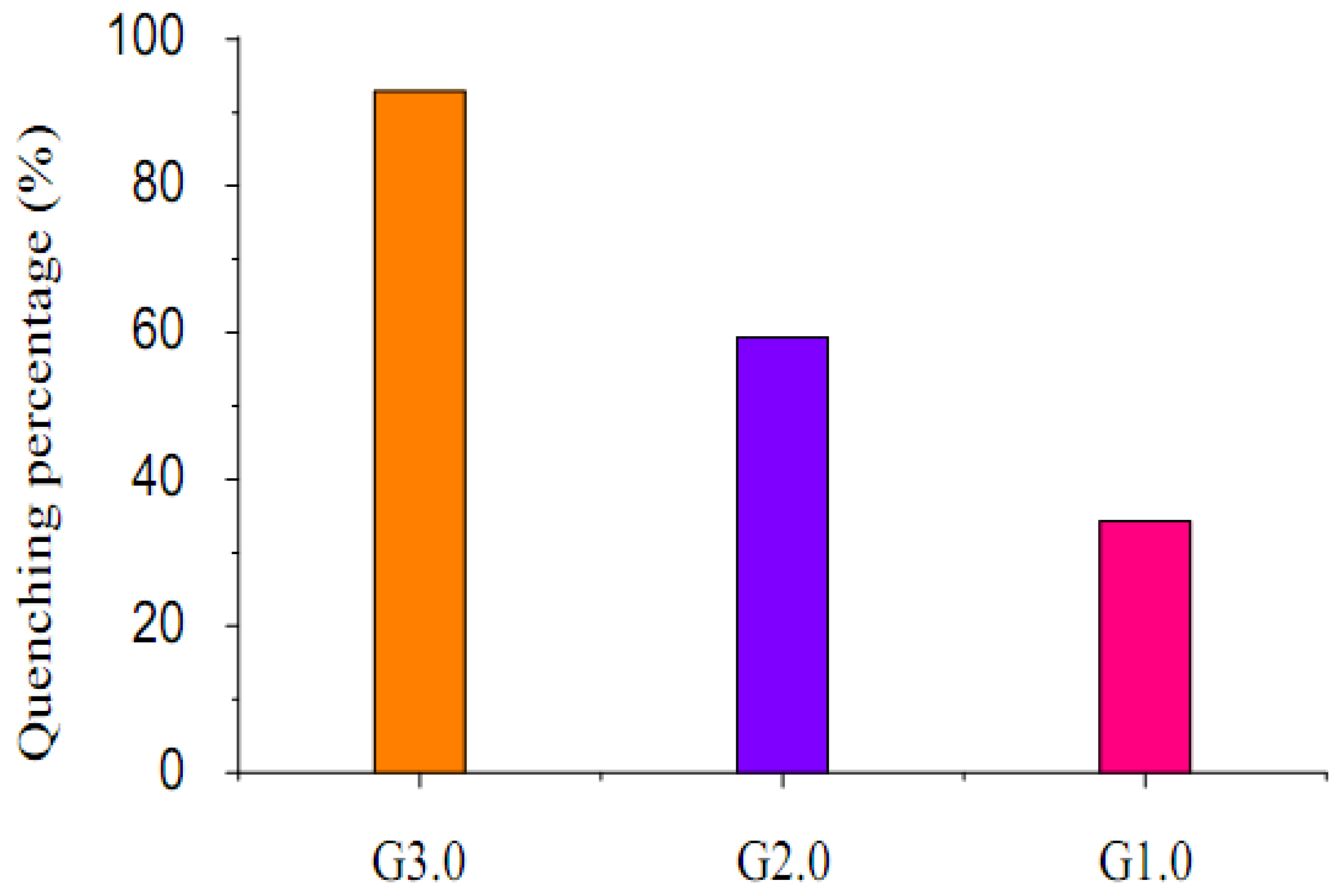
3. Experimental
3.1. Materials and instruments
3.2. Synthesis of G1.0
3.3. Synthesis of G2.0
3.4. Synthesis of G3.0
4. Conclusions
- Sample Availability: Samples of the compounds including G0, G1.0 and G3.0 are available from the authors.
