Synthesis and Antimicrobial Activity of New 4-Heteroarylamino Coumarin Derivatives Containing Nitrogen and Sulfur as Heteroatoms
Abstract
:1. Introduction
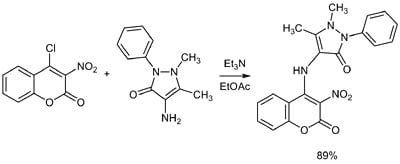
2. Results and Discussion

| Compound | Ar | Formula | Mp/ oC | Colour | Yield/ % |
|---|---|---|---|---|---|
| 5a |  | C12H9N3O4S | 218-220 | yellow | 75 |
| 5b |  | C13H9N3O4S | 229-232 | orange | 71 |
| 5c |  | C13H9N3O4S | 212-215 | yellow | 72 |
| 5d |  | C11H7N5O4 | 249-252 | yellow | 82 |
| 5e |  | C20H16N4O5 | 240-242 | yellow | 89 |
| 5f |  | C18H13N3O4S | 240-242 | yellow | 66 |
| 5g |  | C16H10N4O4 | 253-255 | yellow | 76 |
| 5h |  | C16H10N4O4 | 255-257 | yellow | 69 |
| Microorganism | compound | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | Tetracycline | Nystatine | |
| B. subtilis | 20 | 17 | 20 | 16 | na | 16 | 19 | 19 | 27 | nt |
| Cl. pyogenes | 19 | 21 | 21 | 17 | na | 20 | 20 | 20 | 27 | nt |
| Enterococcussp. | 18 | 19 | 22 | 17 | na | 20 | 20 | 21 | 28 | nt |
| M. flavus | 18 | 19 | 22 | 17 | na | 17 | 18 | 20 | 31 | nt |
| S. lutea | 20 | 22 | 24 | 18 | na | 17 | 23 | 22 | 27 | nt |
| S. aureus | 20 | 20 | 27 | 18 | na | 19 | 23 | 20 | 25 | nt |
| E. coli ATCC 8739 | 18 | 16 | 20 | 15 | na | 18 | 15 | 20 | 27 | nt |
| E. coli ATCC 25922 | 18 | 18 | 24 | 18 | na | 20 | 18 | 20 | 28 | nt |
| K. pneumoniae | 14 | 12 | 14 | 13 | na | 14 | 15 | na | 23 | nt |
| S. enteritidis | 19 | 16 | 22 | 18 | na | 20 | 12 | 17 | 26 | nt |
| P. vulgaris | 20 | 18 | 20 | 17 | na | 18 | 18 | 20 | 26 | nt |
| P. aeruginosa ATCC 27857 | 17 | 19 | 21 | 17 | na | 18 | 17 | 21 | 26 | nt |
| P. aeruginosa ATCC 9027 | 19 | 17 | 22 | 17 | na | 19 | 18 | 15 | 25 | nt |
| A. niger | 12 | 14 | 18 | 14 | na | 13 | 15 | na | nt | 18 |
| C. albicans | 14 | 14 | 15 | 15 | na | 14 | 15 | 15 | nt | 19 |
| S. cerevisiae | na | na | 10 | 10 | na | 14 | na | na | nt | 17 |

3. Experimental Section
3.1. General
3.2. Synthesis of 4-chloro-3-nitrocoumarin 3
3.3. General procedure for the synthesis of 4-heteroarylamino-3-nitrocoumarins 5a–h
3.4. Antimicrobial activity
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds 5a–h are available from the authors.