References
- Baars, M.W.P.L.; Meijer, E.W. Host-guest chemistry of dendritic molecules. Top. Curr. Chem. 2000, 210, 131–182. [Google Scholar] [CrossRef]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1→2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Liu, M.; Kono, K.; Fréchet, J.M.J. Water-soluble dendritic unimolecular micelles: Their potential as drug delivery agents. J. Control. Release 2000, 65, 121–131. [Google Scholar] [CrossRef]
- Liu, M.; Fréchet, J.M.J. Preparation of Water-soluble dendritic unimolecular micelles as potential drug delivery agents. Polym. Mater. Sci. Eng. 1999, 80, 167–168. [Google Scholar]
- Twyman, L.J.; Beezer, A.E.; Esfand, R.; Hardy, M.J.; Mitchell, J.C. The Synthesis of water soluble dendrimers, and their application as possible drug delivery systems. Tetrahedron Lett. 1999, 40, 1743–1746. [Google Scholar] [CrossRef]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Liu, M.; Fréchet, J.M.J. Designing dendrimers for drug delivery. Pharm. Sci. Tech. Today 1999, 2, 393–401. [Google Scholar] [CrossRef]
- Smith, D.K.; Diederich, F. Functional Dendrimers: Unique Biological Mimics. Chem. Eur. J. 1998, 4, 1353–1361. [Google Scholar] [CrossRef]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef]
- Froehling, P.E. Dendrimers and dyes-a review. Dyes Pigments 2001, 48, 187–195. [Google Scholar] [CrossRef]
- Weil, T.; Wiesler, U.M.; Herrmann, A.; Bauer, R.; Hofkens, J.; De Schryver, F.C.; Mullen, K. Polyphenylene dendrimers with different fluorescent chromophores asymmetrically distributed at the periphery. J. Am. Chem. Soc. 2001, 123, 8101–8108. [Google Scholar]
- Gilat, S.L.; Adronov, A.; Fréchet, J.M.J. Modular Approach to the accelerated convergent growth of laser dye-labeled poly(aryl ether) dendrimers using a novel hypermonomer. JOrg. Chem. 1999, 64, 7474–7484. [Google Scholar] [CrossRef]
- Gilat, S.L.; Adronov, A.; Fréchet, J.M.J. Light harvesting and energy transfer in novel convergently constructed dendrimers. Angew. Chem. Int. Ed. 1999, 38, 1422–1427. [Google Scholar]
- Adronov, A.; Fréchet, J.M.J. Light-harvesting dendrimers. Chem. Commun. 2000, 18, 1701–1710. [Google Scholar]
- Jungle, D.M.; McGrath, D.V. Photoresponsive azobenzene-containing dendrimers with multiple discrete states. J. Am. Chem. Soc. 1999, 121, 4912–4913. [Google Scholar]
- Fomie, S.; Rivera, E.; Fomina, L.; Ortiz, A.; Ogawa, T. Polymers from coumarines: 4. Design and synthesis of novel hyperbranched and comb-like coumarin-containing polymers. Polymer 1998, 39, 3551–3558. [Google Scholar] [CrossRef]
- Vogtle, F.; Gorka, M.; Hesse, R.; Ceroni, P.; Maestri, M.; Balzani, V. Photochemical and photophysical properties of poly(propylene amine) dendrimers with peripheral naphthalene and azobenzene groups. Photochem. Photobiol. Sci. 2002, 1, 45–51. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Gestermann, S.; Kauffmann, C.; Gorka, M.; Vogtle, F. Dendrimers as fluorescent sensors with signal amplification. Chem. Commun. 2000, 10, 853–854. [Google Scholar]
- Balzani, V.; Ceroni, P.; Gestermann, S.; Kauffmann, C.; Gorka, M.; Vogtle, F. Effect of protons and metal ions on the fluorescence properties of a polylysin dendrimer containing twenty four dansyl units. J. Chem. Soc. Dalton Trans. 2000, 21, 3765–3771. [Google Scholar]
- Wang, J.; Jiang, M. Dendritic nucleic acid probes for DNA biosensors. J. Am. Chem. Soc. 1998, 120, 8281–8282. [Google Scholar] [CrossRef]
- Chang, A.C.; Gillespie, J.B.; Tabacco, M.B. Enhanced detection of live bacteria using a dendrimer thin film in an optical biosensor. Anal. Chem. 2001, 73, 467–470. [Google Scholar]