References and Notes
- El-Agrody, A.M.; Abd El-Latif, M.S.; El-Hady, N.A.; Fakery, A.H.; Bedair, A.H. Heteroaromatization with 4-hydroxycoumarin part II: synthesis of some new pyrano[2,3-d]pyrimidines, [1,2,4]triazolo[1,5-c]pyrimidines and pyrimido[1,6-b]-[1,2,4]triazine derivatives. Molecules 2001, 6, 519–527. [Google Scholar] [CrossRef]
- Pratibha, S.; Shreeya, P. Synthesis, characterization and antimicrobial studies of some novel 3-arylazo-7-hydroxy-4-methyl-coumarin. Indian J. Chem. 1999, 38, 1139–1142. [Google Scholar]
- Patonay, T.; Litkei, G.Y.; Bognar, R.; Erdei, J.; Misztic, C. Synthesis, antibacterial and antifungal activity of 4-hydroxy-coumarin derivatives, analogues of Novobiocin. Pharmazie 1984, 39, 86–91. [Google Scholar]
- Shaker, R.M. Synthesis and reactions of some new 4H-pyrano[3,2-c]benzopyran-5-one derivatives and their potential biological activities. Pharmazie 1996, 51, 148–151. [Google Scholar]
- El-Farargy, A.F. Synthesis and some reactions of 8-terc-butyl-6-hydroxy-4-methylcoumarin. Egypt. J. Pharm. Sci. 1991, 32, 625. [Google Scholar]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin. Eur. J. Med. Chem. 1995, 30, 531–536. [Google Scholar] [CrossRef]
- Emmanuel-Giota, A.A.; Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Synthesis and biological evalution of several 3-(coumarin-4-yl)tetrahydroisoxazole and 3-(coumarin-4-yl)dihydropyrazole derivatives. J. Heterocyclic Chem. 2001, 38, 717–722. [Google Scholar] [CrossRef]
- Raev, L.; Voinov, E.; Ivanov, I.; Popov, D. Antitumor activity of some coumarin derivatives. Pharmazie 1990, 45, 696. [Google Scholar]
- Nofal, Z.M.; El-Zahar, M.; Abd El-Karim, S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Xie, L.; Tukeuchi, Y.; Consetino, L.M.; Lee, K. Synthesis and structure-activity relationships of (3‘R,4‘R)-(+)-cis-khellactone derivatives as novel potent anti-HIV agents. J. Med. Chem. 1999, 42, 2662–2672. [Google Scholar] [CrossRef]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; John Wiley & Sons Ltd.: Chichester, UK, 1997. [Google Scholar]
- Zahradnik, M. The Production and Application of Fluorescent Brightening Agent; John Wiley & Sons Ltd.: Chichester, England, 1992. [Google Scholar]
- Nora de Souza, M.V. Synthesis and biological activity of natural thiazoles: An important class of heterocyclic compounds. J. Sulfur Chem. 2005, 26, 429–449. [Google Scholar] [CrossRef]
- Geronikaki, A.; Babaev, E.; Dearden, J.; Dehaen, W.; Filimonov, D.; Galaeva, I.; Krajneva, V.; Lagunin, A.; Macaev, F.; Molodavkin, G.; Poroikov, V.; Pogrebnoi, S.; Saloutin, V.; Stepanchikova, A.; Stingaci, E.; Tkach, N.; Vladg, L.; Voronina, T. Design, synthesis, computational and biological evaluation of new anxiolytics. Bioorg.Med. Chem. 2004, 12, 6559–6568. [Google Scholar]
- Sammelson, R.E.; Caboni, P.; Durkin, K.A.; Casida, J.E. GABA receptor antagonists and insecticides: common structural features of 4-alkyl-1-phenylpyrazoles and 4-alkyl-1-phenyltrioxabicyclooctanes. Bioorg. Med. Chem. 2004, 12, 3345–3355. [Google Scholar]
- Kumar, J.S.; Prabhakaran, J.; Arango, V.; Parsey, R.V.; Underwood, M.D.; Simpson, N.R.; Kassir, S.A.; Majo, V.J.; Van Heertum, R.L.; Mann, J.J. Synthesis of [O-methyl-11C]1-(2-chlorophenyl)-5-(4-methoxyphenyl)-4-methyl-1H-pyrazole-3-carboxylic acid piperidin-1-ylamide: a potential PET ligand for CB1 receptors. Bioorg. Med. Chem. Lett. 2004, 14, 2393–2396. [Google Scholar]
- Bekhit, A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 1935–1945. [Google Scholar] [CrossRef]
- Baraldi, P.; Beria, I.; Cozzi, P.; Bianchi, N.; Gambari, R.; Romagnoli, R. Synthesis and growth inhibition activity of α-Bromoacrylic heterocyclic and benzoheterocyclic derivatives of distamycin A modified on the amidino moiety. Bioorg. Med. Chem. 2003, 11, 965–975. [Google Scholar] [CrossRef]
- Debeljak, Z.; Škrbo, A.; Jasprica, I.; Mornar, A.; Plečko, V.; Banjanac, M.; Medić-Sarić, M. QSAR study of antimicrobial activity of some 3-nitrocoumarins and related compound. J. Chem. Inf. Model. 2007, 47, 918–926. [Google Scholar]
- Radulović, N.; Stojanović, G.; Vukićević, R.; Dekić, V.; Dekić, B.; Palić, R. New 3,4-annelated coumarin derivatives: synthesis, antimicrobial activity, antioxidant capacity, and molecular modeling. Monatsh.Chem. 2006, 137, 1477–1486. [Google Scholar] [CrossRef]
- Dekić, S.V.; Dekić, V.S.; Dekić, B.R.; Dekić, M.S. Synthesis of new condensed and cyclized coumarin derivatives. Chem. Pap. 2007, 61, 233–235. [Google Scholar] [CrossRef]
- Dekić, S.V.; Dekić, V.S.; Vučić, B.; Dekić, B.R.; Dekić, M.S. Synthesis of new condensed coumarin derivatives. Facta Universitat., Ser.: Phys., Chem. Technol. 2007, 5, 85–88. [Google Scholar]
- Dekić, M.S.; Dekić, B.R.; Dekić, V.S.; Dekić, S.V. Synthesis and structure of novel 3,4-annelated coumarin derivatives. J. Heterocycl. Chem. 2008, 45, 295–297. [Google Scholar] [CrossRef]
- Dekić, B.; Dekić, V.; Radulović, N.; Vukićević, R.; Palić, R. Synthesis of new antimicrobial 4-aminosubstituted 3-nitrocoumarins. Chem. Pap. 2010, 64, 354–359. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards, Performance Standards For Antimicrobial Disk Susceptibility Test. Approved Standard M2-A6. National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1997. [Google Scholar]
- Savel’ev, V.L.; Artamonova, O.S.; Troitskaya, V.S.; Vinokurov, V.G.; Zagorevskii, V.A. Investigations of pyrans and related compounds. Khim Geterotsikl. 1973, 7, 816–820. [Google Scholar]
- Kaljaj, V.; Trkovnik, M.; Stefanović-Kaljaj, L. Synthesis of new heterocyclocoumarins starting with 3-cyano-4-chlorocoumarin. J. Serb. Chem. Soc. 1987, 52, 183–185. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dekić, B.R.; Radulović, N.S.; Dekić, V.S.; Vukićević, R.D.; Palić, R.M. Synthesis and Antimicrobial Activity of New 4-Heteroarylamino Coumarin Derivatives Containing Nitrogen and Sulfur as Heteroatoms. Molecules 2010, 15, 2246-2256. https://doi.org/10.3390/molecules15042246
Dekić BR, Radulović NS, Dekić VS, Vukićević RD, Palić RM. Synthesis and Antimicrobial Activity of New 4-Heteroarylamino Coumarin Derivatives Containing Nitrogen and Sulfur as Heteroatoms. Molecules. 2010; 15(4):2246-2256. https://doi.org/10.3390/molecules15042246
Chicago/Turabian StyleDekić, Biljana R., Niko S. Radulović, Vidoslav S. Dekić, Rastko D. Vukićević, and Radosav M. Palić. 2010. "Synthesis and Antimicrobial Activity of New 4-Heteroarylamino Coumarin Derivatives Containing Nitrogen and Sulfur as Heteroatoms" Molecules 15, no. 4: 2246-2256. https://doi.org/10.3390/molecules15042246
APA StyleDekić, B. R., Radulović, N. S., Dekić, V. S., Vukićević, R. D., & Palić, R. M. (2010). Synthesis and Antimicrobial Activity of New 4-Heteroarylamino Coumarin Derivatives Containing Nitrogen and Sulfur as Heteroatoms. Molecules, 15(4), 2246-2256. https://doi.org/10.3390/molecules15042246