- Shi, D.X.; Sha, Y.W.; Wang, F. Synthesis and Photophysical properties of poly(ester-amine) dendrimers with focal 4-amino-N-benzylphthalimide, as Sensitive media probes and switchable proton sensors. Macromolecules 2008, 41, 7478–7484. [Google Scholar] [CrossRef]
- Wang, F.; Peng, R.G.; Sha, Y.W. Selective dendritic fluorescent sensors for Zn(II). Molecules 2008, 13, 922–930. [Google Scholar] [CrossRef]
- Shen, L.; Li, F.Y.; Sha, Y.W. Synthesis of fluorescent dendritic 8-hydroxyquinoline ligands and investigation on their coordinated Zn(II) complexes. Tetrahedron Lett. 2004, 45, 3961–3964. [Google Scholar] [CrossRef]
- Sha, Y.W.; Shen, L.; Hong, X.Y. A divergent synthesis of new aliphatic poly(ester-amine) dendrimers bearing peripheral hydroxyl or acrylate groups. Tetrahedron Lett. 2002, 43, 9417–9419. [Google Scholar]
- Hu, S.L.; She, N.F.; Yin, G.D.; Guo, H.Z.; Wu, A.X.; Yang, C.L. Synthesis, structural characterization, and fluorescent chemosensory properties of novel molecular clips based on diethoxycarbonyl glycoluril. Tetrahedron Lett. 2007, 48, 1591–1594. [Google Scholar]
- Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Buschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the development of sensor molecules that display Fe(III)-amplified fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar]
- Ouchetto, H.; Dias, M.; Mornet, R.; Lesuisse, E.; Camadro, J.M. A new route to trihydroxamate-containing artificial siderophores and synthesis of a new fluorescent probe. Bioorg. Med. Chem. 2005, 13, 1799–1803. [Google Scholar] [CrossRef]
- Tumambac, G.E.; Rosencrance, C.M.; Wolf, C. Selective metal ion recognition using a fluorescent 1,8-diquinolylnaphthalene-derived sensor in aqueous solution. Tetrahedron 2004, 60, 11293–11297. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, W.; Quinn, P.J.; Liu, Z.; Hider, R.C. Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors. J. Med. Chem. 2004, 47, 6349–6362. [Google Scholar]
- Nudelman, R.; Ardon, O.; Hadar, Y.; Chen, Y.; Libman, J.; Shanzer, A. Modular fluorescent-labeled siderophore analogues. J. Med. Chem. 1998, 41, 1671–1678. [Google Scholar] [CrossRef]
- Weizman, H.; Ardon, O.; Mester, B.; Libman, J.; Dwir, O.; Hadar, Y.; Chen, Y.; Shanzer, A. Fluorescently-labeled ferrichrome analogs as probes for receptor-mediated, microbial iron uptake. J. Am. Chem. Soc. 1996, 118, 12368–12375. [Google Scholar]
- Peng, R.; Wang, F.; Sha, Y.W. Synthesis of 5-dialkyl(aryl)aminomethyl-8-hydroxyquinoline dansylates as selectivefluorescent sensors of Fe3+. Molecules 2007, 12, 1191–1201. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, W.; Li, J.; Tian, H. Novel red-light emitting metal complex based on asymmetric perylene bisimide and 8-hydroxyquinoline dyads. Synth. Met. 2004, 145, 203–210. [Google Scholar] [CrossRef]
- Job, P. Studies on the formation of complex minerals in solution and on their stability. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
© 2010 by the authors;
Share and Cite
Zhang, Q.; Sha, Y.; Wang, J.-H. 8-Hydroxyquinoline Dansylates Modified with PAMAM Dendrimer as Fluorescent Fe3+ Sensors. Molecules 2010, 15, 2962-2971. https://doi.org/10.3390/molecules15052962
Zhang Q, Sha Y, Wang J-H. 8-Hydroxyquinoline Dansylates Modified with PAMAM Dendrimer as Fluorescent Fe3+ Sensors. Molecules. 2010; 15(5):2962-2971. https://doi.org/10.3390/molecules15052962
Chicago/Turabian StyleZhang, Qi, Yaowu Sha, and Jin-Hui Wang. 2010. "8-Hydroxyquinoline Dansylates Modified with PAMAM Dendrimer as Fluorescent Fe3+ Sensors" Molecules 15, no. 5: 2962-2971. https://doi.org/10.3390/molecules15052962
APA StyleZhang, Q., Sha, Y., & Wang, J.-H. (2010). 8-Hydroxyquinoline Dansylates Modified with PAMAM Dendrimer as Fluorescent Fe3+ Sensors. Molecules, 15(5), 2962-2971. https://doi.org/10.3390/molecules15052962




